JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature
Abstract
Simple Summary
Abstract
1. Introduction
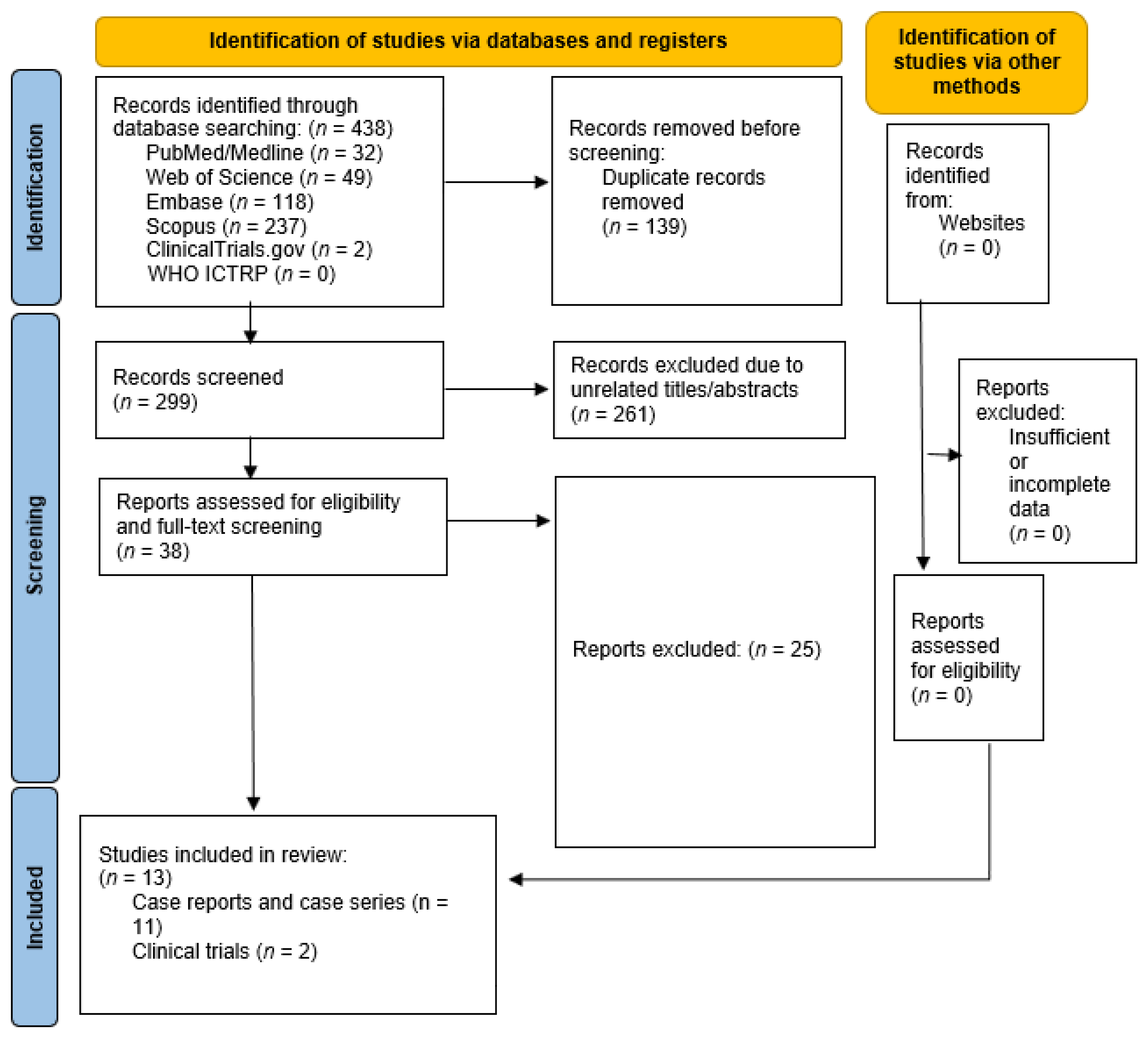
2. Materials and Methods
3. Result
3.1. Studies Evaluating the Efficacy of JAK Inhibitors in the Treatment of CTCL
3.1.1. Clinical Trials
3.1.2. Case Reports/Series
3.2. De Novo CTCL following JAK Inhibitor Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood J. Am. Soc. Hematol. 2019, 133, 1703–1714. [Google Scholar] [CrossRef]
- Korgavkar, K.; Xiong, M.; Weinstock, M. Changing incidence trends of cutaneous T-cell lymphoma. JAMA Dermatol. 2013, 149, 1295–1299. [Google Scholar] [CrossRef]
- Nasimi, M.; Kamyab, K.; Aghahi, T.; Fahim, S.; Ghandi, N. Childhood mycosis fungoides: A clinicopathologic study of 30 cases from Iran. Australas. J. Dermatol. 2019, 61, e259–e261. [Google Scholar] [CrossRef]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.d.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Lymphoid neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Campo, E.; Jaffe, E.S.; Cook, J.R.; Quintanilla-Martinez, L.; Swerdlow, S.H.; Anderson, K.C.; Brousset, P.; Cerroni, L.; de Leval, L.; Dirnhofer, S. The international consensus classification of mature lymphoid neoplasms: A report from the clinical advisory committee. Blood J. Am. Soc. Hematol. 2022, 140, 1229–1253. [Google Scholar] [CrossRef]
- Amorim, G.M.; Corbellini, J.P.N.; Quintella, D.C.; Cuzzi, T.; Ramos-e-Silva, M. Evaluation of the Cutaneous Lymphoma International Prognostic Index in patients with early stage mycosis fungoides. Anais Bras. Dermatol. 2018, 93, 680–685. [Google Scholar] [CrossRef]
- Benton, E.; Crichton, S.; Talpur, R.; Agar, N.; Fields, P.; Wedgeworth, E.; Mitchell, T.; Cox, M.; Ferreira, S.; Liu, P. A cutaneous lymphoma international prognostic index (CLIPi) for mycosis fungoides and Sezary syndrome. Eur. J. Cancer 2013, 49, 2859–2868. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2019, 94, 1027–1041. [Google Scholar] [CrossRef]
- Gilson, D.; Whittaker, S.; Child, F.; Scarisbrick, J.; Illidge, T.M.; Parry, E.; Mohd Mustapa, M.; Exton, L.; Kanfer, E.; Rezvani, K. British Association of Dermatologists and UK Cutaneous Lymphoma Group guidelines for the management of primary cutaneous lymphomas 2018. Br. J. Dermatol. 2019, 180, 496–526. [Google Scholar] [CrossRef]
- Hristov, A.C.; Tejasvi, T.; Wilcox, R.A. Cutaneous T-cell lymphomas: 2021 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2021, 96, 1313–1328. [Google Scholar] [CrossRef]
- Kamijo, H.; Miyagaki, T. Mycosis fungoides and Sézary syndrome: Updates and review of current therapy. Curr. Treat. Options Oncol. 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Dippel, E.; Assaf, C.; Becker, J.C.; von Bergwelt-Baildon, M.; Bernreiter, S.; Cozzio, A.; Eich, H.T.; Elsayad, K.; Follmann, M.; Grabbe, S. S2k-Guidelines–Cutaneous lymphomas (ICD10 C82-C86): Update 2021. J. Der Dtsch. Dermatol. Ges. 2022, 20, 537. [Google Scholar] [CrossRef]
- de Masson, A.; Beylot-Barry, M.; Ram-Wolff, C.; Mear, J.-B.; Dalle, S.; d’Incan, M.; Ingen-Housz-Oro, S.; Orvain, C.; Abraham, J.; Dereure, O. Allogeneic transplantation in advanced cutaneous T-cell lymphomas (CUTALLO): A propensity score matched controlled prospective study. Lancet 2023, 401, 1941–1950. [Google Scholar] [CrossRef]
- Groner, B.; von Manstein, V. Jak Stat signaling and cancer: Opportunities, benefits and side effects of targeted inhibition. Mol. Cell Endocrinol. 2017, 451, 1–14. [Google Scholar] [CrossRef]
- Samuel, C.; Cornman, H.; Kambala, A.; Kwatra, S.G. A Review on the Safety of Using JAK Inhibitors in Dermatology: Clinical and Laboratory Monitoring. Dermatol. Ther. 2023, 13, 729–749. [Google Scholar] [CrossRef]
- Miot, H.A.; Criado, P.R.; de Castro, C.C.S.; Ianhez, M.; Talhari, C.; Ramos, P.M. JAK-STAT pathway inhibitors in dermatology. Anais Bras. Dermatol. 2023, 98, 656–677. [Google Scholar] [CrossRef]
- Solimani, F.; Meier, K.; Ghoreschi, K. Emerging Topical and Systemic JAK Inhibitors in Dermatology. Front. Immunol. 2019, 10, 2847. [Google Scholar] [CrossRef]
- Gallardo, F.; Pujol, R.M. Genetics Abnormalities with Clinical Impact in Primary Cutaneous Lymphomas. Cancers 2022, 14, 4972. [Google Scholar] [CrossRef]
- Tensen, C.P.; Quint, K.D.; Vermeer, M.H. Genetic and epigenetic insights into cutaneous T-cell lymphoma. Blood 2022, 139, 15–33. [Google Scholar] [CrossRef]
- García-Díaz, N.; Piris, M.; Ortiz-Romero, P.L.; Vaqué, J.P. Mycosis Fungoides and Sézary Syndrome: An Integrative Review of the Pathophysiology, Molecular Drivers, and Targeted Therapy. Cancers 2021, 13, 1931. [Google Scholar] [CrossRef]
- Luo, Y.; Vermeer, M.H.; de Haan, S.; Kinderman, P.; de Gruijl, F.R.; van Hall, T.; Tensen, C.P. Socs1-knockout in skin-resident CD4(+) T cells in a protracted contact-allergic reaction results in an autonomous skin inflammation with features of early-stage mycosis fungoides. Biochem. Biophys. Rep. 2023, 35, 101535. [Google Scholar] [CrossRef]
- Bastidas Torres, A.N.; Cats, D.; Out-Luiting, J.J.; Fanoni, D.; Mei, H.; Venegoni, L.; Willemze, R.; Vermeer, M.H.; Berti, E.; Tensen, C.P. Deregulation of JAK2 signaling underlies primary cutaneous CD8(+) aggressive epidermotropic cytotoxic T-cell lymphoma. Haematologica 2022, 107, 702–714. [Google Scholar] [CrossRef]
- Netchiporouk, E.; Litvinov, I.V.; Moreau, L.; Gilbert, M.; Sasseville, D.; Duvic, M. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle 2014, 13, 3331–3335. [Google Scholar] [CrossRef]
- Rendón-Serna, N.; Correa-Londoño, L.A.; Velásquez-Lopera, M.M.; Bermudez-Muñoz, M. Cell signaling in cutaneous T-cell lymphoma microenvironment: Promising targets for molecular-specific treatment. Int. J. Dermatol. 2021, 60, 1462–1480. [Google Scholar] [CrossRef]
- Ghoreschi, K.; Laurence, A.; O’Shea, J.J. Janus kinases in immune cell signaling. Immunol. Rev. 2009, 228, 273–287. [Google Scholar] [CrossRef]
- O’Shea, J.J.; Schwartz, D.M.; Villarino, A.V.; Gadina, M.; McInnes, I.B.; Laurence, A. The JAK-STAT pathway: Impact on human disease and therapeutic intervention. Annu. Rev. Med. 2015, 66, 311–328. [Google Scholar] [CrossRef]
- Ciechanowicz, P.; Rakowska, A.; Sikora, M.; Rudnicka, L. JAK-inhibitors in dermatology: Current evidence and future applications. J. Dermatol. Treat. 2019, 30, 648–658. [Google Scholar] [CrossRef]
- Muddebihal, A.; Khurana, A.; Sardana, K. JAK inhibitors in dermatology: The road travelled and path ahead, a narrative review. Expert Rev. Clin. Pharmacol. 2023, 16, 279–295. [Google Scholar] [CrossRef]
- Herrera-deGuise, C.; Serra-Ruiz, X.; Lastiri, E.; Borruel, N. JAK inhibitors: A new dawn for oral therapies in inflammatory bowel diseases. Front. Med. 2023, 10, 1089099. [Google Scholar] [CrossRef]
- Shawky, A.M.; Almalki, F.A.; Abdalla, A.N.; Abdelazeem, A.H.; Gouda, A.M. A comprehensive overview of globally approved JAK inhibitors. Pharmaceutics 2022, 14, 1001. [Google Scholar] [CrossRef]
- Winthrop, K.L. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat. Rev. Rheumatol. 2017, 13, 234–243. [Google Scholar] [CrossRef]
- Bakr, F.S.; Whittaker, S.J. Advances in the understanding and treatment of Cutaneous T-cell Lymphoma. Front. Oncol. 2022, 12, 1043254. [Google Scholar] [CrossRef]
- Pérez, C.; Mondéjar, R.; García-Díaz, N.; Cereceda, L.; León, A.; Montes, S.; Durán Vian, C.; Pérez Paredes, M.; González-Morán, A.; Alegre de Miguel, V. Advanced-stage mycosis fungoides: Role of the signal transducer and activator of transcription 3, nuclear factor-κB and nuclear factor of activated T cells pathways. Br. J. Dermatol. 2020, 182, 147–155. [Google Scholar] [CrossRef]
- Vadivel, C.K.; Gluud, M.; Torres-Rusillo, S.; Boding, L.; Willerslev-Olsen, A.; Buus, T.B.; Nielsen, T.K.; Persson, J.L.; Bonefeld, C.M.; Geisler, C. JAK3 is expressed in the nucleus of malignant T cells in cutaneous T cell lymphoma (CTCL). Cancers 2021, 13, 280. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Feldman, T.A.; Hess, B.T.; Khodadoust, M.S.; Kim, Y.H.; Munoz, J.; Patel, M.R.; Phillips, T.J.; Smith, S.D.; Smith, S.M. A phase 2 study of the dual SYK/JAK inhibitor cerdulatinib demonstrates good tolerability and clinical response in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood 2019, 134, 466. [Google Scholar] [CrossRef]
- Lee, K.; Evans, M.G.; Yang, L.; Ng, S.; Snowden, C.; Khodadoust, M.; Brown, R.A.; Trum, N.A.; Querfeld, C.; Doan, L.T. Primary cytotoxic T-cell lymphomas harbor recurrent targetable alterations in the JAK-STAT pathway. Blood J. Am. Soc. Hematol. 2021, 138, 2435–2440. [Google Scholar]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Vardhana, S.; Ganesan, N.; Hancock, H. A phase 2 biomarker-driven study of ruxolitinib demonstrates effectiveness of JAK/STAT targeting in T-cell lymphomas. Blood J. Am. Soc. Hematol. 2021, 138, 2828–2837. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Horwitz, S.M.; Feldman, T.A.; Hess, B.T.; Khodadoust, M.S.; Kim, Y.H.; Munoz, J.; Patel, M.R.; Phillips, T.J.; Smith, S.D.; Smith, S.M. The novel SYK/JAK inhibitor cerdulatinib demonstrates good tolerability and clinical response in a phase 2a study in relapsed/refractory peripheral T-cell lymphoma and cutaneous T-cell lymphoma. Blood 2018, 132, 1001. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Ghione, P.; Jacobsen, E.D.; Ruan, J.; Schatz, J.H.; Noor, S.; Myskowski, P.; Hancock, H.; Davey, T.; Obadi, O. Final results of a phase II biomarker-driven study of ruxolitinib in relapsed and refractory T-cell lymphoma. Blood 2019, 134, 4019. [Google Scholar] [CrossRef]
- Moskowitz, A.J.; Jacobsen, E.; Ruan, J.; Schatz, J.H.; Obadi, O.; Motylinski, K.; Jarjies, C.; Galasso, N.; Hancock, H.; Davey, T. Durable responses observed with JAK inhibition in T-cell lymphomas. Blood 2018, 132, 2922. [Google Scholar] [CrossRef]
- Lévy, R.; Fusaro, M.; Guerin, F.; Chetouani, A.; Moshous, D.; Fischer, A.; de Saint Basile, G.; Sepulveda, F.E.; Neven, B. Efficacy of ruxolitinib in subcutaneous panniculitis-like T-cell lymphoma and hemophagocytic lymphohistiocytosis. Blood Adv. 2020, 4, 1383–1387. [Google Scholar] [CrossRef]
- Castillo, D.E.; Romanelli, P.; Lev-Tov, H.; Kerdel, F. A case of erythrodermic mycosis fungoides responding to upadacitinib. JAAD Case Rep. 2022, 30, 91–93. [Google Scholar] [CrossRef]
- Kook, H.D.; Park, S.Y.; Hong, N.; Lee, D.H.; Jung, H.J.; Park, M.Y.; Ahn, J. A Case of Mycosis Fungoides Mimicking Atopic Dermatitis Treated with Upadacitinib. Acta Derm.-Venereol. 2022, 102, 30. [Google Scholar]
- Watson, L.R.; Lew, T.E.; Fox, L.C.; Khot, A.; van der Weyden, C. Ruxolitinib bridging therapy to allogeneic SCT for high-risk refractory subcutaneous panniculitis-like T-cell lymphoma. Leuk. Lymphoma 2022, 63, 3217–3221. [Google Scholar] [CrossRef]
- Hansen, S.; Alduaij, W.; Biggs, C.M.; Belga, S.; Luecke, K.; Merkeley, H.; Chen, L.Y. Ruxolitinib as adjunctive therapy for secondary hemophagocytic lymphohistiocytosis: A case series. Eur. J. Haematol. 2021, 106, 654–661. [Google Scholar] [CrossRef]
- Duan, Y.; Gao, H.; Zhou, C.; Jin, L.; Yang, J.; Huang, S.; Zhang, M.; Zhang, Y.; Wang, T. A retrospective study of 18 children with subcutaneous panniculitis-like T-cell lymphoma: Multidrug combination chemotherapy or immunomodulatory therapy? Orphanet J. Rare Dis. 2022, 17, 432. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, C.J.; Li, D.H.; Cui, L.; Li, W.J.; Ma, H.H.; Zhao, Y.Z.; Wang, D.; Li, Z.G.; Wang, T.Y. Efficacy of ruxolitinib for HAVCR2 mutation-associated hemophagocytic lymphohistiocytosis and panniculitis manifestations in children. Br. J. Haematol. 2023, 202, 135–146. [Google Scholar] [CrossRef]
- Iinuma, S.; Hayashi, K.; Noguchi, A.; Ishida-Yamamoto, A. Lymphomatoid papulosis during upadacitinib treatment for rheumatoid arthritis. Eur. J. Dermatol. 2022, 32, 142. [Google Scholar]
- Knapp III, C.; Steele, E.; Mengden-Koon, S.; Williams, T.; Fett, N. A Case of Tofacitinib-Induced Lymphomatoid Papulosis With Ocular Involvement. Am. J. Dermatopathol. 2022, 44, 523–525. [Google Scholar] [CrossRef]
- Saito, K.; Shimauchi, T.; Kageyama, R.; Furukawa, S.; Suzuki, N.; Ginoza, A.; Moriki, M.; Ito, T.; Honda, T. A case of Sézary syndrome in a patient during treatment with baricitinib for seronegative rheumatoid arthritis. Clin. Exp. Dermatol. 2023, 48, 391–393. [Google Scholar] [CrossRef]
- Cohen, E.; Bozonnat, A.; Battistella, M.; Calvani, J.; Vignon-Pennamen, M.-D.; Rivet, J.; Moins-Teisserenc, H.; Ta, V.-A.; Ram-Wolff, C.; Bouaziz, J.-D. Severe relapses of cutaneous T-cell lymphoma after treatment of chronic graft-versus-host disease with ruxolitinib. J. Eur. Acad. Dermatol. Venereol. 2023, 38, e32–e34. [Google Scholar] [CrossRef]
- Papadavid, E.; Pappa, V.; Kapniari, E.; Nikolaou, V.; Hliakis, T.; Dalamaga, M.; Jonak, C.; Porkert, S.; Engelina, S.; Quaglino, P. Real life data on advanced cutaneous T cell lymphoma patients treated with brentuximab vedotin: Results from a multicenter European EORTC study. Eur. J. Cancer 2019, 119, S34–S35. [Google Scholar] [CrossRef]
- Amagai, R.; Kambayashi, Y.; Ohuchi, K.; Furudate, S.; Hashimoto, A.; Asano, Y.; Fujimura, T. Cutaneous T cell lymphoma treated with mogamulizumab monotherapy and mogamulizumab plus etoposide combined therapy: A real-world case series. Dermatol. Ther. 2022, 35, e15858. [Google Scholar] [CrossRef]
- Ansell, S.M.; Maris, M.B.; Lesokhin, A.M.; Chen, R.W.; Flinn, I.W.; Sawas, A.; Minden, M.D.; Villa, D.; Percival, M.-E.M.; Advani, A.S. Phase I study of the CD47 blocker TTI-621 in patients with relapsed or refractory hematologic malignancies. Clin. Cancer Res. 2021, 27, 2190–2199. [Google Scholar] [CrossRef]
- Cao, X.; Wang, Y.; Zhang, W.; Zhong, X.; Gunes, E.G.; Dang, J.; Wang, J.; Epstein, A.L.; Querfeld, C.; Sun, Z. Targeting macrophages for enhancing CD47 blockade–elicited lymphoma clearance and overcoming tumor-induced immunosuppression. Blood J. Am. Soc. Hematol. 2022, 139, 3290–3302. [Google Scholar] [CrossRef]
- Johnson, L.D.; Banerjee, S.; Kruglov, O.; Viller, N.N.; Horwitz, S.M.; Lesokhin, A.; Zain, J.; Querfeld, C.; Chen, R.; Okada, C. Targeting CD47 in Sézary syndrome with SIRPαFc. Blood Adv. 2019, 3, 1145–1153. [Google Scholar] [CrossRef]
- Querfeld, C.; Thompson, J.A.; Taylor, M.H.; DeSimone, J.A.; Zain, J.M.; Shustov, A.R.; Johns, C.; McCann, S.; Lin, G.H.; Petrova, P.S. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sézary syndrome: A multicentre, phase 1 study. Lancet Haematol. 2021, 8, e808–e817. [Google Scholar] [CrossRef]
- Furumoto, Y.; Gadina, M. The arrival of JAK inhibitors: Advancing the treatment of immune and hematologic disorders. BioDrugs 2013, 27, 431–438. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Chapman, S.; Kwa, M.; Gold, L.S.; Lim, H.W. Janus kinase inhibitors in dermatology: Part I. A comprehensive review. J. Am. Acad. Dermatol. 2022, 86, 406–413. [Google Scholar] [CrossRef]
- Talpur, R.; Sui, D.; Gangar, P.; Dabaja, B.S.; Duvic, M. Retrospective analysis of prognostic factors in 187 cases of transformed mycosis fungoides. Clin. Lymphoma Myeloma Leuk. 2016, 16, 49–56. [Google Scholar] [CrossRef]
- Yi, Y.W.; You, K.S.; Park, J.-S.; Lee, S.-G.; Seong, Y.-S. Ribosomal protein S6: A potential therapeutic target against cancer? Int. J. Mol. Sci. 2021, 23, 48. [Google Scholar] [CrossRef]
- Jin, J.; Cai, Q.; Zhang, L.; Zou, L.; Li, Z.; Wu, H.; Zhou, K.; Qiu, L.; Su, L.; Ding, K. Phase 2 Study of Golidocitinib, a JAK1 Selective Inhibitor, As Maintenance Therapy in Patients with Peripheral T Cell Lymphomas after First-Line Systemic Therapy (JACKPOT26). Blood 2023, 142, 4430. [Google Scholar] [CrossRef]
- Koh, J.; Jang, I.; Mun, S.; Lee, C.; Cha, H.J.; Oh, Y.H.; Kim, J.-M.; Han, J.H.; Paik, J.H.; Cho, J. Genetic profiles of subcutaneous panniculitis-like T-cell lymphoma and clinicopathological impact of HAVCR2 mutations. Blood Adv. 2021, 5, 3919–3930. [Google Scholar] [CrossRef]
- Atzeni, F.; Popa, C.D.; Nucera, V.; Nurmohamed, M.T. Safety of JAK inhibitors: Focus on cardiovascular and thromboembolic events. Expert. Rev. Clin. Immunol. 2022, 18, 233–244. [Google Scholar] [CrossRef]
- Hanzel, J.; Hulshoff, M.S.; Grootjans, J.; D’Haens, G. Emerging therapies for ulcerative colitis. Expert Rev. Clin. Immunol. 2022, 18, 513–524. [Google Scholar] [CrossRef]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Ingrassia, J.P.; Maqsood, M.H.; Gelfand, J.M.; Weber, B.N.; Bangalore, S.; Sicco, K.I.L.; Garshick, M.S. Cardiovascular and Venous Thromboembolic Risk With JAK Inhibitors in Immune-Mediated Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis. JAMA Dermatol. 2023, 160, 28–36. [Google Scholar] [CrossRef]

| CTCL Subtype | Frequency (%) | 5-Years Disease-Specific Survival (%) | Clinical Features | T-Cell Phenotype |
|---|---|---|---|---|
| Mycosis fungoides (MF) Folliculotropic MF Pagetoid reticulosis Granulomatous slack skin | 39 5 <1 <1 | 88 75 100 100 | Patches and plaques; (ulcerating) tumors in advanced stage | CD3+, CD4+, CD8− |
| Sézary syndrome (SS) | 2 | 36 | Triad of pruritic erythroderma, generalized lymphadenopathy, and clonally related neoplastic T cells with cerebriform nuclei (Sézary cells) in the skin, lymph nodes, and peripheral blood | CD4+, CD7−, CD26− |
| Primary cutaneous CD30+ lymphoproliferative disorders (LPDs) Primary cutaneous anaplastic large lymphoma (C-ALCL) Lymphomatoid papulosis (LyP) | 8 12 | 95 99 | Solitary or localized nodules or tumors Chronic course of recurrent, self-healing papulonecrotic, or nodular skin lesions. | CD3+/−, CD4+, CD8−, CD30+ CD4+, CD8− or CD4−, CD8+ or CD4−, CD8− |
| Subcutaneous panniculitis-like T-cell lymphoma | 1 | 87 | Subcutaneous nodules and plaques | CD3+, CD4−, CD8+ |
| Primary cutaneous peripheral T cell lymphoma, rare subtypes Primary cutaneous gamma-delta T cell lymphoma (PCGD-TCL) Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma (PCAECyTCL) Primary cutaneous CD4+ small- or medium-sized LPDs Primary cutaneous acral CD8+ LPD | <1 <1 6 <1 | 11 31 100 100 | Ulcerating plaques and tumors Ulcerating plaques, nodules, and tumors Solitary nodule or tumor on the face or upper trunk Solitary papule or nodule on acral site (ear; nose) | CD3+, CD4−, CD8−/+ CD3+, CD4−, CD8+ CD3+, CD4+, CD8−, CD279/PD-1+ CD3+, CD4−, CD8+ |
| Primary cutaneous peripheral T cell lymphoma, not otherwise specified | 2 | 15 | Localized skin lesions | CD4+ |
| Study | Design | Patient Number | Diagnosis | Drug Name | Dosage | Duration | Outcome | Side Effect |
|---|---|---|---|---|---|---|---|---|
| Horwitz et al., USA 2019 [35] | Clinical trial | 37 | CTCL | Cerdulatinib JAK1, 2, and 3 inhibitor | 30 mg twice daily | NA | ORR was 35% (13/37) MF (ORR of 45%; 9% of the patients achieved CR) versus SS (ORR of 17%, with no CR). | Present |
| Moskowitz et al., USA 2021 [37] | Clinical trial | 10 | 7 MF 1 SPTCL 1 PCALCL 1 PCGDTCL | Ruxolitinib JAK1 and 2 inhibitor | 20 mg twice daily | NA | ORRs for MF, SPTCL, and PCALCL were 14% (1/7), 100%, and 100%, respectively. | Present |
| Study | Design | Patient Number | Diagnosis | Drug Name | Dosage | Duration | Outcome | Side Effect |
|---|---|---|---|---|---|---|---|---|
| Levy et al., France 2020 [42] | Case report | 1 | SPTCL + HLH | Ruxolitinib JAK1 and 2 inhibitor | 15 mg twice daily + 20 mg twice daily | 4 + 14 months | CR | NA |
| Castillo et al., USA 2022 [43] | Case report | 1 | Erythrodermic MF | Upadacitinib JAK1 inhibitor | 15 mg daily | 16 weeks | CR | NA |
| Kook et al., South Korea 2022 [44] | Case report | 1 | MF | Upadacitinib JAK1 inhibitor | NA | 16 weeks | CR | NA |
| Watson et al., Australia 2022 [45] | Case report | 1 | SPTCL | Ruxolitinib JAK1 and 2 inhibitor | 15 mg twice daily | 7 weeks | CR | Present |
| Hansen et al., Canada 2020 [46] | Case series | 1 | SPTCL+ HLH | Ruxolitinib JAK1 and 2 inhibitor | 15 mg twice daily then 10 mg twice daily | 11 months | CR | Present |
| Duan et al., China 2022 [47] | Case series | 4 | SPTCL | Ruxolitinib JAK1 and 2 inhibitor | NA | NA | 75% (3/4) CR 25% (1/4) allo-SCT | NA |
| Zhang et al., China 2023 [48] | Case series | 2 | SPTCL + HLH | Ruxolitinib JAK1 and 2 inhibitor | 10 mg twice daily 5 mg twice daily | 16 months About 8 months | CR CR | NA |
| Study | Design | Patient Number | Diagnosis | Drug Name | Dosage | Duration | Outcome | Side Effect |
|---|---|---|---|---|---|---|---|---|
| Iinuma et al., Japan 2022 [49] | Case report | 1 | Rheumatoid arthritis | Upadacitinib JAK1 inhibitor | 7.5 mg daily | 2 weeks | Incident of LyP | NA |
| Knapp et al., USA 2022 [50] | Case report | 1 | Erythema elevatum diutinum and associated inflammatory arthritis | Tofacitinib JAK1, 2, and 3 inhibitor | NA | 10 weeks | Incident of LyP | NA |
| Saito et al., Japan 2023 [51] | Case report | 1 | Seronegative rheumatoid arthritis | Baricitinib JAK1 and 2 inhibitor | NA | 7 months | Incident of SS | NA |
| Cohen et al., France 2023 [52] | Case report | 2 | Chronic graft-versus-host disease | Ruxolitinib JAK1 and 2 inhibitor | 20 mg daily 30 mg daily | NA NA | CTCL relapse MF relapse | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vahabi, S.M.; Bahramian, S.; Esmaeili, F.; Danaei, B.; Kalantari, Y.; Fazeli, P.; Sadeghi, S.; Hajizadeh, N.; Assaf, C.; Etesami, I. JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature. Cancers 2024, 16, 861. https://doi.org/10.3390/cancers16050861
Vahabi SM, Bahramian S, Esmaeili F, Danaei B, Kalantari Y, Fazeli P, Sadeghi S, Hajizadeh N, Assaf C, Etesami I. JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature. Cancers. 2024; 16(5):861. https://doi.org/10.3390/cancers16050861
Chicago/Turabian StyleVahabi, Seyed Mohammad, Saeed Bahramian, Farzad Esmaeili, Bardia Danaei, Yasamin Kalantari, Patrick Fazeli, Sara Sadeghi, Nima Hajizadeh, Chalid Assaf, and Ifa Etesami. 2024. "JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature" Cancers 16, no. 5: 861. https://doi.org/10.3390/cancers16050861
APA StyleVahabi, S. M., Bahramian, S., Esmaeili, F., Danaei, B., Kalantari, Y., Fazeli, P., Sadeghi, S., Hajizadeh, N., Assaf, C., & Etesami, I. (2024). JAK Inhibitors in Cutaneous T-Cell Lymphoma: Friend or Foe? A Systematic Review of the Published Literature. Cancers, 16(5), 861. https://doi.org/10.3390/cancers16050861







