Global Burden of Pancreatic Cancer Attributable to High Body-Mass Index in 204 Countries and Territories, 1990–2019
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
2.3. Study Variables and Measures
2.4. Statistical Analysis
2.5. Ethical Considerations
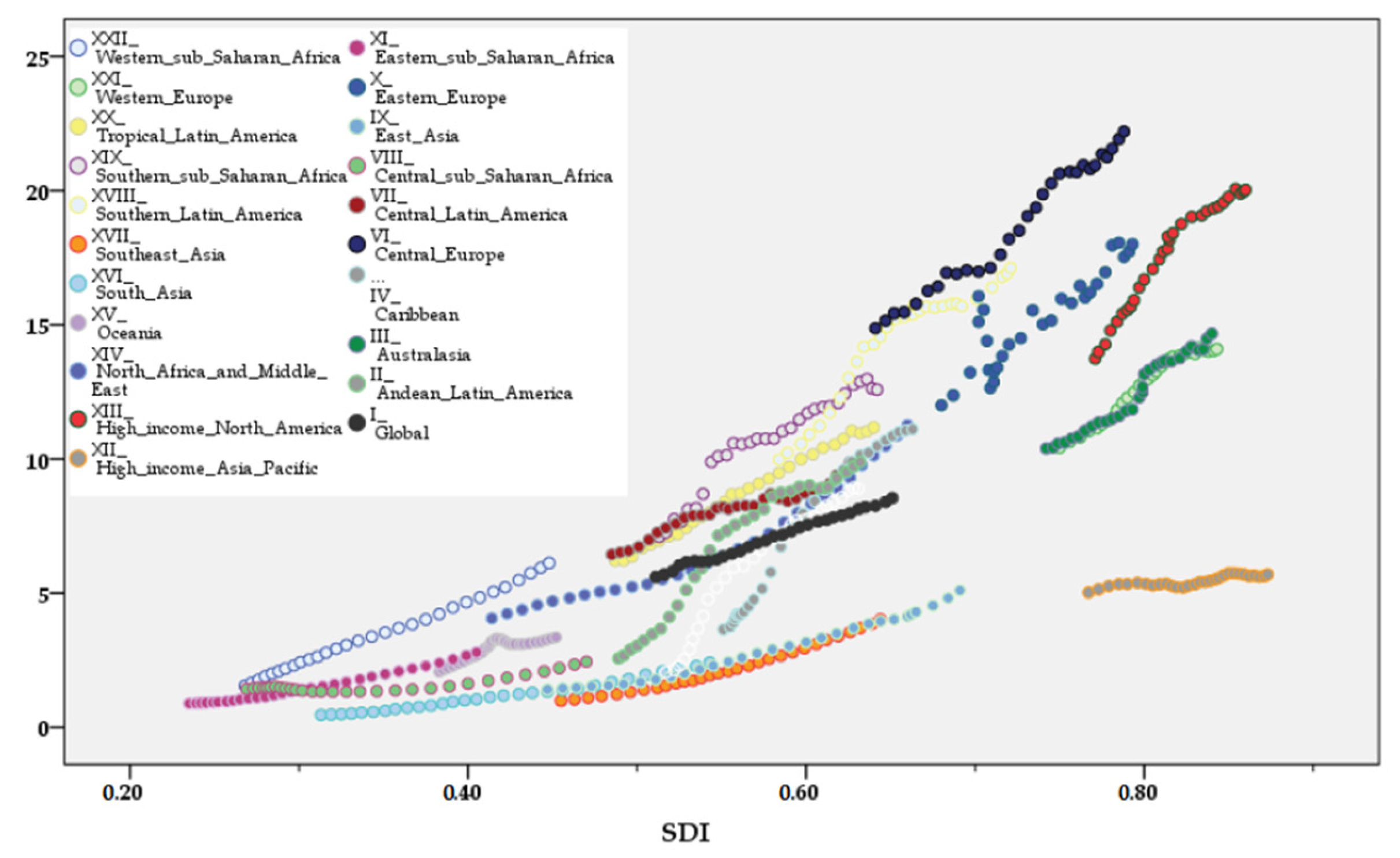
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Bray, F. Cancer Incidence in Five Continents, CI5plus: IARC CancerBase No. 9. Lyon, France: International Agency for Research on Cancer. 2018. Available online: http://ci5.iarc.fr (accessed on 7 December 2023).
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, based on November 2020 SEER Data Submission, Posted to the SEER Web Site, April 2021. Available online: https://seer.cancer.gov/csr/1975_2018/ (accessed on 7 December 2023).
- Ferlay, J.; Partensky, C.; Bray, F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncol. 2016, 55, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Kempf, E.; Priou, S.; Lamé, G.; Laurent, A.; Guével, E.; Tzedakis, S.; Bey, R.; Fuks, D.; Chatellier, G.; Tannier, X.; et al. No changes in clinical presentation, treatment strategies and survival of pancreatic cancer cases during the SARS-CoV-2 outbreak: A retrospective multicenter cohort study on real-world data. Int. J. Cancer 2023, 153, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Kędzierska-Kapuza, K.; Witkowski, G.; Baumgart-Gryn, K.; Szylińska, A.; Durlik, M. Impact of COVID-19 on pancreatic cancer surgery: A high-volume Polish center experience. Adv. Clin. Exp. Med. 2022, 31, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Mattiuzzi, C. The global burden of pancreatic cancer. Arch. Med. Sci. 2020, 16, 820–824. [Google Scholar] [CrossRef]
- Yu, J.; Yang, X.; He, W.; Ye, W. Burden of pancreatic cancer along with attributable risk factors in Europe between 1990 and 2019, and projections until 2039. Int. J. Cancer 2021, 149, 993–1001. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- UN General Assembly. Transforming our World: The 2030 Agenda for Sustainable Development, 21 October 2015, A/RES/70/1. Available online: http://www.refworld.org/docid/57b6e3e44.html (accessed on 7 December 2023).
- Institute for Health Metrics and Evaluation (IHME). GBD Results. Seattle, WA: IHME, University of Washington. 2020. Available online: https://vizhub.healthdata.org/gbd-results/ (accessed on 7 December 2023).
- World Health Organization. International Classification of Disease and Related Health Problems: 10th Revision; World Health Organization: Geneva, Switzerland, 1992. [Google Scholar]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER statement. Lancet 2016, 388, e19–e23. [Google Scholar] [CrossRef]
- GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Clegg, L.X.; Hankey, B.F.; Tiwari, R.; Feuer, E.J.; Edwards, B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009, 28, 3670–3682. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Pancreatic Cancer Collaborators. The global, regional, and national burden of pancreatic cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2019, 4, 934–947. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Demographics Collaborators. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: A comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1160–1203. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P. Pancreatic cancer: A growing burden. Lancet Gastroenterol. Hepatol. 2019, 4, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Fiscella, K. Disparities in Pancreatic Cancer Treatment and Outcomes. Health Equity 2019, 3, 532–540. [Google Scholar] [CrossRef]
- Møller, B.; Jerm, M.B.; Larønningen, S.; Johannesen, T.B.; Seglem, A.H.; Larsen, I.K.; Myklebust, T.Å. The validity of cancer information on death certificates in Norway and the impact of death certificate initiated cases on cancer incidence and survival. Cancer Epidemiol. 2021, 75, 102023. [Google Scholar] [CrossRef]
- Rahu, K.; McKee, M.; Mägi, M.; Rahu, M. The fall and rise of cancer registration in Estonia: The dangers of overzealous application of data protection. Cancer Epidemiol. 2020, 66, 101708. [Google Scholar] [CrossRef] [PubMed]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.H.; Relton, C.L.; Martin, R.M.; Davey Smith, G.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109, djx012. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, C.F.; Garcia, C.; Jacox, J.B.; Lawres, L.; Muzumdar, M.D. Decoding the obesity-cancer connection: Lessons from preclinical models of pancreatic adenocarcinoma. Life Sci. Alliance 2023, 6, e202302228. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, S.; Nakamura, N.; Roach, B.; Bernlohr, D.A.; Ikramuddin, S.; Yamamoto, M. Obesity and Pancreatic Cancer: Recent Progress in Epidemiology, Mechanisms and Bariatric Surgery. Biomedicines 2022, 10, 1284. [Google Scholar] [CrossRef] [PubMed]
- Ruze, R.; Song, J.; Yin, X.; Chen, Y.; Xu, R.; Wang, C.; Zhao, Y. Mechanisms of obesity- and diabetes mellitus-related pancreatic carcinogenesis: A comprehensive and systematic review. Signal Transduct. Target. Ther. 2023, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, S.; Li, Y.; Fan, Z.; Meng, Y.; Zhou, B.; Zhang, G.; Zhan, H. FABP4 in macrophages facilitates obesity-associated pancreatic cancer progression via the NLRP3/IL-1β axis. Cancer Lett. 2023, 575, 216403. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Gordon-Dseagu, V.L.; Devesa, S.S.; Goggins, M.; Stolzenberg-Solomon, R. Pancreatic cancer incidence trends: Evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int. J. Epidemiol. 2018, 47, 427–439. [Google Scholar] [CrossRef]
- Saad, A.M.; Turk, T.; Al-Husseini, M.J.; Abdel-Rahman, O. Trends in pancreatic adenocarcinoma incidence and mortality in the United States in the last four decades; a SEER-based study. BMC Cancer 2018, 18, 688. [Google Scholar] [CrossRef]
- Stolzenberg-Solomon, R.Z.; Amundadottir, L.T. Epidemiology and Inherited Predisposition for Sporadic Pancreatic Adenocarcinoma. Hematol. Oncol. Clin. N. Am. 2015, 29, 619–640. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Farias, A.J.; Streicher, S.A.; Stram, D.O.; Wang, S.; Pandol, S.J.; Le Marchand, L.; Setiawan, V.W. Racial/ethnic disparities in weight or BMI change in adulthood and pancreatic cancer incidence: The multiethnic cohort. Cancer Med. 2021, 10, 4097–4106. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.Z.H.; Ng, K.W.; Ishak, N.D.B.; Lee, S.Y.; Zhang, Z.; Chiang, J.; Ngeow, J.Y.Y. Geographical, ethnic, and genetic differences in pancreatic cancer predisposition. Chin. Clin. Oncol. 2023, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Chen, C.; Guo, J.; Qiao, M.; Lyu, J. Recent estimates and predictions of 5-year survival rate in patients with pancreatic cancer: A model-based period analysis. Front. Med. 2022, 9, 1049136. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Maisonneuve, P.; Bamlet, W.R.; Petersen, G.M.; Li, D.; Risch, H.A.; Yu, H.; Fontham, E.T.; Luckett, B.; Bosetti, C.; et al. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas 2016, 45, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Whitley, A.; Kocián, P.; Nikov, A.; Krejčí, D.; Pehalová, L.; Blaha, M.; Dušek, L.; Gürlich, R. Early-onset pancreatic cancer: A national cancer registry study from the Czech Republic and review of the literature. J. Hepatobiliary Pancreat. Sci. 2023, 30, 1324–1333. [Google Scholar] [CrossRef]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009, 301, 2553–2562. [Google Scholar] [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moura, E.C.; Conde, W.L.; Popkin, B.M. Socioeconomic status and obesity in adult populations of developing countries: A review. Bull. World Health Organ. 2004, 82, 940–946. [Google Scholar] [PubMed]
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Jiang, J.Y.; Liang, M.; Fang, Y.; Yeung, M.S.; Sung, J.J.Y. Global temporal patterns of pancreatic cancer and association with socioeconomic development. Sci. Rep. 2017, 7, 3165. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.F.; Wang, L.; Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021, 9, 373–392. [Google Scholar] [CrossRef] [PubMed]
- Motta, B.M.; Masarone, M.; Torre, P.; Persico, M. From Non-Alcoholic Steatohepatitis (NASH) to Hepatocellular Carcinoma (HCC): Epidemiology, Incidence, Predictions, Risk Factors, and Prevention. Cancers 2023, 15, 5458. [Google Scholar] [CrossRef] [PubMed]
- Casari, I.; Falasca, M. Diet and Pancreatic Cancer Prevention. Cancers 2015, 7, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.O.; Abuhijleh, H.; Tayyem, R. What Dietary Patterns and Nutrients are Associated with Pancreatic Cancer? Literature Review. Cancer Manag. Res. 2023, 15, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Possibility of Pancreatic Cancer Chemoprevention by Anti-Inflammatory/Anti-Diabetic/Anti-Dyslipidemic Drugs. Gan To Kagaku Ryoho 2021, 48, 1429–1434. [Google Scholar]
- Miyaki, C.; Lynch, L.M. An Update on Common Pharmaceuticals in the Prevention of Pancreatic Cancer. Cureus 2022, 14, e25496. [Google Scholar] [CrossRef]
- Hu, J.; Fan, H.D.; Gong, J.P.; Mao, Q.S. The relationship between the use of metformin and the risk of pancreatic cancer in patients with diabetes: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 50. [Google Scholar] [CrossRef]
- Fan, H.; Mao, Q.; Zhang, W.; Fang, Q.; Zou, Q.; Gong, J. The Impact of Bariatric Surgery on Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Obes. Surg. 2023, 33, 1889–1899. [Google Scholar] [CrossRef]
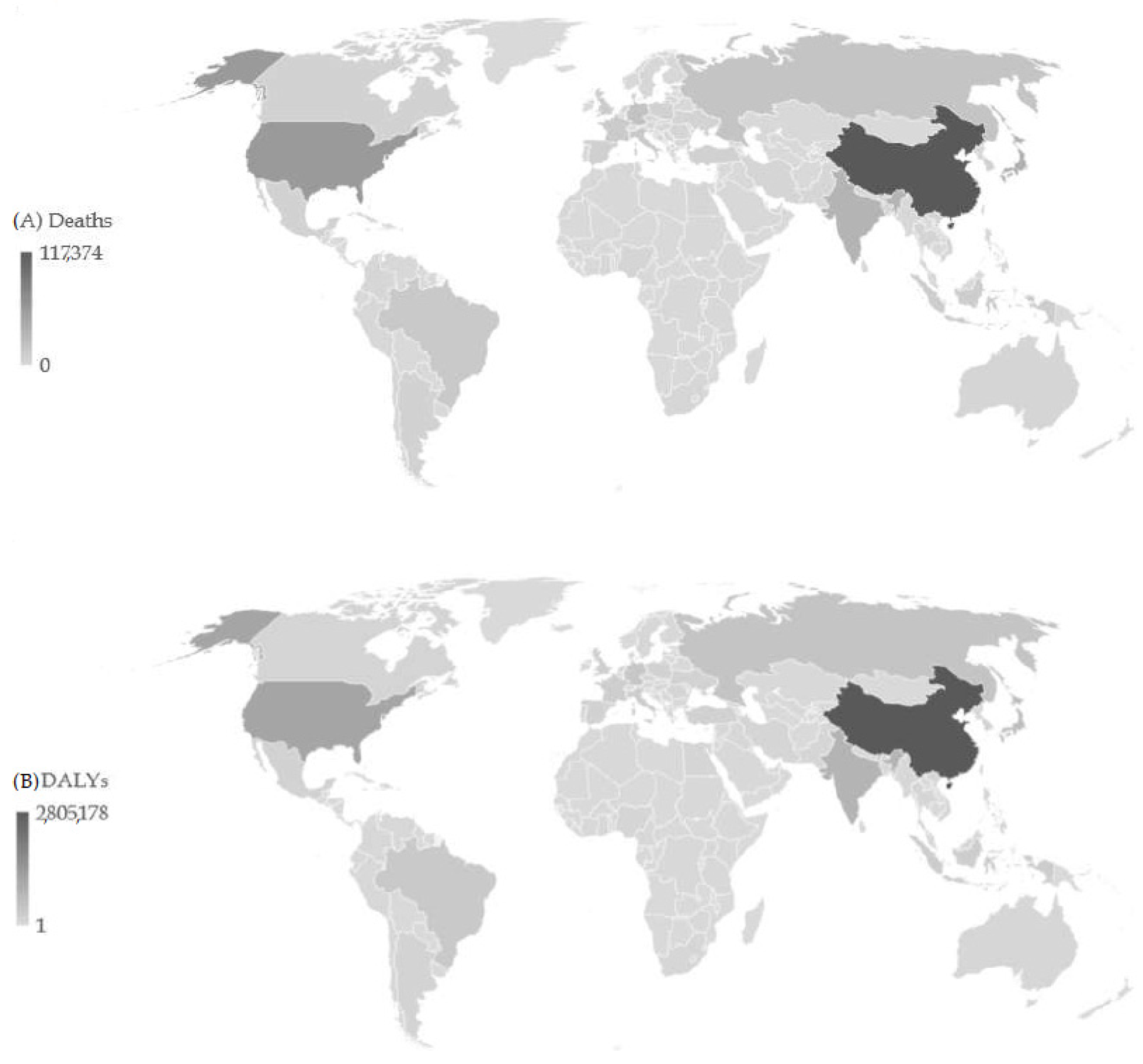
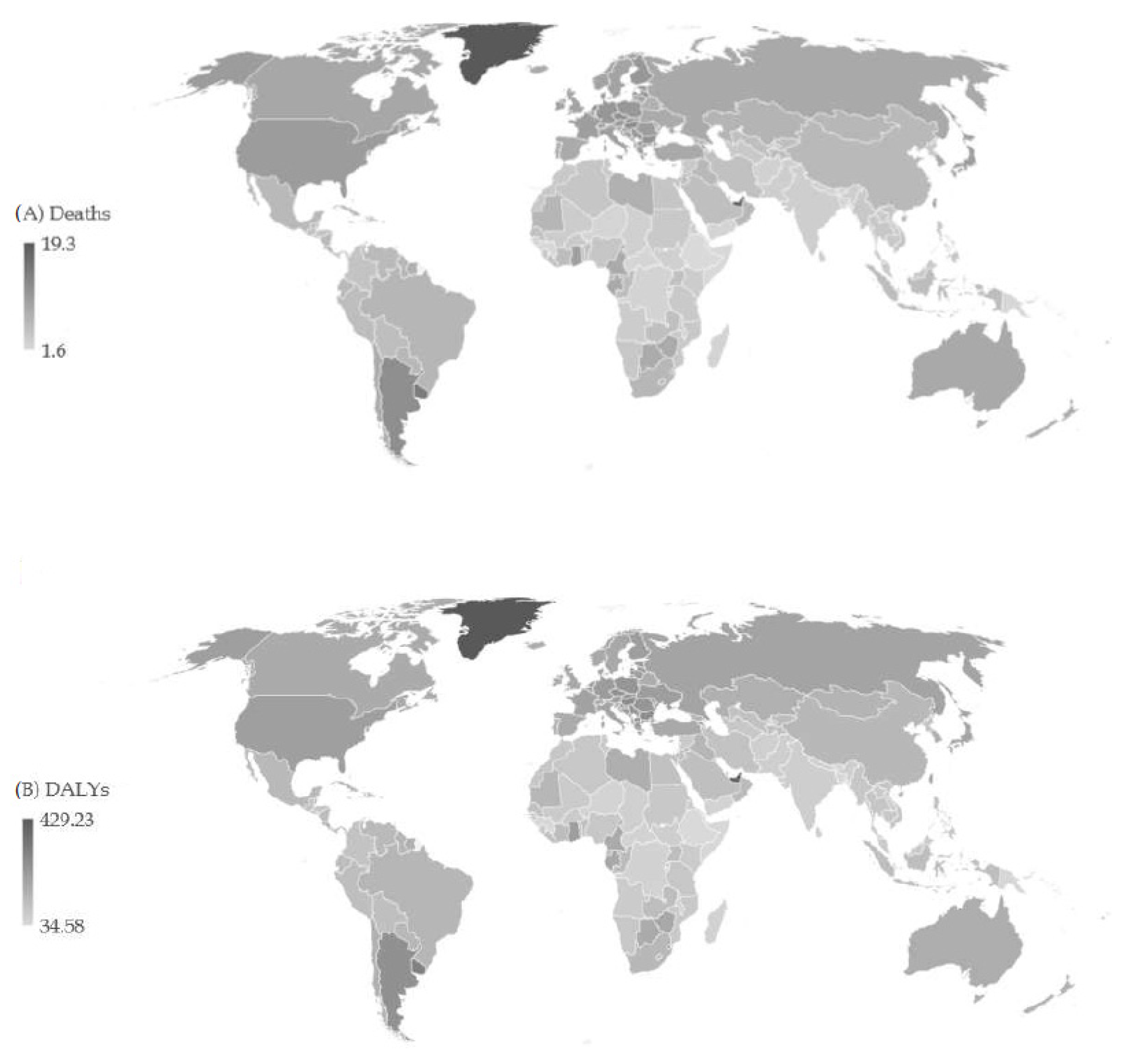
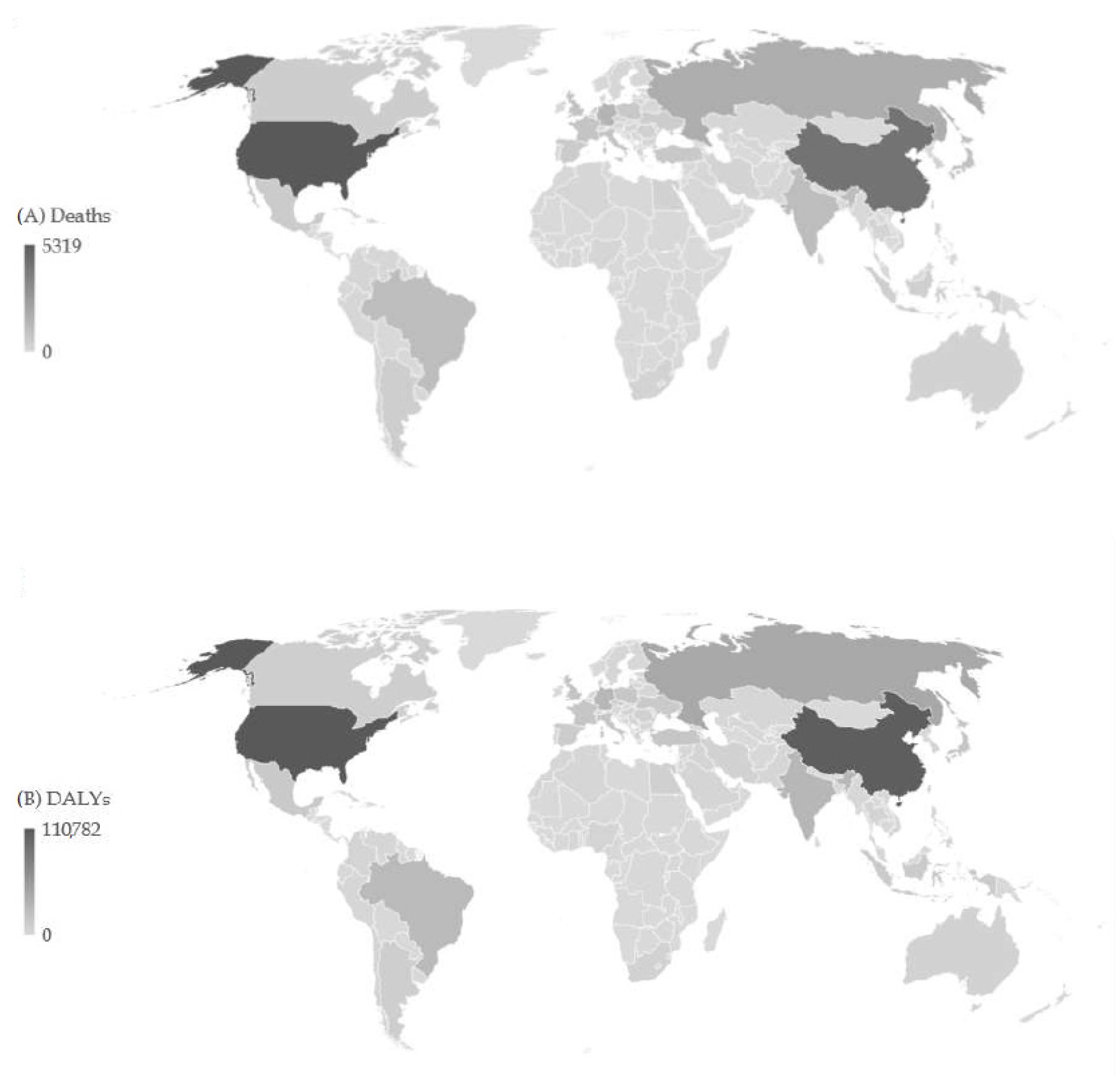
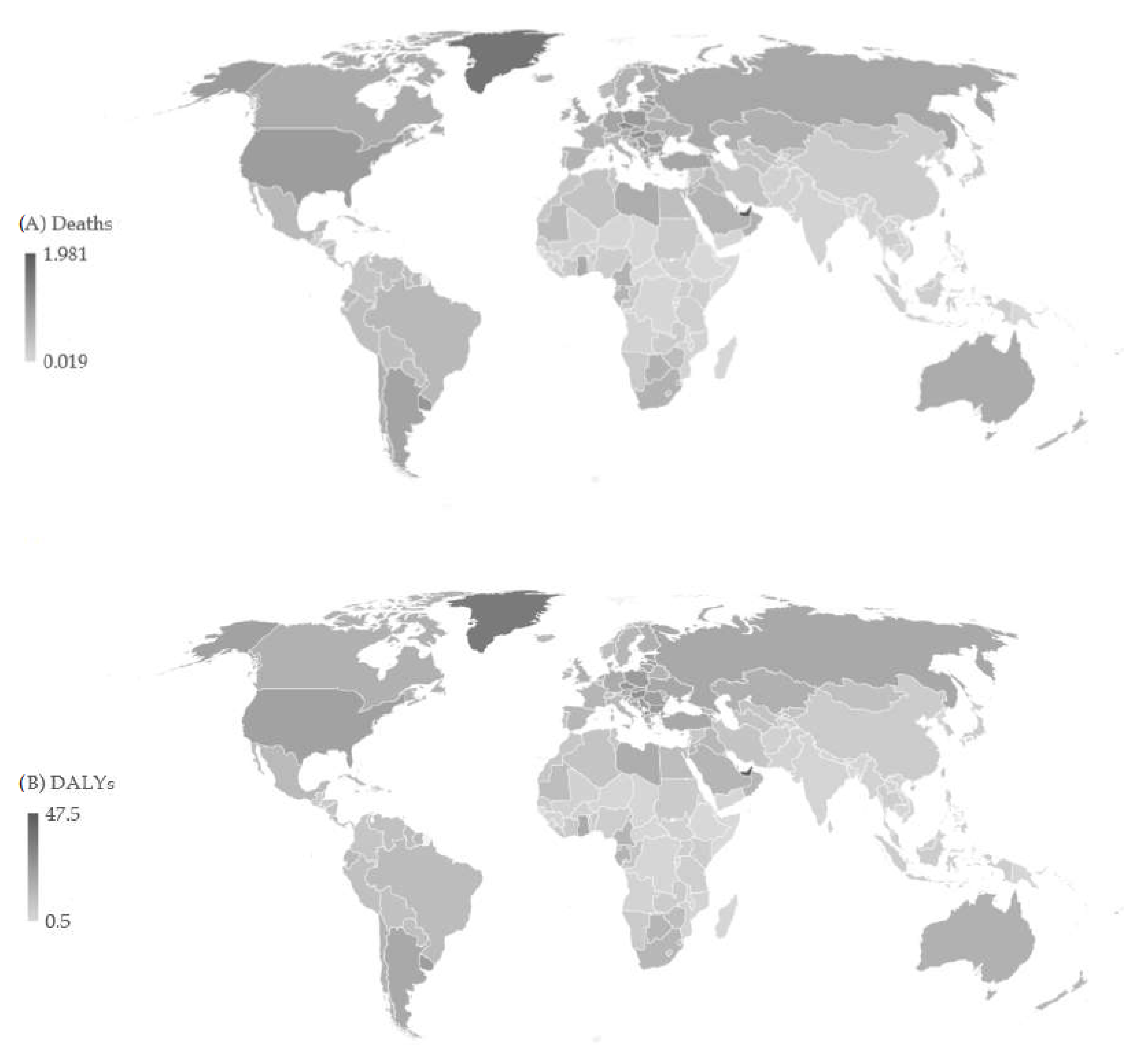
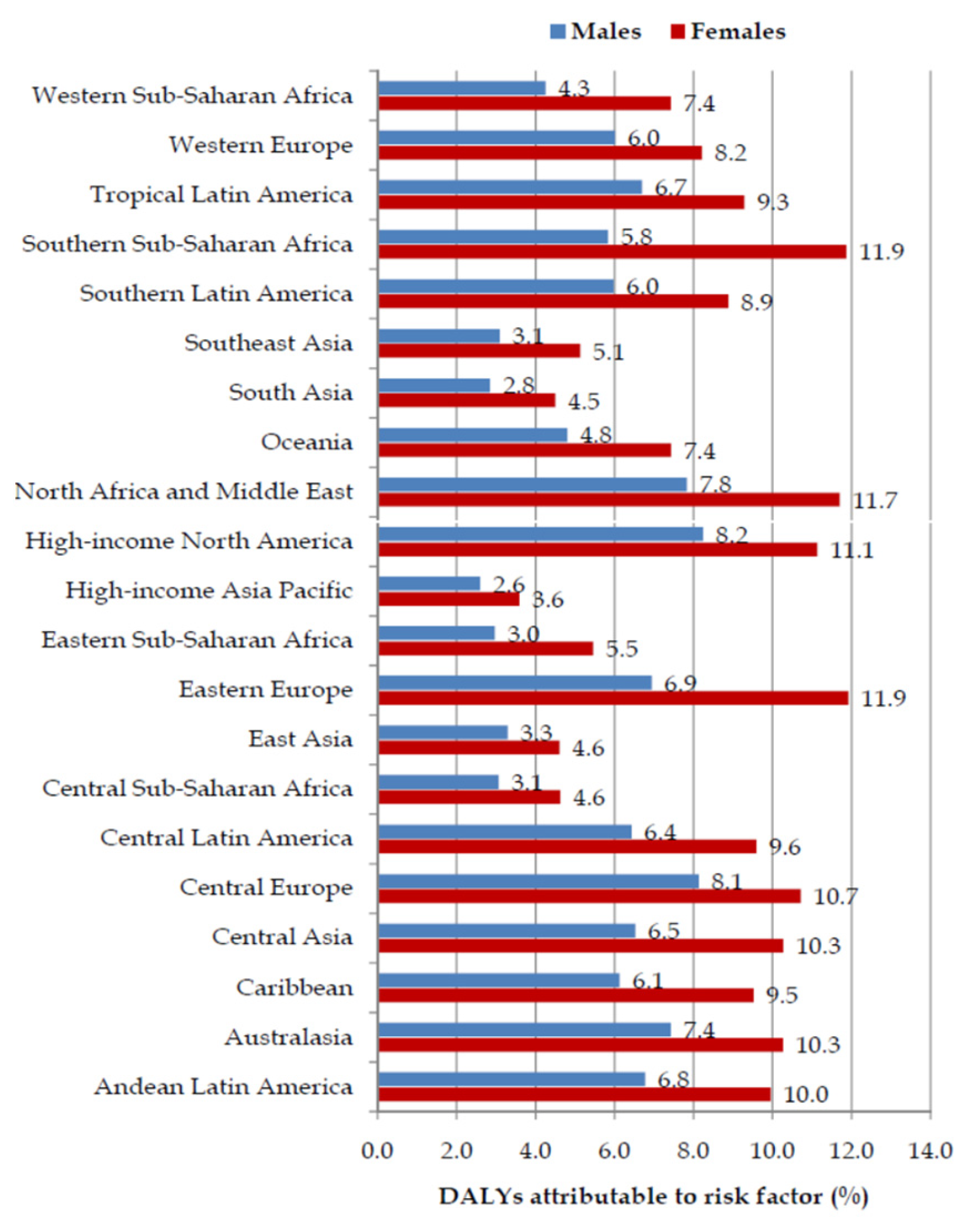

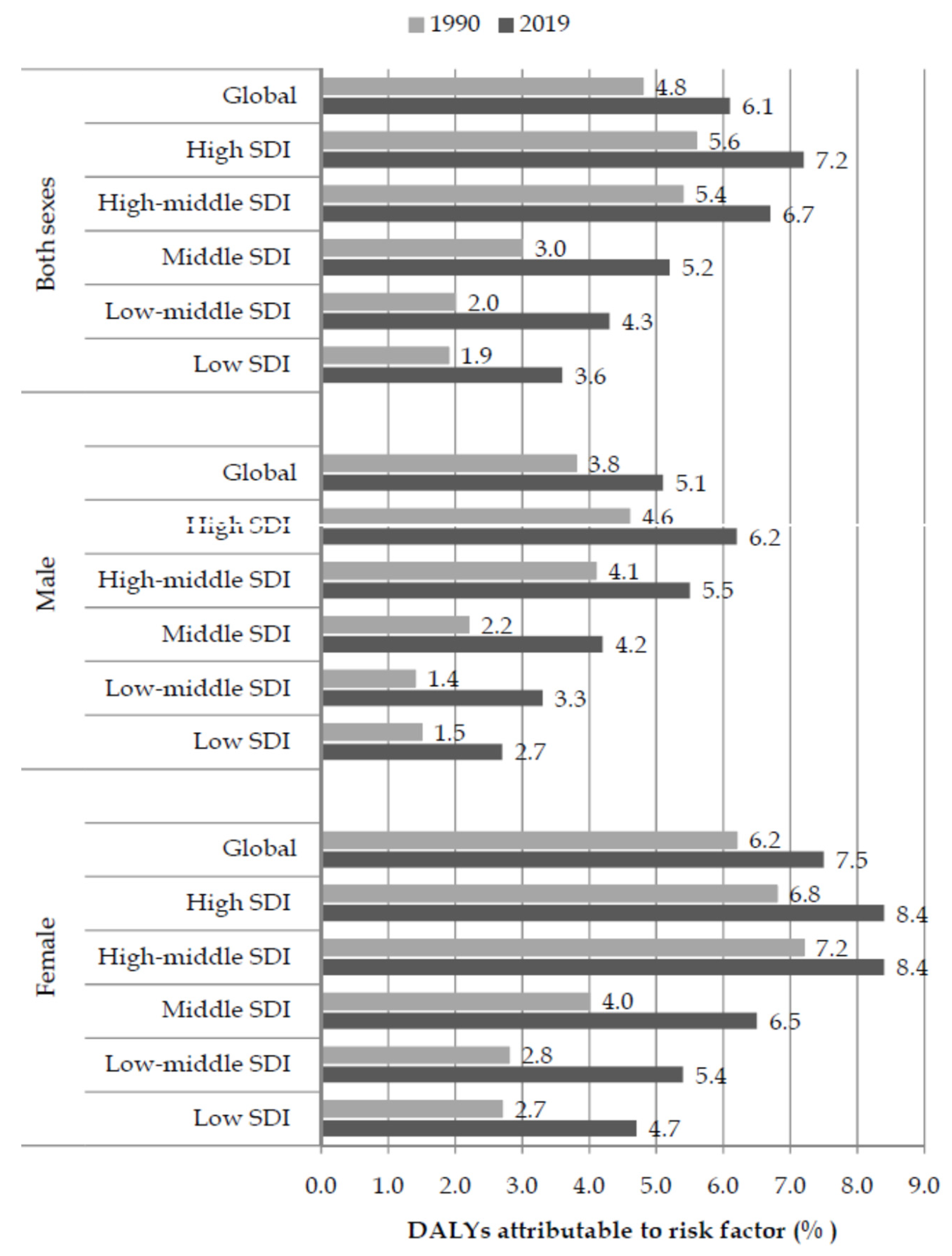
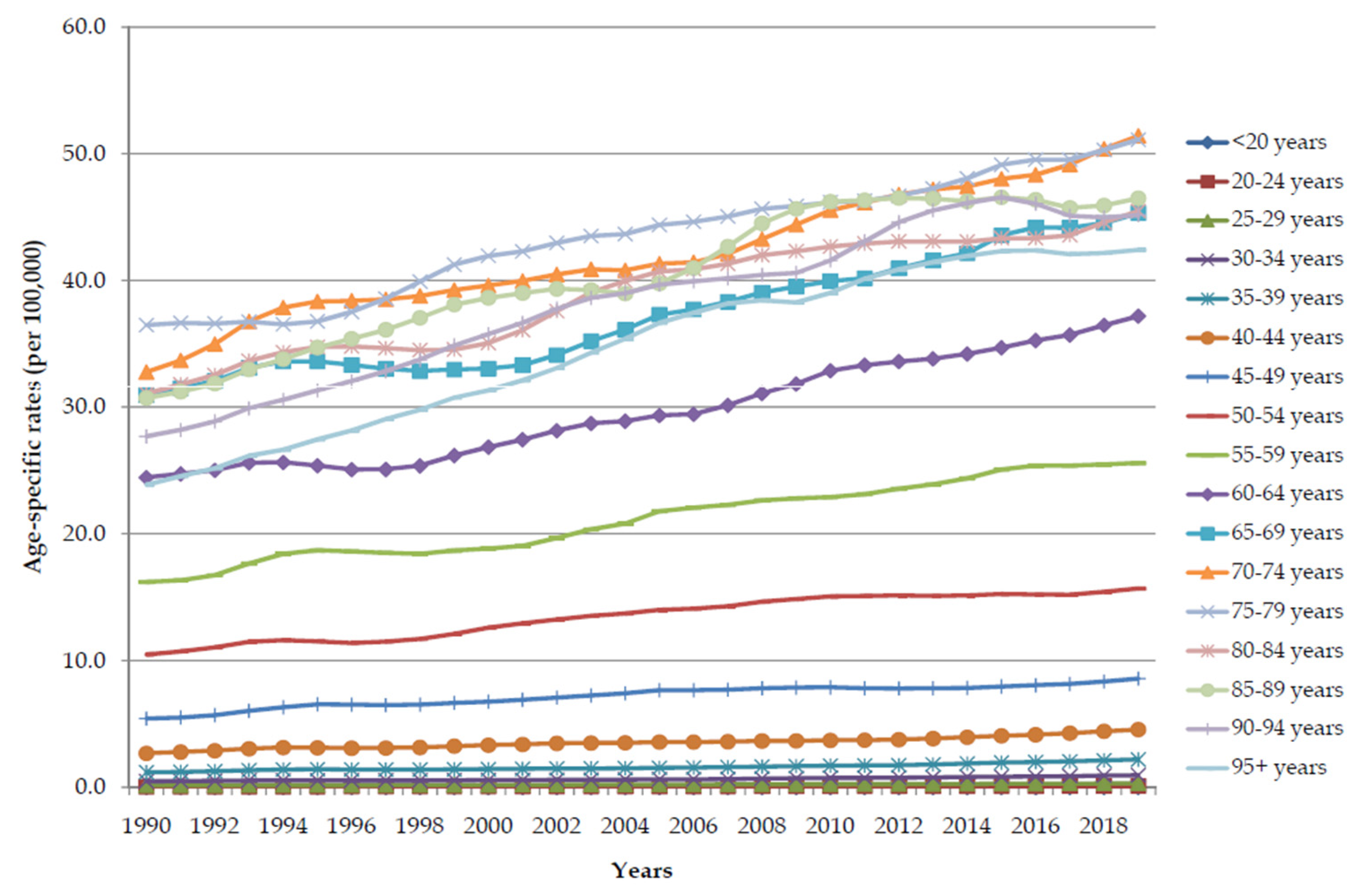
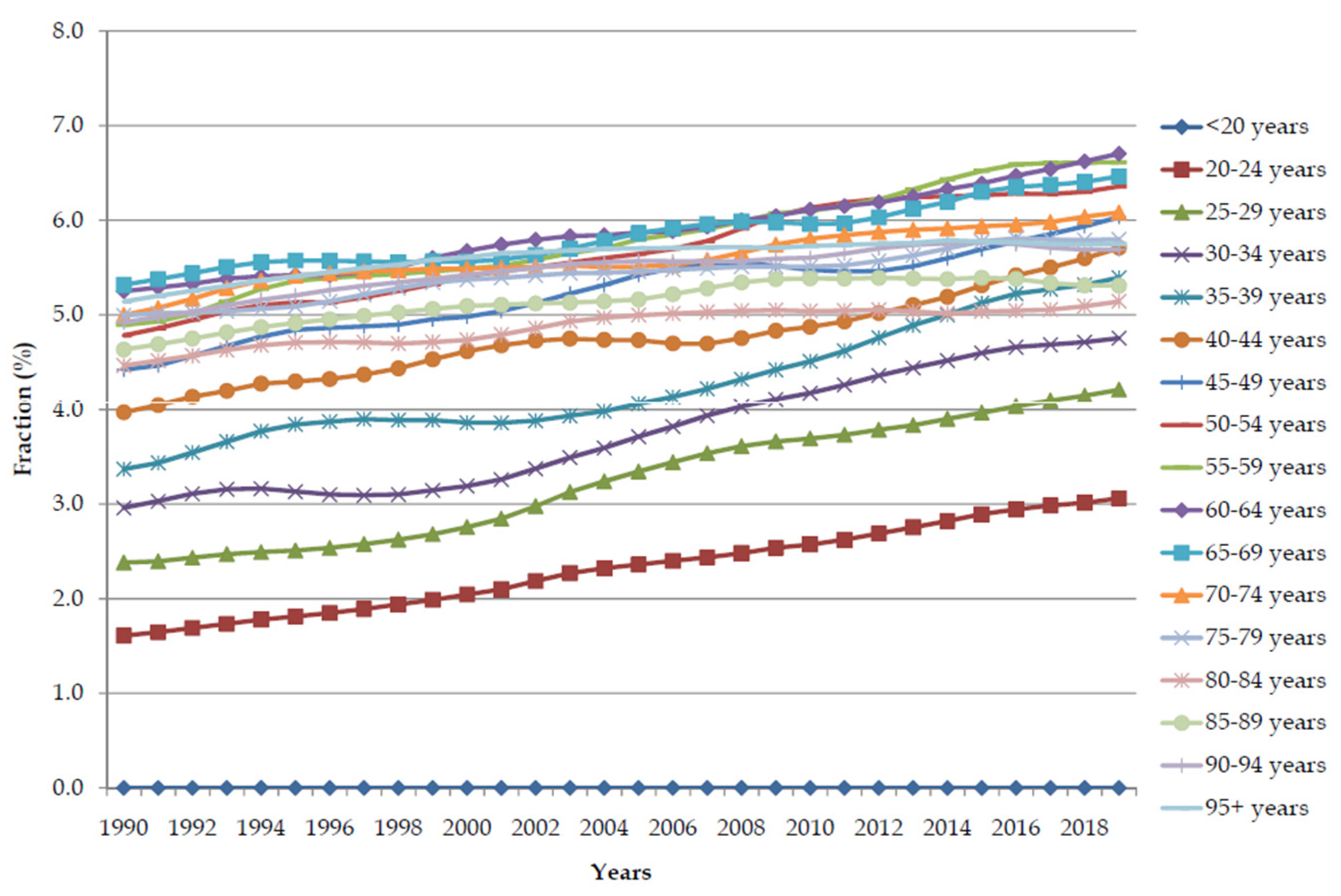
| Deaths in 1990 | ASR in 1990 | Deaths in 2019 | ASR in 2019 | AAPC (ASR) (95%CI) | p Value | |
|---|---|---|---|---|---|---|
| Global | ||||||
| Male | 3841 | 0.22 | 13,598 | 0.36 | 1.7 * (1.7 to 1.8) | <0.001 |
| Female | 5851 | 0.28 | 18,323 | 0.42 | 1.3 * (1.3 to 1.3) | <0.001 |
| Both sexes | 9693 | 0.26 | 31,921 | 0.40 | 1.4 * (1.4 to 1.5) | <0.001 |
| ― SDI regions | ||||||
| High SDI | 4945 | 0.47 | 12,901 | 0.67 | 1.3 * (1.2 to 1.3) | <0.001 |
| High–middle SDI | 3635 | 0.35 | 10,576 | 0.52 | 1.3 * (1.2 to 1.4) | <0.001 |
| Middle SDI | 834 | 0.09 | 5974 | 0.24 | 3.7 * (3.6 to 3.8) | <0.001 |
| Low–middle SDI | 207 | 0.04 | 2005 | 0.15 | 5.0 * (4.9 to 5.1) | <0.001 |
| Low SDI | 69 | 0.03 | 449 | 0.09 | 4.1 * (3.9 to 4.2) | <0.001 |
| ― GBD regions | ||||||
| Andean Latin America | 21 | 0.10 | 239 | 0.43 | 5.3 * (4.6 to 6.0) | <0.001 |
| Australasia | 110 | 0.47 | 347 | 0.69 | 1.4 * (1.3 to 1.4) | <0.001 |
| Caribbean | 22 | 0.09 | 201 | 0.39 | 5.1 * (4.3 to 5.9) | <0.001 |
| Central Asia | 68 | 0.15 | 334 | 0.48 | 5.0 * (4.5 to 5.4) | <0.001 |
| Central Europe | 919 | 0.62 | 2083 | 0.97 | 1.6 * (1.5 to 1.7) | <0.001 |
| Central Latin America | 219 | 0.27 | 995 | 0.43 | 1.3 * (1.2 to 1.5) | <0.001 |
| Central Sub-Saharan Africa | 13 | 0.06 | 51 | 0.10 | 1.4 * (0.8 to 2.0) | <0.001 |
| East Asia | 484 | 0.06 | 4402 | 0.21 | 4.9 * (4.7 to 5.2) | <0.001 |
| Eastern Europe | 1365 | 0.48 | 2593 | 0.75 | 1.3 * (1.1 to 1.6) | <0.001 |
| Eastern Sub-Saharan Africa | 26 | 0.04 | 181 | 0.11 | 4.4 * (4.1 to 4.6) | <0.001 |
| High-income Asia-Pacific | 452 | 0.23 | 1315 | 0.28 | 0.6 * (0.5 to 0.7) | <0.001 |
| High-income North America | 2126 | 0.60 | 5814 | 0.91 | 1.5 * (1.4 to 1.6) | <0.001 |
| North Africa and Middle East | 277 | 0.17 | 2006 | 0.48 | 3.8 * (3.6 to 4.0) | <0.001 |
| Oceania | 2 | 0.08 | 9 | 0.14 | 1.6 * (1.3 to 1.9) | <0.001 |
| South Asia | 103 | 0.02 | 1393 | 0.10 | 6.0 * (5.8 to 6.2) | <0.001 |
| Southeast Asia | 98 | 0.04 | 990 | 0.16 | 5.2 * (5.1 to 5.2) | <0.001 |
| Southern Latin America | 204 | 0.45 | 671 | 0.80 | 1.8 * (1.5 to 2.1) | <0.001 |
| Southern Sub-Saharan Africa | 79 | 0.30 | 300 | 0.57 | 2.2 * (1.9 to 2.4) | <0.001 |
| Tropical Latin America | 233 | 0.27 | 1197 | 0.50 | 2.3 * (2.3 to 2.4) | <0.001 |
| Western Europe | 2814 | 0.48 | 6336 | 0.67 | 1.2 * (1.1 to 1.3) | <0.001 |
| Western Sub-Saharan Africa | 55 | 0.06 | 465 | 0.26 | 5.0 * (4.9 to 5.0) | <0.001 |
| DALYs (Number) in 1990 | ASR in 1990 | DALYs (Number) in 2019 | ASR in 2019 | AAPC (ASR) (95%CI) | p Value | |
|---|---|---|---|---|---|---|
| Global | ||||||
| Male | 98,571 | 5.12 | 329,455 | 8.27 | 1.7 * (1.6 to 1.7) | <0.001 |
| Female | 125,683 | 5.90 | 379,994 | 8.69 | 1.3 * (1.3 to 1.3) | <0.001 |
| Both sexes | 224,255 | 5.60 | 709,449 | 8.54 | 1.4 * (1.4 to 1.5) | <0.001 |
| ― SDI regions | ||||||
| High SDI | 106,212 | 10.37 | 256,852 | 14.62 | 1.2 * (1.2 to 1.3) | <0.001 |
| High–middle SDI | 87,986 | 8.01 | 238,575 | 11.64 | 1.2 * (1.1 to 1.3) | <0.001 |
| Middle SDI | 22,529 | 2.04 | 150,391 | 5.74 | 3.7 * (3.6 to 3.7) | <0.001 |
| Low–middle SDI | 5555 | 0.87 | 51,063 | 3.57 | 5.1 * (5.0 to 5.1) | <0.001 |
| Low SDI | 1892 | 0.74 | 12,202 | 2.18 | 4.0 * (3.9 to 4.2) | <0.001 |
| ― GBD regions | ||||||
| Andean Latin America | 552 | 2.57 | 5611 | 9.88 | 5.0 * (4.3 to 5.6) | <0.001 |
| Australasia | 2420 | 10.39 | 6860 | 14.67 | 1.3 * (1.2 to 1.4) | <0.001 |
| Caribbean | 540 | 2.05 | 4625 | 8.90 | 5.1 * (4.3 to 5.9) | <0.001 |
| Central Asia | 1774 | 3.65 | 8790 | 11.11 | 4.8 * (4.3 to 5.3) | <0.001 |
| Central Europe | 22,298 | 14.87 | 45,286 | 22.20 | 1.4 * (1.3 to 1.5) | <0.001 |
| Central Latin America | 5659 | 6.44 | 23,811 | 9.88 | 1.2 * (1.1 to 1.4) | <0.001 |
| Central Sub-Saharan Africa | 360 | 1.42 | 1455 | 2.44 | 1.4 * (0.8 to 2.0) | <0.001 |
| East Asia | 13,477 | 1.41 | 110,235 | 5.11 | 4.8 * (5.6 to 5.0) | <0.001 |
| Eastern Europe | 34,253 | 12.00 | 60,662 | 18.00 | 1.1 * (0.8 to 1.4) | <0.001 |
| Eastern Sub-Saharan Africa | 733 | 0.89 | 5041 | 2.80 | 4.3 * (4.1 to 4.6) | <0.001 |
| High-income Asia-Pacific | 10,336 | 5.02 | 23,154 | 5.70 | 0.4 * (0.3 to 0.4) | <0.001 |
| High-income North America | 46,249 | 13.74 | 120,686 | 20.03 | 1.3 * (1.2 to 1.4) | <0.001 |
| North Africa and Middle East | 7527 | 4.06 | 52,345 | 11.26 | 3.6 * (3.4 to 3.8) | <0.001 |
| Oceania | 70 | 2.07 | 267 | 3.35 | 1.4 * (1.1 to 1.8) | <0.001 |
| South Asia | 2824 | 0.46 | 35,432 | 2.41 | 6.0 * (5.9 to 6.2) | <0.001 |
| Southeast Asia | 2855 | 0.99 | 26,486 | 4.02 | 4.9 * (4.8 to 5.0) | <0.001 |
| Southern Latin America | 106,212 | 9.98 | 14,078 | 17.12 | 1.7 * (1.4 to 2.0) | <0.001 |
| Southern Sub-Saharan Africa | 87,986 | 7.10 | 7318 | 12.58 | 2.0 * (1.7 to 2.2) | <0.001 |
| Tropical Latin America | 22,529 | 6.21 | 27,596 | 11.18 | 2.2 * (2.1 to 2.3) | <0.001 |
| Western Europe | 5555 | 10.42 | 117,439 | 14.10 | 1.1 * (1.0 to 1.2) | <0.001 |
| Western Sub-Saharan Africa | 1892 | 1.55 | 12,271 | 6.12 | 4.8 * (4.7 to 4.9) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilic, I.; Ilic, M. Global Burden of Pancreatic Cancer Attributable to High Body-Mass Index in 204 Countries and Territories, 1990–2019. Cancers 2024, 16, 719. https://doi.org/10.3390/cancers16040719
Ilic I, Ilic M. Global Burden of Pancreatic Cancer Attributable to High Body-Mass Index in 204 Countries and Territories, 1990–2019. Cancers. 2024; 16(4):719. https://doi.org/10.3390/cancers16040719
Chicago/Turabian StyleIlic, Irena, and Milena Ilic. 2024. "Global Burden of Pancreatic Cancer Attributable to High Body-Mass Index in 204 Countries and Territories, 1990–2019" Cancers 16, no. 4: 719. https://doi.org/10.3390/cancers16040719
APA StyleIlic, I., & Ilic, M. (2024). Global Burden of Pancreatic Cancer Attributable to High Body-Mass Index in 204 Countries and Territories, 1990–2019. Cancers, 16(4), 719. https://doi.org/10.3390/cancers16040719






