Surgical De-Escalation for Re-Excision in Patients with a Margin Less Than 2 mm and a Diagnosis of DCIS
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
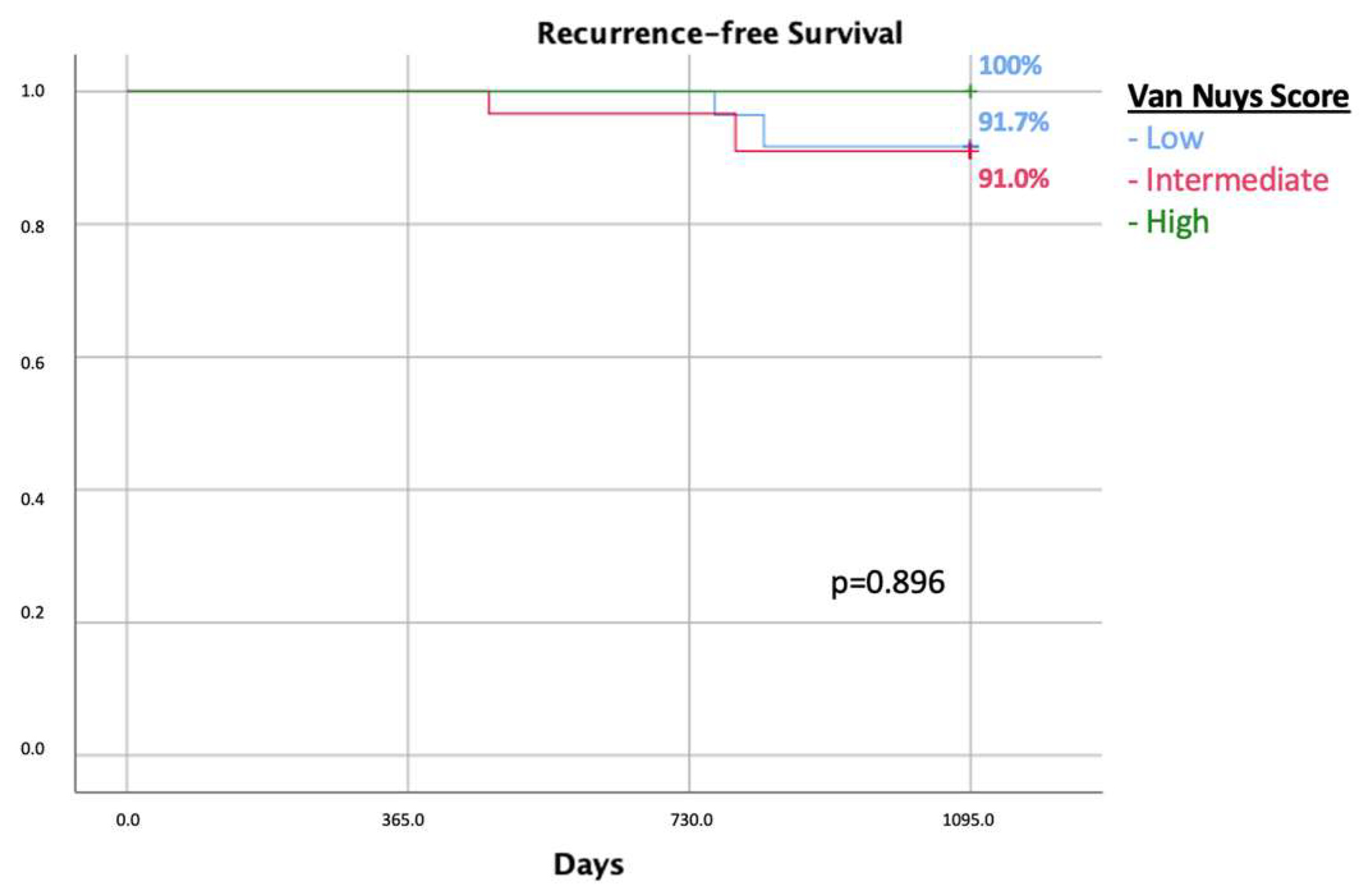
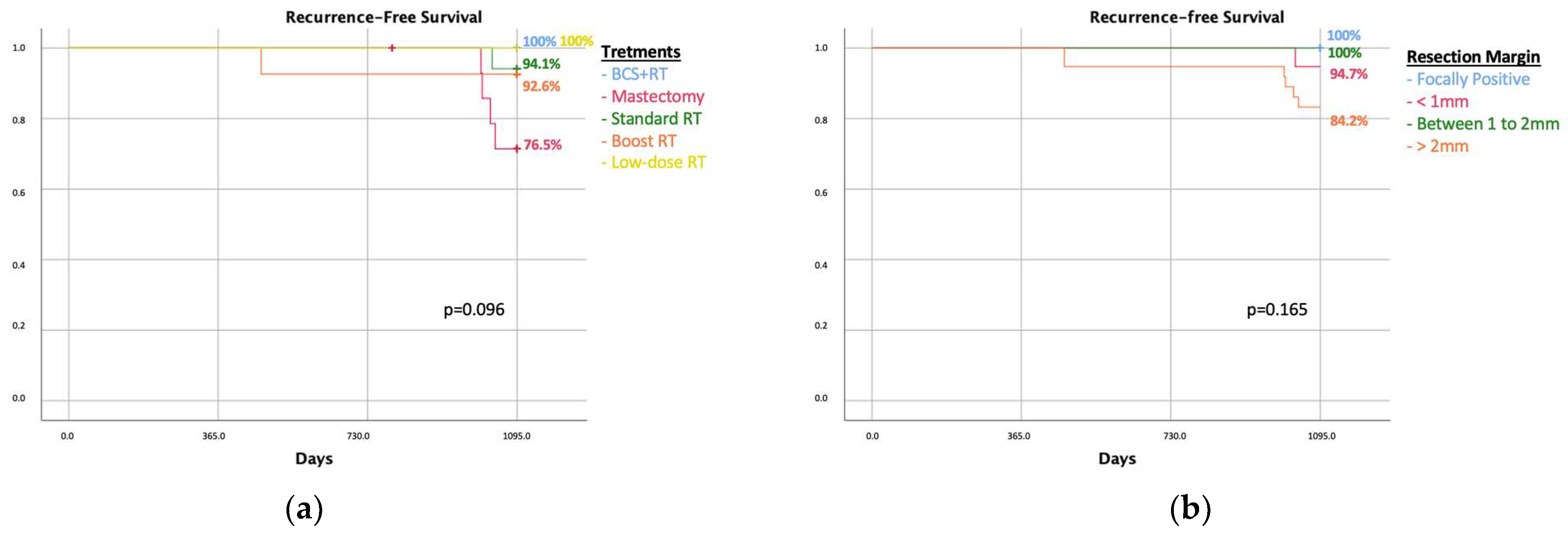
3. Results
Resection Margins < 2 mm
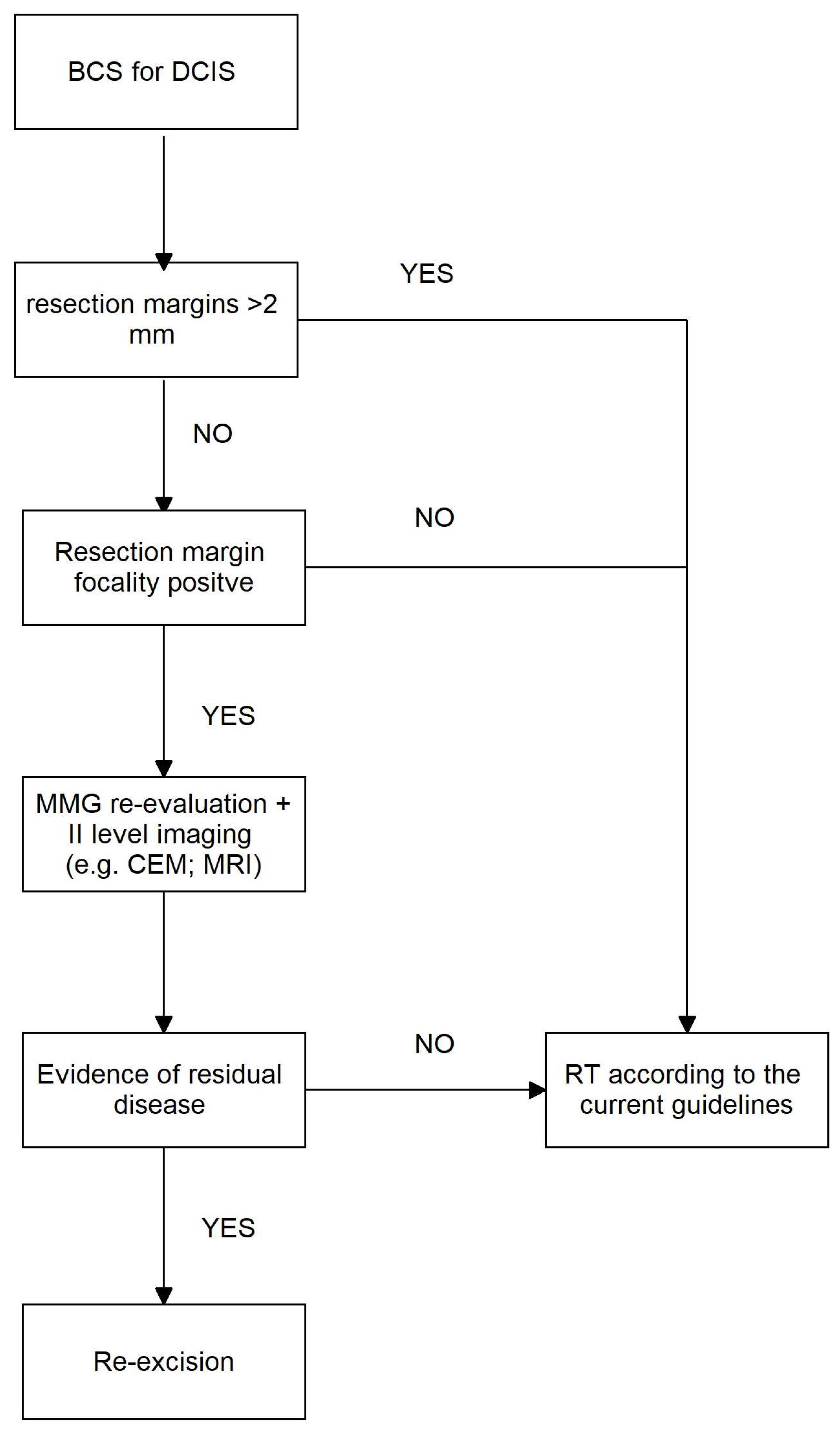
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchheit, J.T.; Schacht, D.; Kulkarni, S.A. Update on Management of Ductal Carcinoma in Situ. Clin. Breast Cancer 2023, in press. [CrossRef] [PubMed]
- Buonomo, O.C.; Pellicciaro, M.; Materazzo, M.; Berardi, S.; Gigliotti, P.E.; Caspi, J.; Meucci, R.; Perretta, T.; Portarena, I.; Dauri, M.; et al. Surgical Treatments for Ductal Carcinoma In Situ (DCIS) in Elderly Patients. Anticancer. Res. 2023, 43, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Frebault, J.; Bergom, C.; Cortina, C.S.; Shukla, M.E.; Zhang, Y.; Huang, C.-C.; Kong, A.L. Treatment Patterns in Women Age 80 and over with DCIS: A Report from the National Cancer Database. Clin. Breast Cancer 2022, 22, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Caspi, J.; Facchini, B.A.; Noce, A.; Lisi, G.; Tacconi, F.; Eskiu, D.; Cervelli, V.; et al. Awake breast surgery and de-escalation treatment: Strategies for frail and elderly breast cancer patients. World Cancer Res. J. 2023, 10, e2656. [Google Scholar] [CrossRef]
- Pappo, I.; Wasserman, I.; Halevy, A. Ductal Carcinoma In Situ of the Breast in Men: A Review. Clin. Breast Cancer 2005, 6, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, P.; De Scalzi, A. Is Mastectomy a Suitable Treatment in Ductal Carcinoma In Situ? Chirurgia 2021, 116, S83–S87. [Google Scholar] [CrossRef]
- Mason, E.J.; Di Leone, A.; Franco, A.; D’archi, S.; Rianna, C.; Sanchez, A.M.; Murando, F.; Accetta, C.; Scardina, L.; Terribile, D.A.; et al. Oncoplastic Breast Surgery versus Conservative Mastectomy in the Management of Large Ductal Carcinoma In Situ (DCIS): Surgical, Oncological, and Patient-Reported Outcomes. Cancers 2022, 14, 5624. [Google Scholar] [CrossRef]
- Langhans, L.; Jensen, M.-B.; Talman, M.-L.M.; Vejborg, I.; Kroman, N.; Tvedskov, T.F. Reoperation Rates in Ductal Carcinoma In Situ vs Invasive Breast Cancer after Wire-Guided Breast-Conserving Surgery. JAMA Surg. 2017, 152, 378–384. [Google Scholar] [CrossRef]
- Thomas, J.; Evans, A.; Macartney, J.; Pinder, S.E.; Hanby, A.; Ellis, I.; Kearins, O.; Roberts, T.; Clements, K.; Lawrence, G.; et al. Radiological and pathological side estimations of pure ductal carcinoma in situ of the breast: A review of 2564 cases from the Sloane Project. Br. J. Cancer 2010, 102, 285–293. [Google Scholar] [CrossRef]
- Schultek, G.; Gerber, B.; Reimer, T.; Stubert, J.; Hartmann, S.; Martin, A.; Stachs, A. Radiological Underestimation of Tumor Size as a Relevant Risk Factor for Positive Margin Rate in Breast-Conserving Therapy of Pure Ductal Carcinoma In Situ (DCIS). Cancers 2022, 14, 2367. [Google Scholar] [CrossRef]
- Farante, G.; Zurrida, S.; Galimberti, V.; Veronesi, P.; Curigliano, G.; Luini, A.; Goldhirsch, A.; Veronesi, U. The management of ductal intraepithelial neoplasia (DIN): Open controversies and guidelines of the Istituto Europeo di Oncologia (IEO), Milan, Italy. Breast Cancer Res. Treat. 2011, 128, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Oses, G.; Mension, E.; Pumarola, C.; Castillo, H.; Francesc, L.; Torras, I.; Cebrecos, I.; Caparrós, X.; Ganau, S.; Ubeda, B.; et al. Analysis of Local Recurrence Risk in Ductal Carcinoma In Situ and External Validation of the Memorial Sloan Kettering Cancer Center Nomogram. Cancers 2023, 15, 2392. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.; Van Zee, K.J.; Solin, L.J.; Houssami, N.; Chavez-MacGregor, M.; Harris, J.R.; Horton, J.; Hwang, S.; Johnson, P.L.; Marinovich, M.L.; et al. Society of Surgical Oncology–American Society for Radiation Oncology–American Society of Clinical Oncology Consensus Guideline on Margins for Breast-Conserving Surgery With Whole-Breast Irradiation in Ductal Carcinoma in Situ. Pract. Radiat. Oncol. 2016, 6, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Van Zee, K.J.; Subhedar, P.; Olcese, C.B.; Patil, S.; Morrow, M.M. Relationship Between Margin Width and Recurrence of Ductal Carcinoma In Situ: Analysis of 2996 Women Treated With Breast-conserving Surgery for 30 Years. Ann. Surg. 2015, 262, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Tadros, A.B.; Smith, B.D.; Shen, Y.; Lin, H.; Krishnamurthy, S.; Lucci, A.; Barcenas, C.H.M.; Hwang, R.F.; Rauch, G.; Santiago, L.; et al. Ductal Carcinoma In Situ and Margins < 2 mm: Contemporary Outcomes With Breast Conservation. Ann. Surg. 2019, 269, 150–157. [Google Scholar]
- van der Borden, C.L.; Stoffers, S.; Lips, E.H.; Wesseling, J. Avoiding Overtreatment of Ductal Carcinoma in situ. Trends Cancer 2019, 5, 391–393. [Google Scholar] [CrossRef]
- van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal carcinoma in situ: To treat or not to treat, that is the question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef]
- Cutuli, B.; Lemanski, C.; De Lafontan, B.; Chauvet, M.-P.; De Lara, C.T.; Mege, A.; Fric, D.; Richard-Molard, M.; Mazouni, C.; Cuvier, C.; et al. Ductal Carcinoma in Situ: A French National Survey. Analysis of 2125 Patients. Clin. Breast Cancer 2020, 20, e164–e172. [Google Scholar] [CrossRef]
- Schmitz, R.S.; Engelhardt, E.G.; Gerritsma, M.A.; Sondermeijer, C.M.; Verschuur, E.; Houtzager, J.; Griffioen, R.; Retèl, V.; Bijker, N.; Mann, R.M.; et al. Active surveillance versus treatment in low-risk DCIS: Women’s preferences in the LORD-trial. Eur. J. Cancer 2023, 192, 113276. [Google Scholar] [CrossRef]
- Oseni, T.O.; Smith, B.L.; Lehman, C.D.; Vijapura, C.A.; Pinnamaneni, N.; Bahl, M. Do Eligibility Criteria for Ductal Carcinoma In Situ (DCIS) Active Surveillance Trials Identify Patients at Low Risk for Upgrade to Invasive Carcinoma? Ann. Surg. Oncol. 2020, 27, 4459–4465. [Google Scholar] [CrossRef]
- Kunkiel, M.; Niwińska, A. Assessment of the usefulness of prognostic Van Nuys Prognostic Index in the treatment in ductal carcinoma in situ in 15-year observation. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Chien, J.-C.; Huang, W.-T.; Shih, L.-C.; Liu, W.-C.; Chen, Y.-C.; Chou, K.-J.; Shiue, Y.-L.; Lin, P.-C. Local treatment options for young women with ductal carcinoma in situ: A systematic review and meta-analysis comparing breast conserving surgery with or without adjuvant radiotherapy, and mastectomy. Breast 2022, 63, 29–36. [Google Scholar] [CrossRef]
- Chiappa, C.; Bonetti, A.; Jaber, G.J.; De Berardinis, V.; Bianchi, V.; Rovera, F. Pure Ductal Carcinoma In Situ of the Breast: Analysis of 270 Consecutive Patients Treated in a 9-Year Period. Cancers 2021, 13, 431. [Google Scholar] [CrossRef]
- Poelhekken, K.; Lin, Y.; Greuter, M.J.; van der Vegt, B.; Dorrius, M.; de Bock, G.H. The natural history of ductal carcinoma in situ (DCIS) in simulation models: A systematic review. Breast 2023, 71, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.J.; Choi, W.J.; Park, S.Y.; Ahn, S.H.; Son, B.H.; Chung, I.Y.; Lee, J.W.; Ko, B.S.; Kim, J.S.; Chae, E.Y.; et al. Prediction of Underestimation Using Contrast-Enhanced Spectral Mammography in Patients Diagnosed as Ductal Carcinoma In Situ on Preoperative Core Biopsy. Clin. Breast Cancer 2021, 22, e374–e386. [Google Scholar] [CrossRef]
- Seigneurin, A.; Labarère, J.; François, O.; Exbrayat, C.; Dupouy, M.; Filippi, M.; Colonna, M. Overdiagnosis and overtreatment associated with breast cancer mammography screening: A simulation study with calibration to population-based data. Breast 2016, 28, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, K.; Mathieu, E.; Brennan, M.; Snook, K.; Hoffman, J.; Campbell, I.; Scarlet, J.; Flay, H.; Wong, A.; Boyle, F.; et al. Patient-reported health-related quality of life outcomes following mastectomy for breast cancer, with immediate, delayed or no breast reconstruction: Four-year follow-up from a prospective cohort study. Breast 2023, 71, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Benson, J.R. Quality of life after breast-conserving surgery for women with non-low-risk ductal carcinoma in situ. Lancet Oncol. 2020, 21, 612–614. [Google Scholar] [CrossRef]
- Narod, S.A.; Sopik, V. Is invasion a necessary step for metastases in breast cancer? Breast Cancer Res. Treat. 2018, 169, 633–637. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Farley, D.R.; Peros, G. Recent advances and current controversies in the management of DCIS of the breast. Cancer Treat. Rev. 2008, 34, 483–497. [Google Scholar] [CrossRef]
- O’brien, K.M.; Sun, J.; Sandler, D.P.; DeRoo, L.A.; Weinberg, C.R. Risk factors for young-onset invasive and in situ breast cancer. Cancer Causes Control. 2015, 26, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Turaka, A.; Freedman, G.M.; Li, T.; Anderson, P.R.; Swaby, R.; Nicolaou, N.; Goldstein, L.; Sigurdson, E.R.; Bleicher, R.J. Young age is not associated with increased local recurrence for DCIS treated by breast-conserving surgery and radiation. J. Surg. Oncol. 2009, 100, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Hashiba, K.A.; Bahl, M. Ipsilateral tumor recurrence risk in women with ductal carcinoma in situ: Application of the Van Nuys Prognostic Index and the Memorial Sloan Kettering Cancer Center nomogram. Breast Cancer Res. Treat. 2023, 202, 185–190. [Google Scholar] [CrossRef]
- DeCensi, A.; Puntoni, M.; Guerrieri-Gonzaga, A.; Caviglia, S.; Avino, F.; Cortesi, L.; Taverniti, C.; Pacquola, M.G.; Falcini, F.; Gulisano, M.; et al. Randomized Placebo Controlled Trial of Low-Dose Tamoxifen to Prevent Local and Contralateral Recurrence in Breast Intraepithelial Neoplasia. J. Clin. Oncol. 2019, 37, 1629–1637. [Google Scholar] [CrossRef]
- Leonard, C.E.; Tole, S.P.; Turner, M.P.; Bennett, J.P.; Howell, K.T.; Carter, D.L. Association of the 12-Gene Breast DCIS Score® Assay With Local Recurrence in Patients With Ductal Carcinoma In Situ Treated on Accelerated Partial Breast Radiotherapy Protocols. Front. Oncol. 2021, 11, 671047. [Google Scholar] [CrossRef]
- Strand, S.H.; Rivero-Gutiérrez, B.; Houlahan, K.E.; Seoane, J.A.; King, L.M.; Risom, T.; Simpson, L.A.; Vennam, S.; Khan, A.; Cisneros, L.; et al. Molecular classification and biomarkers of clinical outcome in breast ductal carcinoma in situ: Analysis of TBCRC 038 and RAHBT cohorts. Cancer Cell 2022, 40, 1521–1536.e7. [Google Scholar] [CrossRef] [PubMed]


| LRR Group (n = 15) | NLR Group (n = 182) | p Value | |
|---|---|---|---|
| Age range | 0.692 | ||
| Age < 40 y | 0 | 4 (2.2%) | |
| Age 40–60 y | 9 (60.0%) | 121 (66.5%) | |
| Age > 60 y | 6 (40.0%) | 57 (31.3%) | |
| Tumor position | 0.007 | ||
| Upper outer quadrant (UOQ) | 0 | 23 (12.9%) | |
| Upper inner quadrant (UIQ) | 4 (26.7%) | 79 (44.4%) | |
| Lower outer quadrant (LOQ) | 0 | 17 (9.6%) | |
| Lower inner quadrant (LIQ) | 5 (33.3%) | 7 (3.9%) | |
| Central portion | 0 | 3 (1.7%) | |
| UOQ–LOQ | 3 (20%) | 3 (1.7%) | |
| UOQ–UIQ | 3 (20%) | 43 (24.2%) | |
| LOQ–LIQ | 0 | 3 (1.7%) | |
| LOQ–LIQ | 0 | 3 (1.7%) | |
| Tumor dimension range | 0.377 | ||
| Lesion < 15 mm | 7 (46.7%) | 107 (58.8%) | |
| Lesion > 15 mm < 40 mm | 8 (53.3%) | 62 (34.1%) | |
| Lesion > 40 mm | 0 | 9 (4.9%) |
| LRR Group (n = 15) | NLR Group (n = 182) | p Value | |
|---|---|---|---|
| Comedonecrosis | 8 (53.3%) | 81 (49.4%) | 0.704 |
| Grading | 21 | 0.056 | |
| Low | 7 (46.7%) | 58 (31.3%) | |
| Intermediate | 0 | 44 (24.4%) | |
| High | 8 (53.3%) | 74 (40%) | |
| Estrogen receptor positivity | 13 (86.6%) | 114 (62.6%) | 0.236 |
| Progesteron receptor positivity | 12 (80%) | 96 (52.7%) | 0.881 |
| Diameter (mm) | 3.7 ± 3.2 | 4.9 ± 6.1 | 0.456 |
| Closer resection margin (mm) | 6.6 ± 4.2 | 6.0 ± 4.0 | 0.543 |
| Resection margin < 2 mm | 2 (13.3%) | 42 (23.1%) | 0.530 |
| Margin status | 0.688 | ||
| Focally positive margin | 0 | 5 (2.7%) | |
| Negative margin < 1 mm | 2 (13.3%) | 19 (10.4%) | |
| Margin between 1 mm and 2 mm | 0 | 17 (9.2%) | |
| Margin > 2 mm | 13 (86.6%) | 141 (77.5%) | |
| Van Nuys prognostic index | 0.518 | ||
| Low | 7 (46.7%) | 77 (41.8%) | |
| Intermediate | 8 (53.3%) | 81(44.5%) | |
| High | 0 | 2 (1.1%) |
| LRR Group (n = 15) | No-Recurrence Group (n = 182) | p Value | |
|---|---|---|---|
| Re-excision | 0 | 12 (18.2%) | 0.305 |
| No hormone therapy | 7 (46.7%) | 83 (45.6%) | 1.000 |
| No radiotherapy | 5 (33.3%) | 30 (16.5%) | 0.148 |
| Radiotheraphy regimen | 0.236 | ||
| None | 5 (33.3%) | 30 (16.5%) | |
| Standard | 10 (66.7%) | 127 (69.8%) | |
| Boost | 0 | 25 (13.7%) |
| BCS+RT (n = 15) | Mastectomy (n = 17) | Standard RT (n = 17) | Boost RT (n = 27) | Low Dose RT (n = 3) | p Value | |
|---|---|---|---|---|---|---|
| Age range | 0.031 | |||||
| Age < 40 y | 0 | 4 (23.5%) | 0 | 4 (14.9%) | 0 | |
| Age 40–60 y | 9 (60%) | 10 (58.8%) | 14 (82.4%) | 17 (61.9%) | 0 | |
| Age > 60 y | 6 (40%) | 3 (17.6%) | 3 (17.6%) | 6 (22.2%) | 3 (100%) | |
| Tumor dimension | 0.006 | |||||
| Lesion < 15 mm | 9 (60%) | 4 (23.5%) | 11 (64.7%) | 9 (33.3%) | 3 (100%) | |
| Lesion > 15 mm < 40 mm | 6 (40%) | 10 (58.8%) | 3 (17.6%) | 14 (51.8%) | 0 | |
| Lesion > 40 mm | 0 | 3 (17.6%) | 3 (17.6%) | 0 | 0 | |
| Multifocal lesion | 0 | 3 (17.6%) | 3 (17.6%) | 0 | 0 | 0.042 |
| Multicentric lesion | 0 | 3 (17.6%) | 3 (17.6%) | 0 | 0 | 0.042 |
| Microcalcifications | 13 (76.5%) | 13 (86.6%) | 17 (100%) | 21 (77.7%) | 0 | 0.001 |
| Comedonecrosis | 12 (80%) | 6 (35.3%) | 14 (82.4%) | 20 (74.1%) | 0 | 0.003 |
| Grading | 0.004 | |||||
| Low | 0 | 0 | 0 | 3 (11.1%) | 3 (100%) | |
| Intermediate | 0 | 11 (74.7%) | 6 (35.3%) | 8 (29.6%) | 0 | |
| High | 12 (100%) | 6 (35.3%) | 11 (64.7%) | 16 (59.3%) | 0 | |
| ER expression | 3 (25%) | 14 (82.4%) | 11 (64.7%) | 17 (63.0%) | 1 (33.3%) | 0.079 |
| PR expression | 3 (25%) | 10 (58.8%) | 11 (64.7%) | 12 (44.4%) | 1 (33.3%) | 0.086 |
| Resection margins | 0.001 | |||||
| Focally positive | 15 (100%) | 17 (100%) | 0 | 5 (18.5%) | 0 | |
| Margins between 0 and 1 mm | 0 | 0 | 14 (82.4%) | 9 (33.3%) | ||
| Margins between 1 and 2 mm | 0 | 0 | 3 (17.6%) | 13 (48.2%) | 3 (100%) | |
| Re-excision margins | 0.001 | |||||
| Focally positive | 0 | 0 | 0 | 5 (18.5%) | 0 | |
| Margins between 0 and 1 mm | 0 | 0 | 14 (82.4%) | 9 (33.3%) | 0 | |
| Margins < 1 mm | 15 (100%) | 17 (100%) | 3 (17.6%) | 13 (48.2%) | 3 (100%) | |
| Van Nuys prognostic index | 0.211 | |||||
| Low | 15 (100%) | 14(82.4%) | 17 (100%) | 26 (96.3%) | 3 (100%) | |
| Intermediate | 0 | 3 (17.6%) | 0 | 1 (3.7%) | 0 | |
| High | 0 | 0 | 0 | 0 | 0 | |
| Recurrence | 0 | 4 (23.5%) | 1 (5.9% | 2 (7.4%) | 0 | 0.231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanni, G.; Pellicciaro, M.; Di Lorenzo, N.; Barbarino, R.; Materazzo, M.; Tacconi, F.; Squeri, A.; D’Angelillo, R.M.; Berretta, M.; Buonomo, O.C. Surgical De-Escalation for Re-Excision in Patients with a Margin Less Than 2 mm and a Diagnosis of DCIS. Cancers 2024, 16, 743. https://doi.org/10.3390/cancers16040743
Vanni G, Pellicciaro M, Di Lorenzo N, Barbarino R, Materazzo M, Tacconi F, Squeri A, D’Angelillo RM, Berretta M, Buonomo OC. Surgical De-Escalation for Re-Excision in Patients with a Margin Less Than 2 mm and a Diagnosis of DCIS. Cancers. 2024; 16(4):743. https://doi.org/10.3390/cancers16040743
Chicago/Turabian StyleVanni, Gianluca, Marco Pellicciaro, Nicola Di Lorenzo, Rosaria Barbarino, Marco Materazzo, Federico Tacconi, Andrea Squeri, Rolando Maria D’Angelillo, Massimiliano Berretta, and Oreste Claudio Buonomo. 2024. "Surgical De-Escalation for Re-Excision in Patients with a Margin Less Than 2 mm and a Diagnosis of DCIS" Cancers 16, no. 4: 743. https://doi.org/10.3390/cancers16040743
APA StyleVanni, G., Pellicciaro, M., Di Lorenzo, N., Barbarino, R., Materazzo, M., Tacconi, F., Squeri, A., D’Angelillo, R. M., Berretta, M., & Buonomo, O. C. (2024). Surgical De-Escalation for Re-Excision in Patients with a Margin Less Than 2 mm and a Diagnosis of DCIS. Cancers, 16(4), 743. https://doi.org/10.3390/cancers16040743







