Real-Life Use of [68Ga]Ga-DOTANOC PET/CT in Confirmed and Suspected NETs from a Prospective 5-Year Electronic Archive at an ENETS Center of Excellence: More Than 2000 Scans in More Than 1500 Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Whole Population
3.1.1. Pathologically Confirmed NETs
3.1.2. Suspected NETs
3.1.3. Subgroup Analysis of Scans (n = 851) Performed in Confirmed NETs, Requested by the Local Oncology Ward
3.1.4. Subgroup Analysis of Scans in Suspected NETs (n = 59), Requested by the Local Oncology Ward
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graf, J.; Pape, U.-F.; Jann, H.; Denecke, T.; Arsenic, R.; Brenner, W.; Pavel, M.; Prasad, V. Prognostic Significance of Somatostatin Receptor Heterogeneity in Progressive Neuroendocrine Tumor Treated with Lu-177 DOTATOC or Lu-177 DOTATATE. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 881–894. [Google Scholar] [CrossRef] [PubMed]
- Binderup, T.; Knigge, U.; Loft, A.; Federspiel, B.; Kjaer, A. 18F-Fluorodeoxyglucose Positron Emission Tomography Predicts Survival of Patients with Neuroendocrine Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2010, 16, 978–985. [Google Scholar] [CrossRef]
- Ezziddin, S.; Attassi, M.; Yong-Hing, C.J.; Ahmadzadehfar, H.; Willinek, W.; Grünwald, F.; Guhlke, S.; Biersack, H.-J.; Sabet, A. Predictors of Long-Term Outcome in Patients with Well-Differentiated Gastroenteropancreatic Neuroendocrine Tumors after Peptide Receptor Radionuclide Therapy with 177Lu-Octreotate. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2014, 55, 183–190. [Google Scholar] [CrossRef]
- Rindi, G.; Mete, O.; Uccella, S.; Basturk, O.; La Rosa, S.; Brosens, L.A.A.; Ezzat, S.; de Herder, W.W.; Klimstra, D.S.; Papotti, M.; et al. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr. Pathol. 2022, 33, 115–154. [Google Scholar] [CrossRef]
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Singh, S.; Poon, R.; Wong, R.; Metser, U. 68Ga PET Imaging in Patients With Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. Clin. Nucl. Med. 2018, 43, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Treglia, G.; Castaldi, P.; Rindi, G.; Giordano, A.; Rufini, V. Diagnostic Performance of Gallium-68 Somatostatin Receptor PET and PET/CT in Patients with Thoracic and Gastroenteropancreatic Neuroendocrine Tumours: A Meta-Analysis. Endocrine 2012, 42, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Geijer, H.; Breimer, L.H. Somatostatin Receptor PET/CT in Neuroendocrine Tumours: Update on Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1770–1780. [Google Scholar] [CrossRef]
- Hope, T.A.; Allen-Auerbach, M.; Bodei, L.; Calais, J.; Dahlbom, M.; Dunnwald, L.K.; Graham, M.M.; Jacene, H.A.; Heath, C.L.; Mittra, E.S.; et al. SNMMI Procedure Standard/EANM Practice Guideline for SSTR PET: Imaging Neuroendocrine Tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2023, 64, 204–210. [Google Scholar] [CrossRef]
- Bozkurt, M.F.; Virgolini, I.; Balogova, S.; Beheshti, M.; Rubello, D.; Decristoforo, C.; Ambrosini, V.; Kjaer, A.; Delgado-Bolton, R.; Kunikowska, J.; et al. Guideline for PET/CT Imaging of Neuroendocrine Neoplasms with 68Ga-DOTA-Conjugated Somatostatin Receptor Targeting Peptides and 18F–DOPA. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1588–1601. [Google Scholar] [CrossRef]
- Treglia, G.; Sadeghi, R.; Giovinazzo, F.; Galiandro, F.; Annunziata, S.; Muoio, B.; Kroiss, A.S. PET with Different Radiopharmaceuticals in Neuroendocrine Neoplasms: An Umbrella Review of Published Meta-Analyses. Cancers 2021, 13, 5172. [Google Scholar] [CrossRef]
- Han, S.; Suh, C.H.; Woo, S.; Kim, Y.J.; Lee, J.J. Performance of (68)Ga-DOTA-Conjugated Somatostatin Receptor-Targeting Peptide PET in Detection of Pheochromocytoma and Paraganglioma: A Systematic Review and Metaanalysis. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2019, 60, 369–376. [Google Scholar] [CrossRef]
- Hennrich, U.; Kopka, K. Lutathera®: The First FDA- and EMA-Approved Radiopharmaceutical for Peptide Receptor Radionuclide Therapy. Pharm. Basel Switz. 2019, 12, 114. [Google Scholar] [CrossRef]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The Joint IAEA, EANM, and SNMMI Practical Guidance on Peptide Receptor Radionuclide Therapy (PRRNT) in Neuroendocrine Tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Hope, T.A. Updates to the Appropriate-Use Criteria for Somatostatin Receptor PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 1764. [Google Scholar] [CrossRef]
- Hope, T.A.; Bergsland, E.K.; Bozkurt, M.F.; Graham, M.; Heaney, A.P.; Herrmann, K.; Howe, J.R.; Kulke, M.H.; Kunz, P.L.; Mailman, J.; et al. Appropriate Use Criteria for Somatostatin Receptor PET Imaging in Neuroendocrine Tumors. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 66–74. [Google Scholar] [CrossRef]
- Zhernosekov, K.P.; Filosofov, D.V.; Baum, R.P.; Aschoff, P.; Bihl, H.; Razbash, A.A.; Jahn, M.; Jennewein, M.; Rosch, F. Processing of Generator-Produced 68Ga for Medical Application. J. Nucl. Med. 2007, 48, 1741–1748. [Google Scholar] [CrossRef]
- Elsinga, P.; Todde, S.; Penuelas, I.; Meyer, G.; Farstad, B.; Faivre-Chauvet, A.; Mikolajczak, R.; Westera, G.; Gmeiner-Stopar, T.; Decristoforo, C.; et al. Guidance on Current Good Radiopharmacy Practice (cGRPP) for the Small-Scale Preparation of Radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1049–1062. [Google Scholar] [CrossRef]
- Pathak, S.; Starr, J.S.; Halfdanarson, T.; Sonbol, M.B. Understanding the Increasing Incidence of Neuroendocrine Tumors. Expert Rev. Endocrinol. Metab. 2023, 18, 377–385. [Google Scholar] [CrossRef]
- Ambrosini, V.; Zanoni, L.; Filice, A.; Lamberti, G.; Argalia, G.; Fortunati, E.; Campana, D.; Versari, A.; Fanti, S. Radiolabeled Somatostatin Analogues for Diagnosis and Treatment of Neuroendocrine Tumors. Cancers 2022, 14, 1055. [Google Scholar] [CrossRef]
- Clift, A.K.; Kidd, M.; Bodei, L.; Toumpanakis, C.; Baum, R.P.; Oberg, K.; Modlin, I.M.; Frilling, A. Neuroendocrine Neoplasms of the Small Bowel and Pancreas. Neuroendocrinology 2020, 110, 444–476. [Google Scholar] [CrossRef]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Taïeb, D.; Hicks, R.J.; Hindié, E.; Guillet, B.A.; Avram, A.; Ghedini, P.; Timmers, H.J.; Scott, A.T.; Elojeimy, S.; Rubello, D.; et al. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for Radionuclide Imaging of Phaeochromocytoma and Paraganglioma. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2112–2137. [Google Scholar] [CrossRef]
- Kos-Kudła, B.; Castaño, J.P.; Denecke, T.; Grande, E.; Kjaer, A.; Koumarianou, A.; de Mestier, L.; Partelli, S.; Perren, A.; Stättner, S.; et al. European Neuroendocrine Tumour Society (ENETS) 2023 Guidance Paper for Nonfunctioning Pancreatic Neuroendocrine Tumours. J. Neuroendocrinol. 2023, 35, e13343. [Google Scholar] [CrossRef]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; di Castelguidone, E.D.L.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The Multidisciplinary Team for Gastroenteropancreatic Neuroendocrine Tumours: The Radiologist’s Challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef]
- Tabacchi, E.; Fortunati, E.; Argalia, G.; Zanoni, L.; Calabrò, D.; Telo, S.; Campana, D.; Lamberti, G.; Ricci, C.; Casadei, R.; et al. [68Ga]Ga-DOTANOC Uptake at Pancreatic Head/Uncinate Process: Is It a Persistent Diagnostic Pitfall Over Time? Cancers 2022, 14, 3541. [Google Scholar] [CrossRef]
- Mitjavila, M.; Jimenez-Fonseca, P.; Belló, P.; Pubul, V.; Percovich, J.C.; Garcia-Burillo, A.; Hernando, J.; Arbizu, J.; Rodeño, E.; Estorch, M.; et al. Efficacy of [(177)Lu]Lu-DOTATATE in Metastatic Neuroendocrine Neoplasms of Different Locations: Data from the SEPTRALU Study. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2486–2500. [Google Scholar] [CrossRef]
- Ambrosini, V.; Campana, D.; Nanni, C.; Cambioli, S.; Tomassetti, P.; Rubello, D.; Fanti, S. Is 68Ga-DOTA-NOC PET/CT Indicated in Patients with Clinical, Biochemical or Radiological Suspicion of Neuroendocrine Tumour? Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1278–1283. [Google Scholar] [CrossRef]
- Zandee, W.T.; Merola, E.; Poczkaj, K.; de Mestier, L.; Klümpen, H.-J.; Geboes, K.; de Herder, W.W.; Munir, A. Evaluation of Multidisciplinary Team Decisions in Neuroendocrine Neoplasms: Impact of Expert Centres. Eur. J. Cancer Care 2022, 31, e13639. [Google Scholar] [CrossRef]
- Ambrosini, V.; Kunikowska, J.; Baudin, E.; Bodei, L.; Bouvier, C.; Capdevila, J.; Cremonesi, M.; de Herder, W.W.; Dromain, C.; Falconi, M.; et al. Consensus on Molecular Imaging and Theranostics in Neuroendocrine Neoplasms. Eur. J. Cancer 2021, 146, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Kunikowska, J.; Ambrosini, V.; Herrmann, K. EANM Focus 3: The International Conference on Molecular Imaging and Theranostics in Neuroendocrine Tumours-the Consensus in a Nutshell. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1276–1277. [Google Scholar] [CrossRef] [PubMed]
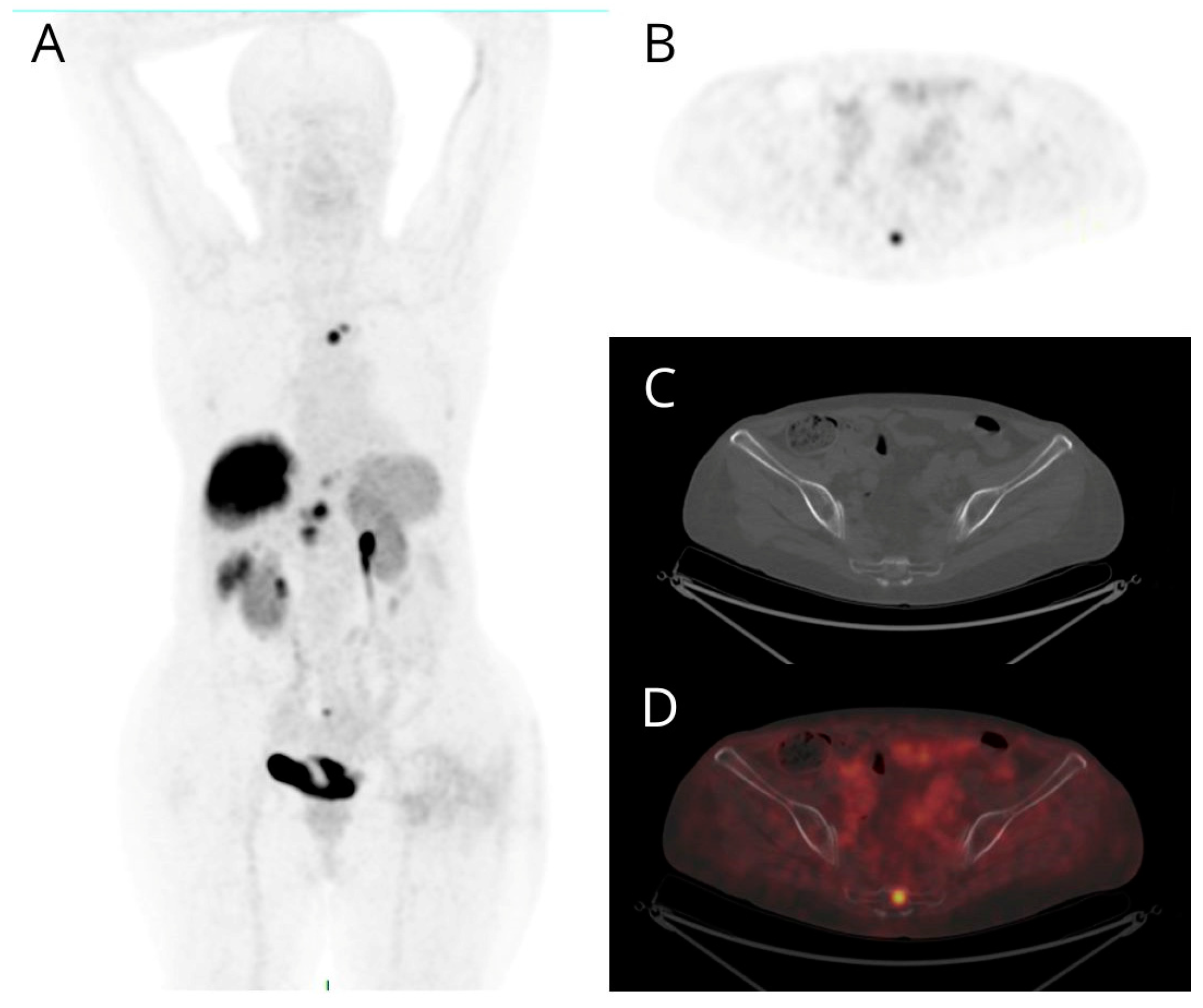
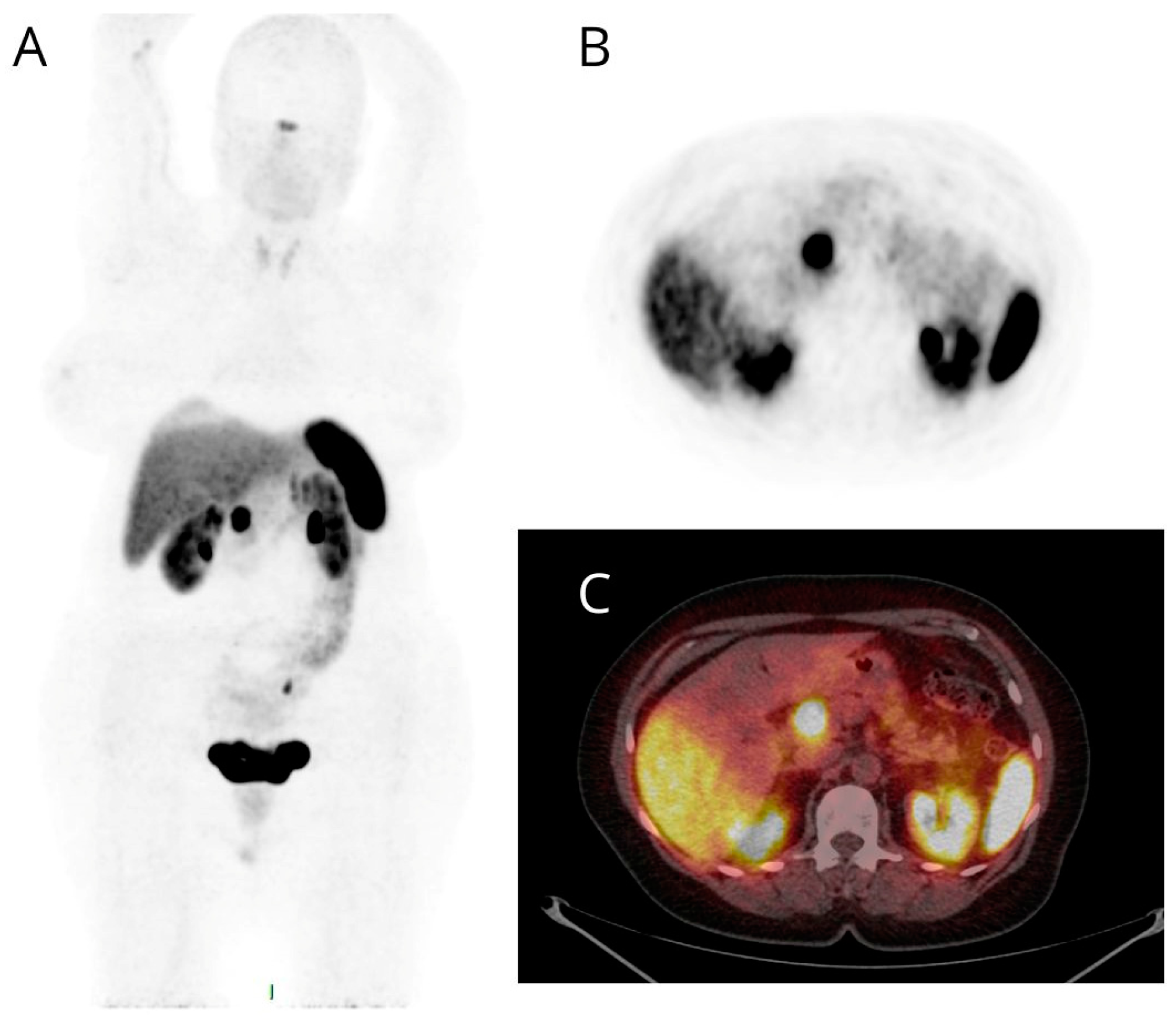
| n | % | PET/CT | |||
|---|---|---|---|---|---|
| pos | neg | Positivity Rate | |||
| Scans Classified by Primary Tumor Site | n | n | % | ||
| GEP | 1135 | 67 | 785 | 350 | 69.2 |
| Lung | 188 | 11 | 111 | 77 | 59.0 |
| Pheochromocytoma | 13 | 1 | 9 | 4 | 69.2 |
| Paraganglioma | 26 | 2 | 21 | 5 | 80.8 |
| Medullary thyroid cancer | 14 | 1 | 11 | 3 | 78.6 |
| Insulinoma | 6 | 0 | 2 | 4 | 33.3 |
| Meningioma | 11 | 1 | 11 | 0 | 100.0 |
| Ear | 18 | 1 | 8 | 10 | 44.4 |
| Breast | 10 | 1 | 4 | 6 | 40.0 |
| Ovary | 4 | 0 | 3 | 1 | 75.0 |
| Thymus | 11 | 1 | 6 | 5 | 54.5 |
| MEN | 69 | 4 | 57 | 12 | 82.6 |
| Neuroblastoma | 1 | 0 | 1 | 0 | 100.0 |
| Salivary glands | 4 | 0 | 1 | 3 | 25.0 |
| MEN + GIST | 4 | 0 | 4 | 0 | 100.0 |
| NA | 171 | 10 | 122 | 49 | 71.3 |
| Total | 1685 | 100 | 1156 | 529 | 68.6 |
| n | % | PET/CT | |||
| pos | neg | Positivity Rate | |||
| Scans Classified by Indication of PET/CT | n | n | % | ||
| Staging | 503 | 30 | 289 | 214 | 57.5 |
| Pre-surgical | 254 | 186 | 68 | 73.2 | |
| Post-surgical | 249 | 103 | 146 | 41.4 | |
| PRRT selection | 36 | 2 | 34 | 2 | 94.4 |
| Treatment response assessment | |||||
| Interim | 299 | 18 | 277 | 22 | 92.6 |
| Post-treatment | 99 | 6 | 88 | 11 | 88.9 |
| Suspected relapse | 195 | 12 | 127 | 68 | 65.1 |
| Follow-up | 479 | 28 | 284 | 195 | 59.3 |
| Unknow primary tumor | 74 | 4 | 57 | 17 | 77.0 |
| Total | 1685 | 100 | 1156 | 529 | 68.6 |
| n | % | |
|---|---|---|
| Scans Classified by Primary Tumor Site | ||
| Pancreas | 419 | 36.9 |
| Ileum | 529 | 46.6 |
| Pancreas and ileum | 2 | 0.2 |
| Duodenum | 38 | 3.3 |
| Jejunum | 13 | 1.1 |
| Colon | 17 | 1.5 |
| Sigma | 6 | 0.5 |
| Rectum | 15 | 1.3 |
| Stomach | 41 | 3.6 |
| Cecum | 6 | 0.5 |
| Vater’s papilla | 5 | 0.4 |
| Appendix | 26 | 2.3 |
| Gallbladder | 5 | 0.4 |
| Liver | 4 | 0.4 |
| Ileocecal valve | 9 | 0.8 |
| Total | 1135 | 100.0 |
| n | % | |
| Scans Classified by Grading | ||
| G1 | 432 | 38.1 |
| G2 | 499 | 44.0 |
| G3 | 22 | 1.9 |
| NET_grade not specified | 33 | 2.9 |
| NA | 149 | 13.1 |
| Total | 1135 | 100.0 |
| n | PET/CT | |||
|---|---|---|---|---|
| pos | neg | Detection Rate | ||
| Scans Classified by Primary Tumor Site | n | n | % | |
| GEP | 633 | 468 | 165 | 73.9 |
| Lung | 75 | 53 | 22 | 70.7 |
| Pheochromocytoma | 3 | 2 | 1 | 66.7 |
| Paraganglioma | 1 | 0 | 1 | 0.0 |
| Ear | 7 | 4 | 3 | 57.1 |
| Breast | 8 | 2 | 6 | 25.0 |
| Ovary | 1 | 0 | 1 | 0.0 |
| Thymus | 2 | 0 | 2 | 0.0 |
| MEN | 53 | 43 | 10 | 81.1 |
| Salivary glands | 1 | 0 | 1 | 0.0 |
| NA | 67 | 51 | 16 | 76.1 |
| Total | 851 | 623 | 228 | 73.2 |
| n | PET/CT | |||
| pos | neg | Detection Rate | ||
| Scans Classified by Indication of PET/CT | n | n | % | |
| Staging | ||||
| Pre-surgical | 77 | 65 | 12 | 84.4 |
| Post-surgical | 127 | 57 | 70 | 44.9 |
| PRRT selection | 22 | 22 | 0 | 100.0 |
| Treatment response assessment | ||||
| Interim | 172 | 162 | 10 | 94.2 |
| Post-treatment | 52 | 48 | 4 | 92.3 |
| Suspected relapse | 86 | 63 | 23 | 73.3 |
| Follow-up | 282 | 179 | 103 | 63.5 |
| Unknow primary tumor | 33 | 27 | 6 | 81.8 |
| Total | 851 | 623 | 228 | 73.2 |
| n | Grade | PET pos | PET neg | Detection Rate | |||
|---|---|---|---|---|---|---|---|
| Scans Classified by Primary Tumor Site | n | % | n | n | n | % | |
| Pancreas | 239 | 37.8 | G1 | 81 | 57 | 24 | 70.4 |
| G2 | 135 | 101 | 34 | 74.8 | |||
| G3 | 11 | 8 | 3 | 72.7 | |||
| NET-NA | 12 | 9 | 3 | 75.0 | |||
| Ileum | 326 | 51.5 | G1 | 134 | 102 | 32 | 76.1 |
| G2 | 164 | 122 | 42 | 74.4 | |||
| NET-NA | 28 | 24 | 4 | 85.7 | |||
| Pancreas and ileum | 1 | 0.2 | G1 | 1 | 1 | 0 | 100.0 |
| Duodenum | 22 | 3.5 | G1 | 7 | 5 | 2 | 71.4 |
| G2 | 15 | 6 | 9 | 40.0 | |||
| NET-NA | 1 | 1 | 0 | 100.0 | |||
| Jejunum | 9 | 1.4 | G1 | 7 | 7 | 0 | 100.0 |
| G2 | 1 | 1 | 0 | 100.0 | |||
| Colon | 4 | 0.6 | G1 | 3 | 2 | 1 | 66.7 |
| G2 | 1 | 0 | 1 | 0.0 | |||
| Sigma | 2 | 0.3 | G2 | 1 | 0 | 1 | 0.0 |
| NET-NA | 1 | 1 | 0 | 100.0 | |||
| Rectum | 7 | 1.1 | G2 | 6 | 6 | 0 | 100.0 |
| G3 | 1 | 1 | 0 | 100.0 | |||
| Gastric | 7 | 1.1 | G1 | 2 | 1 | 1 | 50.0 |
| G2 | 5 | 3 | 2 | 60.0 | |||
| Cecum | 5 | 0.8 | G2 | 5 | 5 | 0 | 100.0 |
| Vater’s papilla | 2 | 0.3 | G1 | 2 | 1 | 1 | 50.0 |
| Appendix | 2 | 0.3 | G1 | 2 | 0 | 2 | 0.0 |
| Gallbladder | 1 | 0.2 | G2 | 1 | 0 | 1 | 0.0 |
| Liver_CUP | 1 | 0.2 | G2 | 1 | 1 | 0 | 100.0 |
| Ileal valve | 5 | 0.8 | G1 | 2 | 2 | 0 | 100.0 |
| G2 | 3 | 1 | 2 | 33.3 | |||
| total | 633 | 100.0 | 633 | 468 | 165 | 73.9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonazzi, N.; Fortunati, E.; Zanoni, L.; Argalia, G.; Calabrò, D.; Tabacchi, E.; Allegri, V.; Campana, D.; Andrini, E.; Lamberti, G.; et al. Real-Life Use of [68Ga]Ga-DOTANOC PET/CT in Confirmed and Suspected NETs from a Prospective 5-Year Electronic Archive at an ENETS Center of Excellence: More Than 2000 Scans in More Than 1500 Patients. Cancers 2024, 16, 701. https://doi.org/10.3390/cancers16040701
Bonazzi N, Fortunati E, Zanoni L, Argalia G, Calabrò D, Tabacchi E, Allegri V, Campana D, Andrini E, Lamberti G, et al. Real-Life Use of [68Ga]Ga-DOTANOC PET/CT in Confirmed and Suspected NETs from a Prospective 5-Year Electronic Archive at an ENETS Center of Excellence: More Than 2000 Scans in More Than 1500 Patients. Cancers. 2024; 16(4):701. https://doi.org/10.3390/cancers16040701
Chicago/Turabian StyleBonazzi, Norma, Emilia Fortunati, Lucia Zanoni, Giulia Argalia, Diletta Calabrò, Elena Tabacchi, Vincenzo Allegri, Davide Campana, Elisa Andrini, Giuseppe Lamberti, and et al. 2024. "Real-Life Use of [68Ga]Ga-DOTANOC PET/CT in Confirmed and Suspected NETs from a Prospective 5-Year Electronic Archive at an ENETS Center of Excellence: More Than 2000 Scans in More Than 1500 Patients" Cancers 16, no. 4: 701. https://doi.org/10.3390/cancers16040701
APA StyleBonazzi, N., Fortunati, E., Zanoni, L., Argalia, G., Calabrò, D., Tabacchi, E., Allegri, V., Campana, D., Andrini, E., Lamberti, G., Di Franco, M., Casadei, R., Ricci, C., Mosconi, C., Fanti, S., & Ambrosini, V. (2024). Real-Life Use of [68Ga]Ga-DOTANOC PET/CT in Confirmed and Suspected NETs from a Prospective 5-Year Electronic Archive at an ENETS Center of Excellence: More Than 2000 Scans in More Than 1500 Patients. Cancers, 16(4), 701. https://doi.org/10.3390/cancers16040701









