Oligometastases of Esophageal Squamous Cell Carcinoma: A Review
Abstract
Simple Summary
Abstract
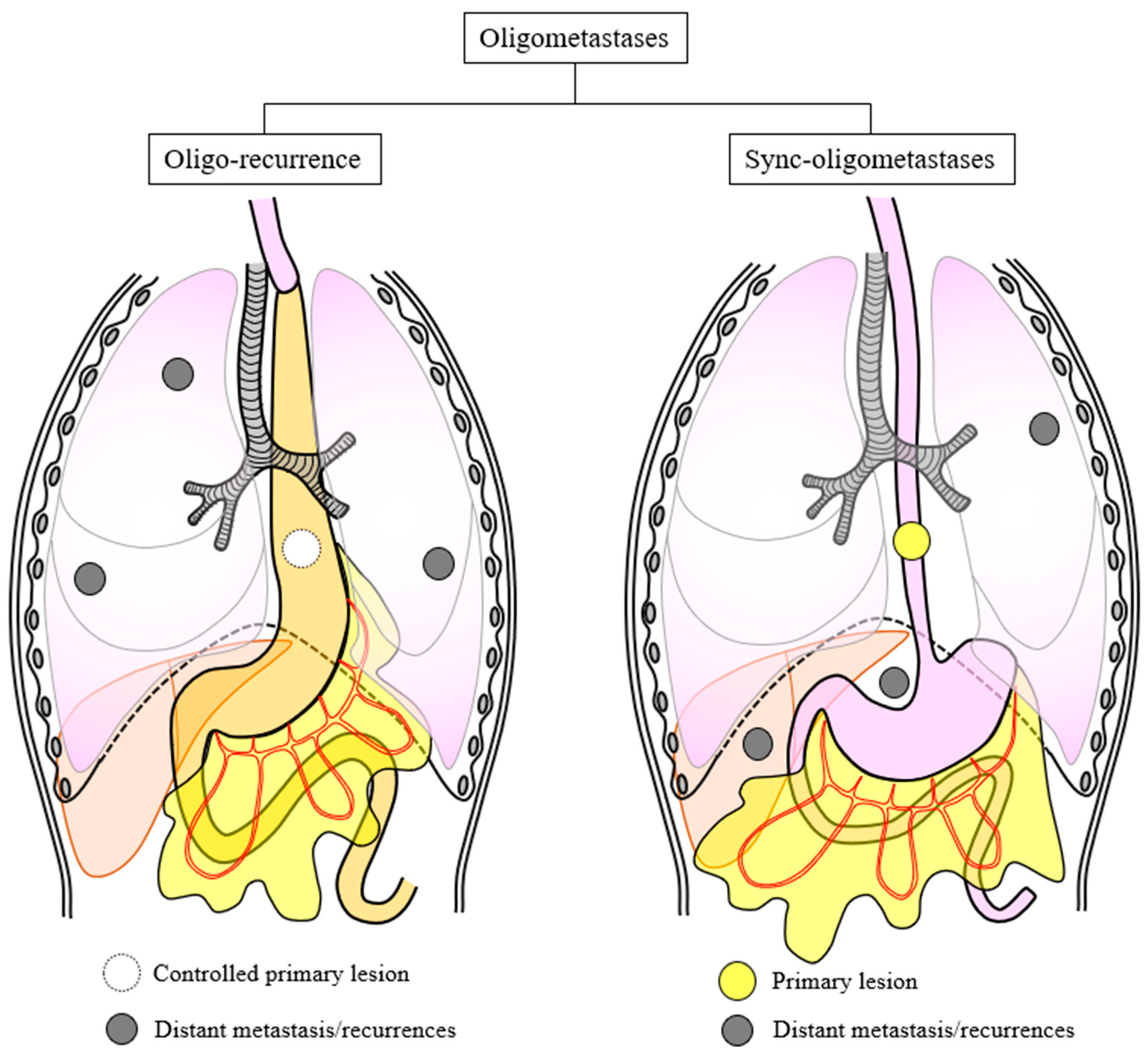
1. Introduction
2. Materials and Methods
3. Results
3.1. Surgical Resection for Oligometastatic Esophageal Squamous Cell Carcinoma
3.2. Radiation or Chemoradiation for Oligometastatic Esophageal Squamous Cell Carcinoma
3.3. Treatment Strategy for Sync-Oligometastases of Esophageal Squamous Cell Carcinoma
3.4. Phase II Non-Randomized Trials for Oligometastatic Esophageal Squamous Cell Carcinoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef]
- Lagergren, J.; Smyth, E.; Cunningham, D.; Lagergren, P. Oesophageal cancer. Lancet 2017, 390, 2383–2396. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan esophageal society: Part 1. Esophagus 2023, 20, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, Y.; Ishihara, R.; Ishikawa, H.; Ito, Y.; Oyama, T.; Oyama, T.; Kato, K.; Kato, H.; Kawakubo, H.; Kawachi, H.; et al. Esophageal cancer practice guidelines 2022 edited by the Japan Esophageal Society: Part 2. Esophagus 2023, 20, 373–389. [Google Scholar] [CrossRef]
- Nakamura, K.; Kato, K.; Igaki, H.; Ito, Y.; Mizusawa, J.; Ando, N.; Udagawa, H.; Tsubosa, Y.; Daiko, H.; Hironaka, S.; et al. Japan Esophageal Oncology Group/Japan Clinical Oncology Group. Three-arm phase III trial comparing cisplatin plus 5-FU (CF) versus docetaxel, cisplatin plus 5-FU (DCF) versus radiotherapy with CF (CF-RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn. J. Clin. Oncol. 2013, 43, 752–755. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Hoeppner, J.; Lordick, F.; Brunner, T.; Glatz, T.; Bronsert, P.; Röthling, N.; Schmoor, C.; Lorenz, D.; Ell, C.; Hopt, U.T.; et al. ESOPEC: Prospective randomized controlled multicenter phase III trial comparing perioperative chemotherapy (FLOT protocol) to neoadjuvant chemoradiation (CROSS protocol) in patients with adenocarcinoma of the esophagus (NCT02509286). BMC Cancer 2016, 16, 503. [Google Scholar] [CrossRef]
- Reynolds, J.V.; Preston, S.R.; O’Neill, B.; Lowery, M.A.; Baeksgaard, L.; Crosby, T.; Cunningham, M.; Cuffe, S.; Griffiths, G.O.; Parker, I.; et al. Neo-AEGIS Investigators and Trial Group. Trimodality therapy versus perioperative chemotherapy in the management of locally advanced adenocarcinoma of the oesophagus and oesophagogastric junction (Neo-AEGIS): An open-label, randomised, phase 3 trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Kato, H.; Igaki, H.; Shinoda, M.; Ozawa, S.; Shimizu, H.; Nakamura, T.; Yabusaki, H.; Aoyama, N.; Kurita, A.; et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann. Surg. Oncol. 2012, 19, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, M.H.; Harker, M.; van de Water, C.; van Dieren, J.; Hugen, N.; Nagtegaal, I.D.; Rosman, C.; van der Post, R.S. Metastatic pattern in esophageal and gastric cancer: Influenced by site and histology. World J. Gastroenterol. 2020, 21, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- Tachimori, Y.; Ozawa, S.; Numasaki, H.; Ishihara, R.; Matsubara, H.; Muro, K.; Oyama, T.; Toh, Y.; Udagawa, H.; Uno, T. Registration Committee for Esophageal Cancer of the Japan Esophageal Society. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus 2019, 16, 221–245. [Google Scholar] [CrossRef] [PubMed]
- Hellman, S.; Weichselbaum, R.R. Oligometastases. J. Clin. Oncol. 1995, 13, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Lievens, Y.; Guckenberger, M.; Gomez, D.; Hoyer, M.; Iyengar, P.; Kindts, I.; Méndez Romero, A.; Nevens, D.; Palma, D.; Park, C.; et al. Defining oligometastatic disease from a radiation oncology perspective: An ESTRO-ASTRO consensus document. Radiother. Oncol. 2020, 148, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Guckenberger, M.; Lievens, Y.; Bouma, A.B.; Collette, L.; Dekker, A.; deSouza, N.M.; Dingemans, A.C.; Fournier, B.; Hurkmans, C.; Lecouvet, F.E.; et al. Characterisation and classification of oligometastatic disease: A European Society for Radiotherapy and Oncology and European Organisation for Research and Treatment of Cancer consensus recommendation. Lancet Oncol. 2020, 21, e18–e28. [Google Scholar] [CrossRef] [PubMed]
- Niibe, Y.; Hayakawa, K. Oligometastases and oligo-recurrence: The new era of cancer therapy. Jpn. J. Clin. Oncol. 2010, 40, 107–111. [Google Scholar] [CrossRef]
- Niibe, Y.; Chang, J.Y. Novel insights of oligometastases and oligo-recurrence and review of the literature. Pulm. Med. 2012, 2012, 261096. [Google Scholar] [CrossRef]
- Niibe, Y.; Nishimura, T.; Inoue, T.; Karasawa, K.; Shioyama, Y.; Jingu, K.; Shirato, H. Oligo-recurrence predicts favorable prognosis of brain-only oligometastases in patients with non-small cell lung cancer treated with stereotactic radiosurgery or stereotactic radiotherapy: A multi-institutional study of 61 subjects. BMC Cancer 2016, 16, 659. [Google Scholar] [CrossRef]
- Niibe, Y.; Yamamoto, T.; Onishi, H.; Yamashita, H.; Katsui, K.; Matsumoto, Y.; Oh, R.J.; Aoki, M.; Shintani, T.; Yamada, K.; et al. Pulmonary oligometastases treated by stereotactic body radiation therapy: A nationwide survey of 1378 patients. Anticancer Res. 2020, 40, 393–399. [Google Scholar] [CrossRef]
- Yamashita, H.; Jingu, K.; Niibe, Y.; Katsui, K.; Matsumoto, T.; Nishina, T.; Terahara, A. Definitive salvage radiation therapy and chemoradiation therapy for lymph node oligo-recurrence of esophageal cancer: A Japanese multi-institutional study of 237 patients. Radiat. Oncol. 2017, 12, 38. [Google Scholar] [CrossRef]
- Hamai, Y.; Hihara, J.; Emi, M.; Furukawa, T.; Ibuki, Y.; Yamakita, I.; Kurokawa, T.; Okada, M. Treatment Outcomes and Prognostic Factors After Recurrence of Esophageal Squamous Cell carcinoma. World J. Surg. 2018, 42, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, X.; Song, H.; Wu, A.J.; Ku, G.Y.; Lee, P.; Slingerland, M.; Koyanagi, K.; Ke, S.; Qiu, H.; et al. Written on behalf of AME Radiation Oncology Collaborative Group. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for esophageal squamous cell cancer patients presenting with oligometastases. J. Thorac. Dis. 2019, 11, 1536–1545. [Google Scholar] [CrossRef]
- Lin, C.Y.; Fang, H.Y.; Lein, M.Y.; Lin, C.C.; Bai, L.Y.; Tsai, M.H.; Chen, C.C.; Hsieh, T.C.; Wang, Y.C.; Liang, J.A.; et al. Clinical outcomes and prognostic factors of patients with esophageal squamous cell carcinoma with oligo-recurrence treated with radical re-irradiation. Anticancer Res. 2020, 40, 2387–2392. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, Z.; Chen, Y.; Deng, J.; Ai, D.; Liu, Q.; Wang, S.; Wu, S.; Chen, J.; Zhao, K. Phase 2 study of stereotactic body radiation therapy for patients with oligometastatic esophageal squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, 707–715. [Google Scholar] [CrossRef]
- Morinaga, T.; Iwatsuki, M.; Yamashita, K.; Harada, K.; Kurashige, J.; Nagai, Y.; Iwagami, S.; Baba, Y.; Yoshida, N.; Baba, H. Oligometastatic recurrence as a prognostic factor after curative resection of esophageal squamous cell carcinoma. Surg. Today 2021, 51, 798–806. [Google Scholar] [CrossRef]
- Yamashita, H.; Ogita, M.; Aoki, S.; Abe, O.; Nakagawa, K. Linear accelerator-based stereotactic body radiation therapy in the treatment of oligometastatic disease. Mol Clin Oncol. 2020, 13, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wen, Y.; Xiang, Z.; Du, H.; Geng, L.; Yang, X.; Zhang, Y.; Bai, J.; Dai, T.; Feng, G.; et al. Radical radiotherapy for metachronous oligometastasis after initial treatment of esophageal cancer. Radiother. Oncol. 2021, 154, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, Y.; Shindoh, J.; Ueno, M.; Iizuka, T.; Udagawa, H. clinicopathologic characteristics of oligometastases from esophageal cancer and long-term outcomes of resection. Ann. Surg. Oncol. 2020, 27, 651–659. [Google Scholar] [CrossRef]
- Li, B.; Li, W.; Fan, B.; Zou, B.; Jiang, C.; Sun, X.; Yu, J.; Wang, L. Efficacy of radiotherapy in oligometastatic esophageal squamous cell cancer patients: New evidence from a retrospective study. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e611. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, J.; Li, B.; Ye, J.; Wei, S.; Wang, Y.; Yang, H.; Zhu, Z.; Lai, S.; Li, L.; et al. Local therapy for oligometastatic esophageal squamous cell carcinoma: A prospective, randomized, Phase II clinical trial. Future Oncol. 2021, 17, 1285–1293. [Google Scholar] [CrossRef]
- Shi, Z.; Zhu, X.; Ruan, C.; Wei, G.; Li, J.; Qiu, H.; Gao, L.; Cai, G.; Zhangcai, Y.; Li, B.; et al. Evaluation of concurrent chemoradiotherapy for survival outcomes in patients with synchronous oligometastatic esophageal squamous cell carcinoma. JAMA Netw. Open 2022, 5, e2244619. [Google Scholar] [CrossRef]
- Tsai, P.C.; Chien, H.C.; Hsu, P.K.; Hung, J.J.; Huang, C.S.; Hsu, W.H.; Hsu, H.S. Post-recurrence survival analysis in patients with oligo-recurrence after curative esophagectomy. BMC Cancer 2022, 22, 637. [Google Scholar] [CrossRef]
- Zhao, W.; Ke, S.; Cai, X.; Zuo, Z.; Shi, W.; Qiu, H.; Cai, G.; Gong, Y.; Wu, Y.; Ruan, S.; et al. Radiotherapy plus camrelizumab and irinotecan for oligometastatic esophageal squamous cell carcinoma patients after first-line immunotherapy plus chemotherapy failure: An open-label, single-arm, phase II trial. Radiother. Oncol. 2023, 184, 109679. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Niibe, Y.; Yamamoto, T.; Katsui, K.; Jingu, K.; Kanazawa, S.; Terahara, A.; Nakagawa, K. Lung stereotactic radiotherapy for oligometastases: Comparison of oligo-recurrence and sync-oligometastases. Jpn. J. Clin. Oncol. 2016, 46, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Kudou, K.; Saeki, H.; Nakashima, Y.; Kimura, Y.; Oki, E.; Mori, M.; Shimokawa, M.; Kakeji, Y.; Toh, Y.; Doki, Y.; et al. Clinical outcomes of surgical resection for recurrent lesion after curative esophagectomy for esophageal squamous cell carcinoma: A nationwide, large-scale retrospective study. Esophagus 2022, 19, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Mine, S.; Yamada, K.; Shigaki, H.; Baba, Y.; Yoshida, N.; Kajiyama, K.; Yamamoto, N.; Sano, T.; Baba, H. Outcomes of lymphadenectomy for lymph node recurrence after esophagectomy or definitive chemoradiotherapy for squamous cell carcinoma of the esophagus. Gen. Thorac. Cardiovasc. Surg. 2014, 62, 685–692. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, K.; Guo, W.; Yang, S.; Zhu, X.; Xiang, J.; Zhang, Y.; Li, H. Salvage lymphadenectomy versus salvage radiotherapy/chemoradiotherapy for recurrence in cervical lymph node after curative resection of esophageal squamous cell carcinoma. Ann. Surg. Oncol. 2015, 22, 624–629. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, S.; Wang, F.; Liu, S. Salvage lymphadenectomy for isolated cervical lymph node recurrence after curative resection of thoracic esophageal squamous cell carcinoma. Ann. Transl. Med. 2019, 7, 238. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517, Erratum in Lancet Oncol. 2019, 20, e613. [Google Scholar] [CrossRef]
- Huang, J.; Xu, J.; Chen, Y.; Zhuang, W.; Zhang, Y.; Chen, Z.; Chen, J.; Zhang, H.; Niu, Z.; Fan, Q.; et al. ESCORT Study Group. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2020, 21, 832–842. [Google Scholar] [CrossRef]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.H.; Doi, T.; Moriwaki, T.; Kim, S.B.; Lee, S.H.; et al. KEYNOTE-181 investigators. Randomized phase III KEYNOTE-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Sun, J.M.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.P.; Li, Z.; Kim, S.B.; et al. KEYNOTE-590 Investigators. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771, Erratum in Lancet 2021, 398, 1874. [Google Scholar] [CrossRef]
- Kato, K.; Shah, M.A.; Enzinger, P.; Bennouna, J.; Shen, L.; Adenis, A.; Sun, J.M.; Cho, B.C.; Özgüroğlu, M.; Kojima, T.; et al. KEYNOTE-590: Phase III study of first-line chemotherapy with or without pembrolizumab for advanced esophageal cancer. Future Oncol. 2019, 15, 1057–1066. [Google Scholar] [CrossRef]
- Kojima, T.; Hara, H.; Tsuji, A.; Yasui, H.; Muro, K.; Satoh, T.; Ogata, T.; Ishihara, R.; Goto, M.; Baba, H.; et al. First-line pembrolizumab + chemotherapy in Japanese patients with advanced/metastatic esophageal cancer from KEYNOTE-590. Esophagus 2022, 19, 683–692. [Google Scholar] [CrossRef]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.H.; Adenis, A.; et al. CheckMate 648 Trial Investigators. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Doki, Y.; Ogata, T.; Motoyama, S.; Kawakami, H.; Ueno, M.; Kojima, T.; Shirakawa, Y.; Okada, M.; Ishihara, R.; et al. First-line nivolumab plus ipilimumab or chemotherapy versus chemotherapy alone in advanced esophageal squamous cell carcinoma: A Japanese subgroup analysis of open-label, phase 3 trial (CheckMate 648/ONO-4538-50). Esophagus 2023, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kadono, T.; Yamamoto, S.; Kato, K. Current perspectives of the Japanese Esophageal Oncology Group on the development of immunotherapy for esophageal cancer. Jpn. J. Clin. Oncol. 2022, 52, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Kato, K.; Daiko, H.; Kojima, T.; Hara, H.; Abe, T.; Tsubosa, Y.; Nagashima, K.; Aoki, K.; Mizoguchi, Y.; et al. Feasibility study of nivolumab as neoadjuvant chemotherapy for locally esophageal carcinoma: FRONTiER (JCOG1804E). Future Oncol. 2020, 16, 1351–1357. [Google Scholar] [CrossRef] [PubMed]
- Dagoglu, N.; Karaman, S.; Caglar, H.B.; Oral, E.N. Abscopal effect of radiotherapy in the immunotherapy era: Systematic review of reported cases. Cureus 2019, 11, e4103. [Google Scholar] [CrossRef] [PubMed]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef]
- Biswas, R.; Jindel, R.; Halder, A.; Sen, K.; Kabasi, A. Abscopal effect of radiation in metastatic esophageal carcinoma: Fourth reported case. Int. Cancer Conf. J. 2023, 12, 200–204. [Google Scholar] [CrossRef]
- Matsuda, S.; Tsushima, T.; Kato, K.; Hsu, C.H.; Lee, J.M.; Wong, I.Y.; Wang, H.C.; Kang, C.H.; Guo, X.; Yamamoto, S.; et al. Defining conversion therapy for esophageal squamous cell carcinoma. Ann. Gastroenterol. Surg. 2022, 7, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kato, K.; Hamamoto, Y.; Tsubosa, Y.; Ogawa, H.; Ito, Y.; Hara, H.; Ura, T.; Kojima, T.; Chin, K.; et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br. J. Cancer 2016, 115, 1328–1334. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Kato, K.; Hamamoto, Y.; Tsubosa, Y.; Ogawa, H.; Ito, Y.; Hara, H.; Ura, T.; Kojima, T.; Chin, K.; et al. A 3-year overall survival update from a phase 2 study of chemoselection with DCF and subsequent conversion surgery for locally advanced unresectable esophageal cancer. Ann. Surg. Oncol. 2020, 27, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, M.; Kawakubo, H.; Mayanagi, S.; Yoshida, K.; Irino, T.; Fukuda, K.; Nakamura, R.; Wada, N.; Takeuchi, H.; Kitagawa, Y. The benefits of docetaxel plus cisplatin and 5-fluorouracil induction therapy in conversion to curative treatment for locally advanced esophageal squamous cell carcinoma. World J. Surg. 2019, 43, 2006–2015. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Yasuda, T.; Kato, H.; Nozaki, I.; Sato, H.; Miyata, Y.; Kuroki, Y.; Kato, K.; Hamamoto, Y.; Tsubosa, Y.; et al. Concordance of clinical diagnosis of T classification among physicians for locally advanced unresectable thoracic esophageal cancer. Int. J. Clin. Oncol. 2018, 23, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Terada, M.; Hara, H.; Daiko, H.; Mizusawa, J.; Kadota, T.; Hori, K.; Ogawa, H.; Ogata, T.; Sakanaka, K.; Sakamoto, T.; et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn. J. Clin. Oncol. 2019, 49, 1055–1060. [Google Scholar] [CrossRef]
- Higuchi, T.; Shoji, Y.; Koyanagi, K.; Tajima, K.; Kanamori, K.; Ogimi, M.; Yatabe, K.; Ninomiya, Y.; Yamamoto, M.; Kazuno, A.; et al. Multimodal treatment strategies to improve the prognosis of locally advanced thoracic esophageal squamous cell carcinoma: A narrative review. Cancers 2022, 15, 10. [Google Scholar] [CrossRef]
- Bedenne, L.; Michel, P.; Bouché, O.; Milan, C.; Mariette, C.; Conroy, T.; Pezet, D.; Roullet, B.; Seitz, J.F.; Herr, J.P.; et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol. 2007, 25, 1160–1168. [Google Scholar] [CrossRef]
- Stahl, M.; Stuschke, M.; Lehmann, N.; Meyer, H.J.; Walz, M.K.; Seeber, S.; Klump, B.; Budach, W.; Teichmann, R.; Schmitt, M.; et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol. 2005, 23, 2310–2317. [Google Scholar] [CrossRef]
- Ng, H.Y.; Ko, J.M.Y.; Lam, K.O.; Kwong, D.L.W.; Lo, A.W.I.; Wong, I.Y.H.; Wong, C.L.Y.; Chan, S.Y.; Chan, K.K.; Law, T.T.; et al. Circulating tumor DNA dynamics as prognostic markers in locally advanced and metastatic esophageal squamous cell carcinoma. JAMA Surg. 2023, 158, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Kotani, D.; Oki, E.; Nakamura, Y.; Yukami, H.; Mishima, S.; Bando, H.; Shirasu, H.; Yamazaki, K.; Watanabe, J.; Kotaka, M.; et al. Molecular residual disease and efficacy of adjuvant chemotherapy in patients with colorectal cancer. Nat. Med. 2023, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
| Authors | Year | Country | Inclusion | Patients | Oligometastasis | Histology | Treatment | Key Results | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Type | Center | Period | Type | Definition | SCC | Other | ||||||
| Yamashita [20] | 2017 | Japan | RNR | Multi | 2000–2015 | 237 | OR | <5 lymph node | 231 | 6 | CRT vs. RT | 3 year OS: 36.7% vs. 20.8% (p = 0.00005) |
| Hamai [21] | 2018 | Japan | RNR | Single | 1990–2013 | 133 | OR | ns | 133 | 0 | Various | Median DFI: 9.1 m |
| Chen [22] | 2019 | China | RNR | Multi | 2012–2015 | 461 | SO | <3 metastases | 446 | 15 | CRT vs. RT | DCR: 81.6 vs. 64.5% (p < 0.001) |
| Lin [23] | 2020 | Taiwan | RNR | Multi | 2012–2018 | 30 | OR | <5 metastases | 30 | 0 | Re-irradiation | 5 year OS: 21% |
| Liu [24] | 2020 | China | P2 NR | Single | 2015–2018 | 34 | OR | <3 metastases | 34 | 0 | RT | Median PFS: 13.3 m |
| Morinaga [25] | 2020 | Japan | RNR | Single | 2005–2019 | 43 | OR | <5 metastases (single organ) | 43 | 0 | CLT vs. CT | 5 year OS: 39.2 vs. 14.4 m (p = 0.038) |
| Yamashita [26] | 2020 | Japan | RNR | Single | 2012–2017 | 18 | OR | <5 cm, <3 lesions | 18 | 0 | RT | OS: SCC < other cancers |
| Li [27] | 2020 | China | RNR | Single | ns | 239 | OR | <5 metastases <3 lesions | 239 | 0 | CRT vs. CT | OS: 21.3 vs. 12.7 m (p < 0.001) PFS: 9.5 vs. 3.8 m (p < 0.001) |
| Ohkura [28] | 2020 | Japan | RNR | Single | 2011–2017 | 119 | OR | <5 metastases (single domain) | ns | ns | SR vs. non | 3 year OS: 64.3% vs. 9.8% 5 year OS: 55.6% vs. 0% |
| Li [29] | 2021 | China | RNR | Single | 2009–2018 | 82 | OR | <5 metastases | 78 | 4 | RT vs. non | Median OS: 14 vs. 7 m (p = 0.0016) |
| Liu [30] | 2021 | China | P2 NR | Single | 2019–2022 | 102 | OR | <4 metastases <3 (single organ) | 102 | 0 | RT | On going |
| Shi [31] | 2022 | China | RNR | Multi | 2012–2018 | 532 | SO | <5 metastases | 532 | 0 | CRT vs. CT | OS: 18.5 m vs. 15.2 m (p < 0.001) PFS: 9.7 m vs. 7.6 m (p < 0.001) |
| Tsai [32] | 2022 | Taiwan | RNR | Single | 2004–2017 | 63 | OR | <5 metastases | 60 | 3 | SR vs. non | 3 year PRS rate: 42.9% vs. 23.5% |
| Zhao [33] | 2023 | China | P2 NR | Multi | 2018–2021 | 49 | SO | <5 metastases | 49 | 0 | RT + ICI | PFS 6.9 m (95% CI 4.6–9.3) OS 12.8 m (95% CI 10.1–15.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Tanaka, Y.; Yokoi, R.; Tsuchiya, H.; Sengoku, Y.; Fukada, M.; Yasufuku, I.; Asai, R.; Tajima, J.Y.; Kiyama, S.; et al. Oligometastases of Esophageal Squamous Cell Carcinoma: A Review. Cancers 2024, 16, 704. https://doi.org/10.3390/cancers16040704
Sato Y, Tanaka Y, Yokoi R, Tsuchiya H, Sengoku Y, Fukada M, Yasufuku I, Asai R, Tajima JY, Kiyama S, et al. Oligometastases of Esophageal Squamous Cell Carcinoma: A Review. Cancers. 2024; 16(4):704. https://doi.org/10.3390/cancers16040704
Chicago/Turabian StyleSato, Yuta, Yoshihiro Tanaka, Ryoma Yokoi, Hiroshi Tsuchiya, Yuki Sengoku, Masahiro Fukada, Itaru Yasufuku, Ryuichi Asai, Jesse Yu Tajima, Shigeru Kiyama, and et al. 2024. "Oligometastases of Esophageal Squamous Cell Carcinoma: A Review" Cancers 16, no. 4: 704. https://doi.org/10.3390/cancers16040704
APA StyleSato, Y., Tanaka, Y., Yokoi, R., Tsuchiya, H., Sengoku, Y., Fukada, M., Yasufuku, I., Asai, R., Tajima, J. Y., Kiyama, S., Kato, T., Murase, K., & Matsuhashi, N. (2024). Oligometastases of Esophageal Squamous Cell Carcinoma: A Review. Cancers, 16(4), 704. https://doi.org/10.3390/cancers16040704




