Current and Emerging Radiotherapy Options for Uveal Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Molecular Pathogenesis of Uveal Melanoma
3. Clinical Workup
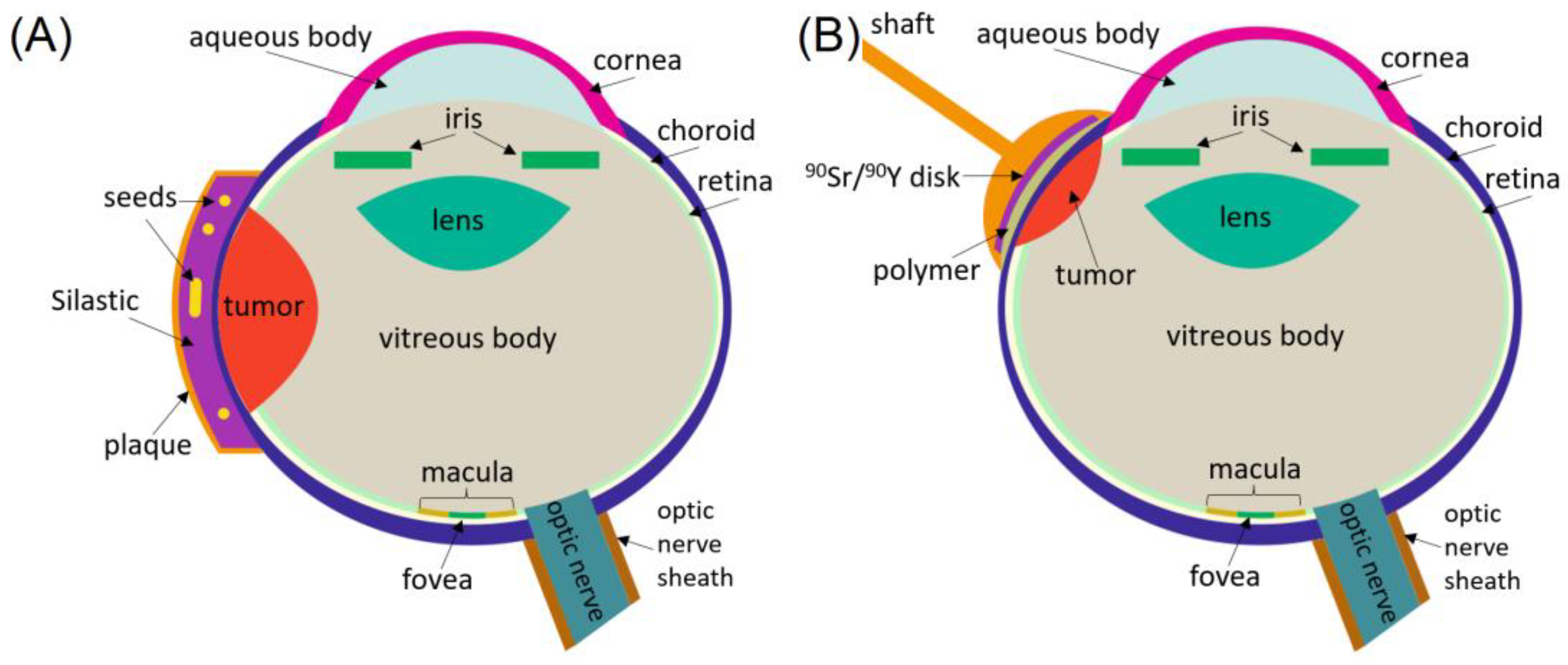
4. Brachytherapy
4.1. LDR Brachytherapy with Low-Energy Photons
4.2. LDR Brachytherapy with Beta-Particles
4.3. HDR Brachytherapy with Photons
4.4. HDR Brachytherapy with Beta-Particles
5. External Beam Radiation Therapy
5.1. Gamma Stereotactic Radiosurgery
5.2. Linac EBRT
5.3. Protons
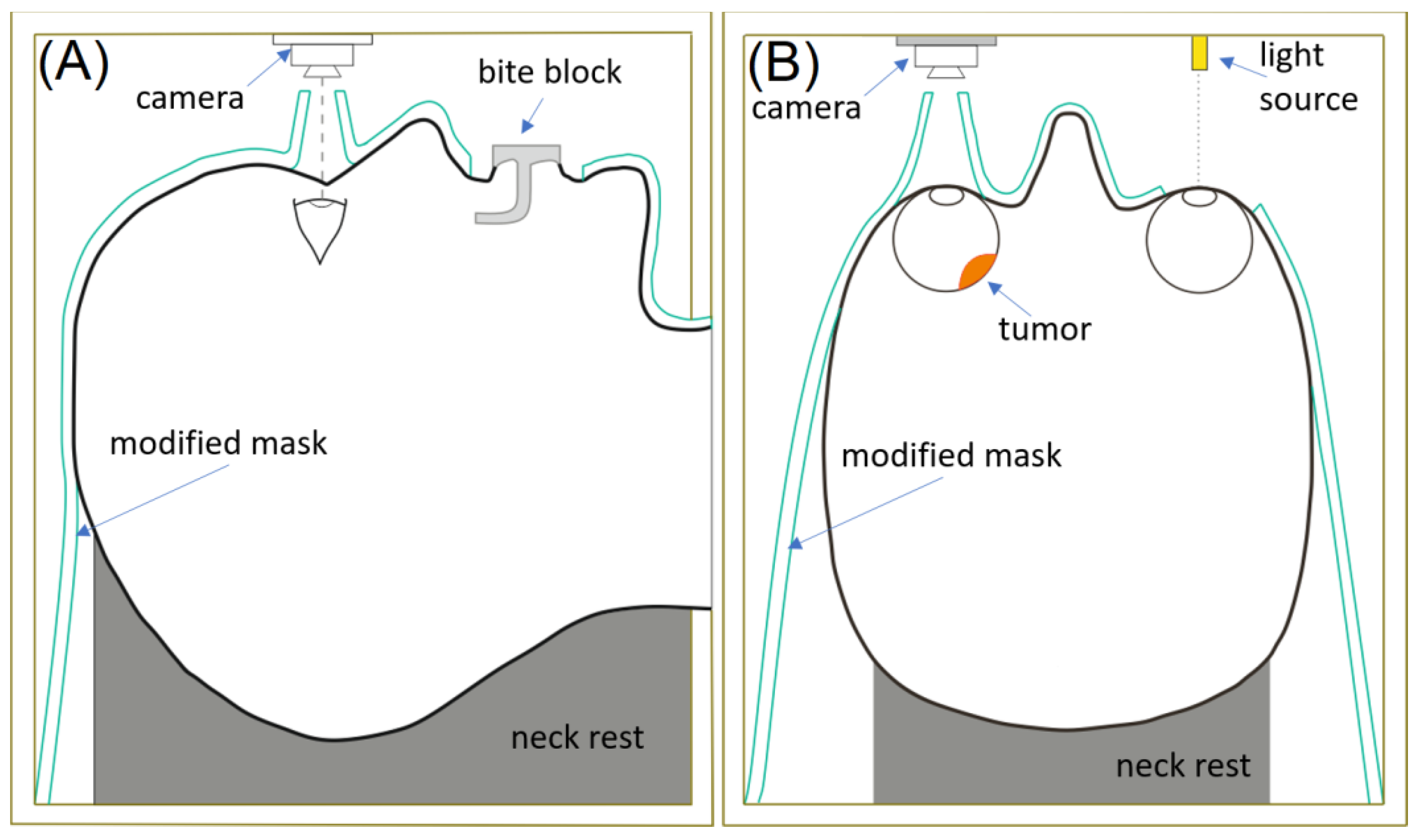
6. Target Imaging, Patient Immobilization and Treatment Delivery Verification
6.1. Brachytherapy
6.2. EBRT
7. Radiation Safety
8. Future Directions
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, Y.; Lou, L.; Wang, Y.; Miao, Q.; Jin, K.; Chen, M.; Ye, J. Epidemiological Study of Uveal Melanoma from US Surveillance, Epidemiology, and End Results Program (2010–2015). J. Ophthalmol. 2020, 2020, 3614039. [Google Scholar] [CrossRef]
- Finger, P.T. Radiation therapy for choroidal melanoma. Surv. Ophthalmol. 1997, 3, 142. [Google Scholar] [CrossRef]
- Zimmerman, L.E.; McLean, I.W.; Foster, W.D. Does enucleation of the eye containing amalignant melanoma prevent or accelerate the dissemination of tumour cells. Br. J. Ophthalmol. 1978, 62, 420–425. [Google Scholar] [CrossRef]
- Chiu-Tsao, S.T.; Astrahan, M.A.; Finger, P.T.; Followill, D.S.; Meigooni, A.S.; Melhus, C.S.; Mourtada, F.; Napolitano, M.E.; Nath, R.; Rivard, M.J.; et al. Dosimetry of 125I and 103Pd COMS eye plaques for intraocular tumors: Report of Task Group 129 by the AAPM and ABS. Med. Phys. 2012, 39, 6161–6184. [Google Scholar] [CrossRef] [PubMed]
- American Brachytherapy Society—Ophthalmic Oncology Task Force. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy 2014, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ballin, R.; Lommatzsch, P.K.; Drost, H.; Ratajek, B. A beta ray applicator (106Ru/106Rh) in the treatment of ciliary body melanomas. Klin. Monatsbl. Augenheilkd. 1985, 187, 144–146. [Google Scholar] [CrossRef]
- Ginderdeuren, R.V.; Limbergen, E.V.; Spileers, W. 18 Years experience with high dose rate strontium-90. Br. J. Ophthalmol. 2005, 89, 1306–1310. [Google Scholar] [CrossRef]
- Gallenga, C.E.; Franco, E.; Adamo, G.; Violanti, S.; Tassinari, P.; Tognon, M.; Perri, P. Genetic Basis and Molecular Mechanisms of Uveal Melanoma Metastasis: A Focus on Prognosis. Front. Oncol. 2022, 11, 828112. [Google Scholar] [CrossRef] [PubMed]
- Taskaeva, I.; Shatruk; Bgatova, N.; Yeremina, A.; Trunov, A.; Kononova, N.; Chernykh, V. Autophagy and vesicular trafficking in human uveal melanoma: A histopathological study. Microsc. Res. Techol. 2024, 87, 122–132. [Google Scholar] [CrossRef]
- NCCN Guidelines for Uveal Melanoma V. 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488 (accessed on 1 March 2024).
- Rivard, M.J.; Melhus, C.S.; Sioshansi, S.; Morr, J. The impact of prescription depth, dose rate, plaque size, and source loading on the central axis using 103Pd, 125I, and 131Cs. Brachytherapy 2008, 7, 327–335. [Google Scholar] [CrossRef]
- Leonard, K.L.; Gagne, N.L.; Mignano, J.E.; Duker, J.S.; Bannon, E.A.; Rivard, M.J. A 17-year retrospective study of institutional results for eye plaque brachytherapy of uveal melanoma using (125)I, (103)Pd, and (131)Cs and historical perspective. Brachytherapy 2011, 10, 331–339. [Google Scholar] [CrossRef]
- Papakostas, P.; Tsaousi, G.; Stavrou, G.; Rachovitsas, D.; Tsiropoulos, G.; Rova, C.; Konstantinidis, I.; Michalopoulos, A.; Grosomanidis, V.; Kotzampassi, K. Percutaneous endoscopic gastrostomy feeding of locally advanced oro-pharygo-laryngeal cancer patients: Blenderized or commercial food? Oral Oncol. 2017, 74, 135–141. [Google Scholar] [CrossRef]
- Kang, D.W.; Lee, S.C.; Park, Y.G.; Chang, J.H. Long-term results of Gamma Knife surgery for uveal melanomas. J. Neurosurg. 2012, 117, 108–114. [Google Scholar] [CrossRef]
- Liegl, R.; Schmelter, V.; Fuerweger, C.; Ehret, F.; Priglinger, S.; Muacevic, A.; Foerster, P. Robotic CyberKnife Radiosurgery for the Treatment of Choroidal and Ciliary Body Melanoma. Am. J. Ophthalmol. 2023, 250, 177–185. [Google Scholar] [CrossRef]
- Buonanno, F.; Conson, M.; Ribeiro, C.D.A.; Oliviero, C.; Itta, F.; Liuzzi, R.; Pacelli, R.; Cella, L.; Clemente, S. Local tumor control and treatment related toxicity after plaque brachytherapy for uveal melanoma: A systematic review and a data pooled analysis. Radiother. Onc. 2022, 166, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Pagliara, M.M.; Tagliaferri, L.; Azario, L.; Lenkowicz, J.; Lanza, A.; Autorino, R.; Caputo, C.G.; Gambacorta, M.A.; Valentini, V.; Blasi, M.A. Ruthenium brachytherapy for uveal melanomas: Factors affecting the development of radiation complications. Brachytherapy 2018, 17, 432–438. [Google Scholar] [CrossRef]
- Chia, S.; Smith, H.B.; Hammer, H.M.; Kemp, E.G. Incidence and indications for pars plana vitrectomy following the treatment of posterior uveal melanomas in Scotland. Eye 2015, 29, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Beykin, G.; Peer, G.J.; Hemo, Y.; Frenkel, S.; Chowers, I. Pars plana vitrectomy to repair retinal detachment following brachytherapy for uveal melanoma. Br. J. Ophthalmol. 2013, 97, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Stadigh, A.E.; Puska, P.M.; Kivelä, T.T. Incidence and Risk Factors for Secondary Glaucoma in Eyes with Uveal Melanoma. Ophthalmol. Glaucoma. 2023, 6, 29–41. [Google Scholar] [CrossRef]
- Peddada, K.V.; Sangani, R.; Menon, H.; Verma, V. Complications and adverse events of plaque brachytherapy for ocular melanoma. J. Contemp. Brachytherapy. 2019, 11, 392–397. [Google Scholar] [CrossRef]
- Chiu-Tsao, S.T.; Anderson, L.L.; OBrien, K.; Stabile, L.; Liu, J.C. Dosimetry for 125I seed (model 6711) in eye plaques. Med. Phys. 1993, 20, 383–389. [Google Scholar] [CrossRef]
- Thomson, R.M.; Taylor, R.E.; Rogers, D.W. Monte Carlo dosimetry for 125I and 103Pd eye plaque brachytherapy. Med. Phys. 2008, 35, 5530–5543. [Google Scholar] [CrossRef] [PubMed]
- Thomason, R.M.; Furutani, K.M.; Kaulich, T.W.; Mourtada, F.; Rivard, M.J.; Soares, C.G.; Vanneste, F.M.; Melhus, C.S. AAPM recommendations on medical physics practice for ocular plaque brachytherapy: Report of task group 221. Med. Phys. 2020, 47, e92–e124. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, S.-C.; Kim, Y. Optic disc dose reduction in ocular brachytherapy using 125I notched COMS plaques: A simulation study based on current clinical practice. J. Appl. Clin. Med. Phys. 2020, 21, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.eyephysics.com/ (accessed on 1 March 2024).
- Rivard, M.J.; Chiu-Tsao, S.T.; Finger, P.T.; Meigooni, A.S.; Melhus, C.S.; Mourtada, F.; Napolitano, M.E.; Rogers, D.W.O.; Thomson, R.M.; Nath, R. Comparison of dose calculation methods for brachytherapy of intraocular tumors. Med. Phys. 2011, 38, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.M.; Ballester, F.; Beaulieu, L.; Morrison, H.; Poher, A.; Rivard, M.J.; Sloboda, R.S.; Vijande, J.; Thomson, R.M. Generation and comparison of 3D dosimetric reference datasets for COMS eye plaque brachytherapy using model-based dose calculations. Med. Phys. 2023, 51, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Gagne, N.L.; Leonard, K.L.; Rivard, M.J. Radiobiology for eye plaque brachytherapy and evaluation of implant duration and radionuclide choice using an objective function. Med. Phys. 2012, 39, 3332–3342. [Google Scholar] [CrossRef] [PubMed]
- Gagne, N.L.; Leonard, K.L.; Huber, K.E.; Mignano, J.E.; Duker, J.S.; Laver NV Rivard, M.J. BEDVH-A method for evaluating biologically effective dose volume histograms: Application to eye plaque brachytherapy implants. Med. Phys. 2012, 39, 976–983. [Google Scholar] [CrossRef]
- Gagne, N.L.; Cutright, D.R.; Rivard, M.J. Keeping an eye on the ring: COMS plaque loading optimization for improved dose conformity and homogeneity. J. Contemp. Brachytherapy 2012, 4, 165–175. [Google Scholar] [CrossRef]
- Weersink, R.A.; Patterson, S.; Ballantyne, H.; Di Tomasso, A.; Borg, J.; Vitkin, A.; Rink, A.; Beiki-Ardakani, A. An improved treatment planning and quality assurance process for Collaborative Ocular Melanoma Study eye plaque brachytherapy. Brachytherapy 2019, 18, 658–667. [Google Scholar] [CrossRef]
- Del Río, H.M.; Lora, A.O.; Reina, A.B.; León, J.A.T. A Monte Carlo dose calculation system for ophthalmic brachytherapy based on a realistic eye model. Med. Phys. 2021, 48, 4542–4559. [Google Scholar] [CrossRef] [PubMed]
- Hermida-López, M. Calculation of dose distributions for 12 106Ru/106Rh ophthalmic applicator models with the PENELOPE Monte Carlo code. Med. Phys. 2013, 40, 101705. [Google Scholar] [CrossRef] [PubMed]
- Dosimetry of Beta Rays and Low-Energy Photons for Brachytherapy with Sealed Sources. J. ICRU 2004, 4, 1–2. [CrossRef]
- Lommatzsch, P.K.; Werschnik, C.; Schuster, E. Long-term follow-up of Ru-106/Rh-106 brachytherapy for posterior uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2000, 238, 129–137. [Google Scholar] [CrossRef]
- Harkenrider, M.; Albuquerque, K.; Brown, D.; Kamrava, M.; King, M.; Mourtada, F.; Orio, P.; Patel, R.; Price, M.; Rassiah, P.; et al. ACR–ABS–ASTRO practice parameter for the performance of radionuclide-based high-dose-rate brachytherapy. Brachytherapy 2021, 20, 1071–1082. [Google Scholar] [CrossRef]
- Missotten, L.; Dirven, W.; Van der Schueren, A.; Leys, A.; De Meester, G.; Van Limbergen, E. Results of treatment of choroidal malignant melanoma with high-dose-rate strontium-90 brachytherapy. A retrospective study of 46 patients treated between 1983 and 1995. Graefes Arch. Clin. Exp. Ophthalmol. 1998, 236, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Dupere, J.M.; Munro, J.J., 3rd; Medich, D.C. Shielded high dose rate ocular brachytherapy using Yb-169. Phys. Med. Biol. 2021, 66, 7. [Google Scholar] [CrossRef]
- Kirwan, J.F.; Constable, P.H.; Murdoch, I.E.; Khaw, P.T. Beta irradiation: New uses for an old treatment: A review. Eye 2003, 17, 207–215. [Google Scholar] [CrossRef]
- Finger, P.T.; Stewart, R.; Rivard, M.J.; Beers, R.J.; Kamen, J.; Lama, S.; Chin, K.J.; Mohney, K.; Welles, T.S.; Sauerwein, W.A.G.; et al. First clinical implementation of Yttrium-90 Disc Brachytherapy after FDA clearance. Brachytherapy 2023, 22, 416–427. [Google Scholar] [CrossRef]
- Reynolds, M.M.; Arnett, A.L.; Parney, I.F.; Kumar, R.; Laack, N.N.; Maloney, P.R.; Kozelsky, T.F.; Garces, Y.I.; Foote, R.L.; Pulido, J.S. Gamma knife radiosurgery for the treatment of uveal melanoma and uveal metastases. Int. J. Retin. Vitr. 2017, 3, 17. [Google Scholar] [CrossRef]
- Sarici, A.M.; Pazarli, H. Gamma-knife-based stereotactic radiosurgery for medium- and large-sized posterior uveal melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2013, 251, 285–294. [Google Scholar] [CrossRef]
- Simonová, G.; Novotný, J., Jr.; Liscák, R.; Pilbauer, J. Leksell gamma knife treatment of uveal melanoma. J. Neurosurg. 2002, 97, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Fakiris, A.J.; Lo, S.S.; Henderson, M.A.; Witt, T.C.; Worth, R.M.; Danis, R.P.; Des Rosiers, P.M.; Timmerman, R.D. Gamma-knife-based stereotactic radiosurgery for uveal melanoma. Stereotact. Funct. Neurosurg. 2007, 85, 106–112. [Google Scholar] [CrossRef]
- Schirmer, C.M.; Chan, M.; Mignano, J.; Duker, J.; Melhus, C.S.; Williams, L.B.; Wu, J.K.; Yao, K.C. Dose de-escalation with gamma knife radiosurgery in the treatment of choroidal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Dinca, E.B.; Yianni, J.; Rowe, J.; Radatz, M.W.; Preotiuc-Pietro, D.; Rundle, P.; Rennie, I.; Kemeny, A.A. Survival and complications following γ knife radiosurgery or enucleation for ocular melanoma: A 20-year experience. Acta Neurochir. 2012, 154, 605–610. [Google Scholar] [CrossRef]
- Haas, L.; Elewaut, A.; Gerard, C.L.; Umkehrer, C.; Leiendecker, L.; Pedersen, M.; Krecioch, I.; Hoffmann, D.; Novatchkova, M.; Kuttke, M.; et al. Acquired resistance to anti-MAPK targeted therapy confers an immune-evasive tumor microenvironment and cross-resistance to immunotherapy in melanoma. Nat. Cancer. 2021, 2, 693–708. [Google Scholar] [CrossRef]
- Logani, S.; Helenowski, T.K.; Thakrar, H.; Pothiawala, B. Gamma Knife radiosurgery in the treatment of ocular melanoma. Stereotact. Funct. Neurosurg. 1993, 61, 38–44. [Google Scholar] [CrossRef]
- Modorati, G.; Miserocchi, E.; Galli, L.; Picozzi, P.; Rama, P. Gamma knife radiosurgery for uveal melanoma: 12 years of experience. Br. J. Ophthalmol. 2009, 93, 40–44. [Google Scholar] [CrossRef]
- Carvounis, P.E.; Katz, B. Gamma knife radiosurgery in neuro-ophthalmology. Curr. Opin. Ophthalmol. 2003, 14, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Lišc, Á.R.; Vladyka, V. Radiosurgery in ocular disorders: Clinical applications. Prog. Neurol. Surg. 2007, 20, 324–339. [Google Scholar]
- Marchini, G.; Babighian, S.; Tomazzoli, L.; Gerosa, M.A.; Nicolato, A.; Bricolo, A.; Piovan, E.; Zampieri, P.G.; Alessandrini, F.; Benati, A. Stereotactic radiosurgery of uveal melanomas: Preliminary results with Gamma Knife treatment. Stereotact. Funct. Neurosurg. 1995, 64, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Akbaba, S.; Foerster, R.; Nicolay, N.H.; Arians, N.; Bostel, T.; Debus, J.; Hauswald, H. Linear accelerator-based stereotactic fractionated photon radiotherapy as an eye-conserving treatment for uveal melanoma. Radiat. Oncol. 2018, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Nowak, P.J.; de Pan, C.; Marijnissen, J.P.; Paridaens, D.A.; Levendag, P.; Luyten, G.P. Effectiveness of fractionated stereotactic radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Nowak, P.; Luyten, G.P.M.; Marijnissen, H.; de Pan, C.; Levendag, P. A modified relocatable stereotactic frame for irradiation of eye melanoma: Design and evaluation of treatment accuracy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Bogner, J.; Petersch, B.; Georg, D.; Dieckmann, K.; Zehetmayer, M.; Pötter, R.A. noninvasive eye fixation and computer-aided eye monitoring system for linear accelerator-based stereotactic radiotherapy of uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.; Bogner, J.; Georg, D.; Zehetmayer, M.; Kren, G.; Pötter, R. A linac-based stereotactic irradiation technique of uveal melanoma. Radiother. Oncol. 2001, 61, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Pastyr, O.; Hartmann, G.H.; Schlegel, W.; Schabbert, S.; Treuer, H.; Lorenz, W.J.; Sturm, V. Stereotactically guided convergent beam irradiation with a linear accelerator: Localization-technique. Acta Neurochir. 1989, 99, 61–64. [Google Scholar] [CrossRef]
- Gorovets, D.; Gagne, N.L.; Melhus, C.S. Dosimetric and radiobiologic comparison of 103Pd COMS plaque brachytherapy and Gamma Knife radiosurgery for choroidal melanoma. Brachytherapy 2017, 16, 433–443. [Google Scholar] [CrossRef]
- Petti, P.L.; Rivard, M.J.; Alvarez, P.E.; Bednarz, G.; Bourland, J.D.; DeWerd, L.A.; Drzymala, R.E.; Johansson, J.; Kunugi, K.; Ma, L.; et al. Recommendations on the practice of calibration, dosimetry, and quality assurance for gamma stereotactic radiosurgery: Report of AAPM Task Group 178. Med. Phys. 2021, 48, 733–770. [Google Scholar] [CrossRef]
- Bensoussan, E.; Thariat, J.; Maschi, C.; Delas, J.; Schouver, E.D.; Hérault, J.; Baillif, S.; Caujolle, J.P. Outcomes After Proton Beam Therapy for Large Choroidal Melanomas in 492 Patients. Am. J. Ophthalmol. 2016, 165, 78–87. [Google Scholar] [CrossRef]
- Conway, J.R.; Dietlein, F.; Taylor-Weiner, A.; AlDubayan, S.; Vokes, N.; Keenan, T.; Reardon, B.; He, M.X.; Margolis, C.A.; Weirather, J.L.; et al. Integrated molecular drivers coordinate biological and clinical states in melanoma. Nat. Genet. 2020, 52, 1373–1383. [Google Scholar] [CrossRef]
- Mosci, C.; Lanza, F.B.; Barla, A.; Mosci, S.; Hérault, J.; Anselmi, L.; Truini, M. Comparison of clinical outcomes for patients with large choroidal melanoma after primary treatment with enucleation or proton beam radiotherapy. Ophthalmologica 2012, 227, 190–196. [Google Scholar] [CrossRef]
- Verma, V.; Mehta, M.P. Clinical Outcomes of Proton Radiotherapy for Uveal Melanoma. Clin. Oncol. (R. Coll. Radiol.) 2016, 28, 17–27. [Google Scholar] [CrossRef]
- Weber, D.C.; Bogner, J.; Verwey, J.; Georg, D.; Dieckmann, K.; Escudé, L.; Caro, M.; Pötter, R.; Goitein, G.; Lomax, A.J.; et al. Proton beam radiotherapy versus fractionated stereotactic radiotherapy for uveal melanomas: A comparative study. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 373–384. [Google Scholar] [CrossRef]
- Conway, R.M.; Poothullil, A.M.; Daftari, I.K.; Weinberg, V.; Chung, J.E.; OBrien, J.M. Estimates of Ocular and Visual Retention Following Treatment of Extra-Large Uveal Melanomas by Proton Beam Radiotherapy. Arch. Ophthalmol. 2006, 124, 838–843. [Google Scholar] [CrossRef]
- Höcht, S.; Stark, R.; Seiler, F.; Heufelder, J.; Bechrakis, N.E.; Cordini, D.; Marnitz, S.; Kluge, H.; Foerster, M.H.; Hinkelbein, W. Proton or stereotactic photon irradiation for posterior uveal melanoma? A planning intercomparison. Strahlenther. Onkol. 2005, 181, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.; Zografos, L.; Schalenbourg, A.; Beati, D.; Böhringer, T.; Chamot, L.; Goitein, G. Eye retention after proton beam radiotherapy for uveal melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 867–880. [Google Scholar] [CrossRef]
- Wuestemeyer, H.; Sauerwein, W.; Meller, D.; Chauvel, P.; Schueler, A.; Steuhl, K.P.; Bornfeld, N.; Anastassiou, G. Proton radiotherapy as an alternative to exenteration in the management of extended conjunctival melanoma. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Chauvel, P.; Sauerwein, W.; Bornfeld, N.; Friedrichs, W.; Brassart, N.; Courdi, A.; Hérault, J.; Pignol, J.P.; Bondiau, P.Y.; Malandain, G. Clinical and technical requirements for proton treatment planning of ocular diseases. The SERAG (South Europe Radiotherapy Group). Front. Radiat. Ther. Oncol. 1997, 30, 133–142. [Google Scholar]
- Suit, H.D. Protons to replace photons in external beam radiation therapy? Clin. Oncol. (R. Coll. Radiol.) 2003, 15, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Suit, H.D.; Urie, M. Proton beams in radiation therapy. J. Natl. Cancer Inst. 1992, 84, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Koehler AMSchneider, R.J.; Sisterson, J.M. Range modulators for protons and heavy ions. Nucl. Instrum. Methods 1975, 131, 437–440. [Google Scholar] [CrossRef]
- Gragoudas, E.S. Proton beam irradiation of uveal melanomas: The first 30 years. The Weisenfeld Lecture. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4666–4673. [Google Scholar] [CrossRef] [PubMed]
- Gradoudas, E.S.; Goitein, M.; Koehler, A.; Constable, I.J.; Wagner, M.S.; Verhey, L.; Tepper, J.; Suit, H.D.; Brockhurst, R.J.; Schneider, R.J.; et al. Proton irradiation of choroidal melanomas. Preliminary results. Arch. Ophthalmol. 1978, 96, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Goitein, M.; Koehler, A.M.; Verhey, L.; Tepper, J.; Suit, H.D.; Brockhurst, R.; Constable, I.J. Proton irradiation of small choroidal malignant melanomas. Am. J. Ophthalmol. 1977, 83, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Goitein, M.; Seddon, J.; Verhey, L.; Munzenrider, J.; Urie, M.; Suit, H.D.; Blitzer, P.; Johnson, K.N.; Koehler, A. Preliminary results of proton beam irradiation of macular and paramacular melanomas. Br. J. Ophthalmol. 1984, 68, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Goitein, M.; Verhey, L.; Munzenreider, J.; Urie, M.; Suit, H.D.; Koehler, A. Proton beam irradiation of uveal melanomas. Results of 5 1/2-year study. Arch. Ophthalmol. 1982, 100, 928–934. [Google Scholar] [CrossRef]
- Suit, H.D.; Goitein, M.; Tepper, J.; Koehler, A.M.; Schmidt, R.A.; Schneider, R. Exploratory study of proton radiation therapy using large field techniques and fractionated dose schedules. Cancer 1975, 35, 1646–1657. [Google Scholar] [CrossRef]
- Wilson, M.W.; Hungerford, J.L. Comparison of episcleral plaque and proton beam radiation therapy for the treatment of choroidal melanoma. Ophthalmology 1999, 106, 1579–1587. [Google Scholar] [CrossRef]
- Courdi, A.; Caujolle, J.P.; Grange, J.D.; Diallo-Rosier, L.; Sahel, J.; Bacin, F.; Zur, C.; Gastaud, P.; Iborra-Brassart, N.; Hérault, J.; et al. Results of proton therapy of uveal melanomas treated in Nice. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 5–11. [Google Scholar] [CrossRef]
- Fuss, M.; Loredo, L.N.; Blacharski, P.A.; Grove, R.I.; Slater, J.D. Proton radiation therapy for medium and large choroidal melanoma: Preservation of the eye and its functionality. Int. J. Radiat. Oncol. Biol. Phys. 2001, 49, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Papakostas, T.D.; Lane, A.M.; Morrison, M.; Gragoudas, E.S.; Kim, I.K. Long-term Outcomes After Proton Beam Irradiation in Patients with Large Choroidal Melanomas. JAMA Ophthalmol. 2017, 135, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Finger, P.T.; Chin, K.J.; Duvall, G. Palladium-103 for Choroidal Melanoma Study Group. Palladium-103 ophthalmic plaque radiation therapy for choroidal melanoma: 400 treated patients. Ophthalmology 2009, 116, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Solnik, M.; Paduszynska, N.; Czarnecka, A.M.; Synoradzki, K.J.; Yousef, Y.A.; Choragiewicz, T.; Rejdak, R.; Toro, M.D.; Zweifel, S.; Dyndor, K.; et al. Imaging of Uveal Melanoma—Current Standard and Methods in Development. Cancers 2022, 14, 3147. [Google Scholar] [CrossRef] [PubMed]
- Gagne, N.L.; Rivard, M.J. Quantifying the dosimetric influences of radiation coverage and brachytherapy implant placement uncertainty on eye plaque size selection. Brachytherapy 2013, 12, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Houdek, P.V.; Schwade, J.G.; Medina, A.J.; Poole, C.A.; Olsen, K.R.; Nicholson, D.H.; Byrne, S.; Quencer, R.; Hinks, R.S.; Pisciotta, V. MR technique for localization and verification procedures in episcleral brachytherapy. Int. J. Radiat. Oncol. Biol. Phys. 1989, 17, 1111–1114. [Google Scholar] [CrossRef]
- Zoberi, J.E.; Garcia-Ramirez, J.; Hedrick, S.; Rodriguez, V.; Bertelsman, C.G.; Mackey, S.; Hu, Y.; Gach, H.M.; Rao, P.K.; Grigsby, P.W. MRI-based treatment planning and dose delivery verification for intraocular melanoma brachytherapy. Brachytherapy 2018, 17, 31–39. [Google Scholar] [CrossRef]
- Dunavoelgyi, R.; Dieckmann, K.; Gleiss, A.; Sacu, S.; Kircher, K.; Georgopoulos, M.; Georg, D.; Zehetmayer, M.; Poetter, R. Local tumor control, visual acuity, and survival after hypofractionated stereotactic photon radiotherapy of choroidal melanoma in 212 patients treated between 1997 and 2007. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 199–205. [Google Scholar] [CrossRef]
- Xu, T.T.; Pulido, J.S.; Parney, I.F.; Ida, C.M.; Dalvin, L.A.; Olsen, T.W. Carbon Fiducial Markers for Tumor Localization in Stereotactic Irradiation of Uveal Melanoma. Ocul. Oncol. Pathol. 2021, 7, 368–375. [Google Scholar] [CrossRef]
- Laube, T.; Flühs, D.; Kessler, C.; Bornfeld, N. Determination of surgeons absorbed dose in iodine 125 and ruthenium 106 ophthalmic plaque surgery. Ophthalmology 2000, 107, 366–368. [Google Scholar] [CrossRef]
- Classic, K.L.; Furutani, K.M.; Stafford, S.L.; Pulido, J.S. Radiation dose to the surgeon during plaque brachytherapy. Retina 2012, 32, 1900–1905. [Google Scholar] [CrossRef]
- Rivard, M.J.; Butler, W.M.; DeWerd, L.A.; Huq, M.S.; Ibbott, G.S.; Meigooni, A.S.; Melhus, C.S.; Mitch, M.G.; Nath, R.; Williamson, J.F. Supplement to the 2004 update of the AAPM Task Group No. 43 Report. Med. Phys. 2007, 34, 2187–2205. [Google Scholar] [CrossRef]
- Melhus, C.S.; Rivard, M.J. COMS eye plaque brachytherapy dosimetry simulations for 125I, 103Pd and 131Cs. Med. Phys. 2008, 35, 3364–3371. [Google Scholar] [CrossRef]
- Lesperance, M.; Martinov, M.; Thomson, M. Monte Carlo dosimetry for 103Pd, 125I, 131Cs ocular brachytherapy with various plaque models using an eye phantom. Med. Phys. 2014, 41, 031706. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Rogers, D.W.O.; Bazlova-Carter, M.; WE, B.o.l.c.h.; Heath, E.C.; McNitt-Gray, M.F.; Sempau, J.; Williamson, J.F. RECORDS: Improved Reporting of montE CarlO RaDiation transport Studies: Report of the AAPM Research Committee Task 352 Group 268. Med. Phys. 2018, 45, e1–e5. [Google Scholar]
- Deufel, C.L.; McCauley, C.S.; Corbin, K.S.; Dalvin, L.A.; Petersen, I.A. EyeDose: An open-source tool for using published Monte Carlo results to estimate the radiation dose delivered to the tumor and critical ocular structures for 125I Collaborative Ocular Melanoma Study eye plaques. Brachytherapy 2021, 20, 189–199. [Google Scholar] [CrossRef]
- King, M.T.; Mira, K.; Frank, S.J.; Crook, J.M.; Butler, W.B.; Rossi, P.J.; Cox, B.W.; Showalter, T.N.; Mourtada, F.; Potters, L.; et al. Low dose rate brachytherapy for primary treatment of localized prostate cancer: A systemic review and executive summary of an evidence-based consensus statement. Brachytherapy 2021, 20, 1114–1129. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Byrne, M.; Archibald-Heeren, B.; Collett, N.; Liu, G.; Aland, T. Validation of the preconfigured Varian Ethos Acuros XB Beam Model for treatment planning dose calculations: A dosimetric study. J. Appl. Clin. Med. Phys. 2020, 21, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lin, S.C.; Kim, Y. A practical approach to estimating optic disc dose and macula dose without treatment planning in ocular brachytherapy using 125I COMS plaques. Radiat. Oncol. 2018, 13, 221. [Google Scholar] [CrossRef] [PubMed]
| Radiation Technique | Tumor/Treatment Considerations | Radiation Modality | Dose | Local Control (LC) | Complications |
|---|---|---|---|---|---|
| Radioactive seed plaque brachytherapy | Diameter < 19 mm, thickness < 10 mm, >2 mm away from optic nerve, surgery/anesthesia risk | Radioactive seeds (125I, 106Ru, 60Co, 192Ir, and 131Cs) | ~85 Gy in 3–7 days | 5 yr LC 90–95% [11,12] | Cataracts, radiation retinopathy, vitreous hemorrhage, glaucoma, scleral necrosis |
| Proton beam radiation | Proximity to optic nerve, eye immobilization | Protons | 70 Gy in 5 fractions | 10 yr LC 80–90% [13] | Cataracts, glaucoma, vitreous hemorrhage, retinopathy, optic neuropathy, |
| GK-SRS | >2 mm from optic nerve, eye immobilization | 60Co | 20–50 Gy in 1 fraction | 1–5 yr LC 85–95% [14] | Glaucoma, retinopathy, vitreous hemorrhage |
| Linear accelerator-based radiosurgery | Proximity to optic nerve, eye immobilization | Photons | ~22 Gy in 1 fraction, 60 Gy in 3–5 fractions | 5 year LC 80–85% [15] | Cataracts, radiation retinopathy, vitreous hemorrhage, glaucoma |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semeniuk, O.; Yu, E.; Rivard, M.J. Current and Emerging Radiotherapy Options for Uveal Melanoma. Cancers 2024, 16, 1074. https://doi.org/10.3390/cancers16051074
Semeniuk O, Yu E, Rivard MJ. Current and Emerging Radiotherapy Options for Uveal Melanoma. Cancers. 2024; 16(5):1074. https://doi.org/10.3390/cancers16051074
Chicago/Turabian StyleSemeniuk, Oleksii, Esther Yu, and Mark J. Rivard. 2024. "Current and Emerging Radiotherapy Options for Uveal Melanoma" Cancers 16, no. 5: 1074. https://doi.org/10.3390/cancers16051074
APA StyleSemeniuk, O., Yu, E., & Rivard, M. J. (2024). Current and Emerging Radiotherapy Options for Uveal Melanoma. Cancers, 16(5), 1074. https://doi.org/10.3390/cancers16051074






