Scalp Irradiation with 3D-Milled Bolus: Initial Dosimetric and Clinical Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. CT Simulation and 3D-Milled Bolus Formation
2.3. Target Delineation and Treatment Planning
2.4. Study Endpoints
2.5. Patient Characteristics
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network. Squamous Cell Skin Cancer (Version 01.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (accessed on 19 June 2023).
- Cheng, J.; Yan, S. Prognostic variables in high-risk cutaneous squamous cell carcinoma: A review. J. Cutan. Pathol. 2016, 43, 994–1004. [Google Scholar] [CrossRef]
- Rowe, D.E.; Carroll, R.J.; Day, C.L. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Brodland, D.G.; Zitelli, J.A. Surgical margins for excision of primary cutaneous squamous cell carcinoma. J. Am. Acad. Dermatol. 1992, 27, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Eigentler, T.K.; Leiter, U.; Häfner, H.M.; Garbe, C.; Röcken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; MacNeil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region With Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef]
- Holden, C.A.; Spittle, M.F.; Jones, E.W. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer 1987, 59, 1046–1057. [Google Scholar] [CrossRef]
- Alam, M.; Ratner, D. Cutaneous squamous-cell carcinoma. N. Engl. J. Med. 2001, 344, 975–983. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Schwartz, A.M.; Henson, D.E.; Kostun, L.; Hart, A.; Angeles-Albores, D.; Chablé-Montero, F. Cutaneous angiosarcoma. Analysis of 434 cases from the Surveillance, Epidemiology, and End Results Program, 1973–2007. Ann. Diagn Pathol. 2011, 15, 93–97. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 02.2023). Available online: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf (accessed on 19 June 2023).
- Guadagnolo, B.A.; Zagars, G.K.; Araujo, D.; Ravi, V.; Shellenberger, T.D.; Sturgis, E.M. Outcomes after definitive treatment for cutaneous angiosarcoma of the face and scalp. Head Neck 2011, 33, 661–667. [Google Scholar] [CrossRef]
- Porceddu, S.V.; Daniels, C.; Yom, S.S.; Liu, H.; Waldron, J.; Gregoire, V.; Moore, A.; Veness, M.; Yao, M.; Johansen, J.; et al. Head and Neck Cancer International Group (HNCIG) Consensus Guidelines for the Delivery of Postoperative Radiation Therapy in Complex Cutaneous Squamous Cell Carcinoma of the Head and Neck (cSCCHN). Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 641–651. [Google Scholar] [CrossRef]
- Patel, S.H.; Hayden, R.E.; Hinni, M.L.; Wong, W.W.; Foote, R.L.; Milani, S.; Wu, Q.; Ko, S.J.; Halyard, M.Y. Angiosarcoma of the scalp and face: The Mayo Clinic experience. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Paulino, A.F.; McGinn, C.J.; Baker, L.H.; Cohen, D.S.; Morris, J.S.; Rees, R.; Sondak, V.K. Cutaneous angiosarcoma of the scalp: A multidisciplinary approach. Cancer 2003, 98, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.; Toya, R.; Saito, T.; Kuraoka, A.; Shimohigashi, Y.; Nakaguchi, Y.; Maruyama, M.; Murakami, R.; Yamashita, Y.; Oya, N. Plan quality and delivery time comparisons between volumetric modulated arc therapy and intensity modulated radiation therapy for scalp angiosarcoma: A planning study. J. Med. Radiat. Sci. 2018, 65, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Tung, S.S.; Shiu, A.S.; Starkschall, G.; Morrison, W.H.; Hogstrom, K.R. Dosimetric evaluation of total scalp irradiation using a lateral electron-photon technique. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gogineni, E.; Cai, H.; Carillo, D.; Rana, Z.; Bloom, B.; Potters, L.; Gaballa, H.; Ghaly, M. Computed tomography-based flap brachytherapy for non-melanoma skin cancers of the face. J. Contemp. Brachytherapy 2021, 13, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A. Nonmelanoma skin cancer treated with electronic brachytherapy: Results at 1 year. Brachytherapy 2013, 12, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, A.; Riva, G.; Durante, S.; Fodor, C.; Comi, S.; Cambria, R.; Cattani, F.; Spadola, G.; Orecchia, R.; Jereczek-Fossa, B.A. Mould-based surface high-dose-rate brachytherapy for eyelid carcinoma. J. Contemp. Brachytherapy 2019, 11, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, J. Brachytherapy in the treatment of skin cancer: An overview. Postepy Dermatol. Alergol. 2015, 32, 362–367. [Google Scholar] [CrossRef]
- Hunzeker, A.; Mundy, D.W.; Ma, J.; Mullikin, T.C.; Foote, R.L. Intensity-Modulated Proton Therapy (IMPT) Treatment of Angiosarcoma of the Face and Scalp. Int. J. Part Ther. 2021, 8, 304–310. [Google Scholar] [CrossRef]
- Mitra, D.; Devlin, P.M.; Buzurovic, I.; Thornton, K.; Lam, A.C.; Raut, C.P.; Baldini, E.H.; Lam, M.B. Clinical outcomes following high-dose-rate surface applicator brachytherapy for angiosarcoma of scalp and face. J. Contemp. Brachytherapy 2021, 13, 172–178. [Google Scholar] [CrossRef]
- Mitra, D.; Pei, Y.; Buzurovic, I.; Devlin, P.M.; Thornton, K.; Raut, C.P.; Baldini, E.H.; Lam, M.B. Angiosarcoma of the Scalp and Face: A Dosimetric Comparison of HDR Surface Applicator Brachytherapy and VMAT. Sarcoma 2020, 2020, 7615248. [Google Scholar] [CrossRef]
- Fujimoto, K.; Shiinoki, T.; Yuasa, Y.; Hanazawa, H.; Shibuya, K. Efficacy of patient-specific bolus created using three-dimensional printing technique in photon radiotherapy. Phys. Medica 2017, 38, 1–9. [Google Scholar] [CrossRef]
- Rakici, S.Y.; Çinar, Y.; Eren, M. Total Scalp Irradiation: The Comparison of Five Different Plans Using Volumetric Modulated Arc Therapy-Simultaneous Integrated Boost (VMAT-SIB) Technique. Turk. J. Oncol./Türk Onkol. Derg. 2017, 32. [Google Scholar] [CrossRef][Green Version]
- Baltz, G.C.; Chi, P.M.; Wong, P.F.; Wang, C.; Craft, D.F.; Kry, S.F.; Lin, S.S.H.; Garden, A.S.; Smith, S.A.; Howell, R.M. Development and validation of a 3D-printed bolus cap for total scalp irradiation. J. Appl. Clin. Med. Phys. 2019, 20, 89–96. [Google Scholar] [CrossRef]
- Casto, Z.; Liu, M.; Lenards, N.; Hunzeker, A.; Zoller, W.; Blakaj, D.M. Multiple case dosimetric evaluation of VMAT scalp irradiation using 3D milled bolus. Med. Dosim. 2021, 46, 324–327. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, J.; Bai, S.; Jiang, X.; Xu, F. Conventionally-fractionated image-guided intensity modulated radiotherapy (IG-IMRT): A safe and effective treatment for cancer spinal metastasis. Radiat. Oncol. 2008, 3, 11. [Google Scholar] [CrossRef]
- Jensen, A.O.; Svaerke, C.; Farkas, D.; Pedersen, L.; Kragballe, K.; Sorensen, H.T. Skin cancer risk among solid organ recipients: A nationwide cohort study in Denmark. Acta Derm. Venereol. 2010, 90, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Hansen, S.; Moller, B.; Leivestad, T.; Pfeffer, P.; Geiran, O.; Fauchald, P.; Simonsen, S. Skin cancer in kidney and heart transplant recipients and different long-term immunosuppressive therapy regimens. J. Am. Acad. Dermatol. 1999, 40, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.D.; Shanafelt, T.D.; Khezri, F.; Sosa Seda, I.M.; Zubair, A.S.; Baum, C.L.; Arpey, C.J.; Cerhan, J.R.; Call, T.G.; Roenigk, R.K.; et al. Increased incidence and recurrence rates of nonmelanoma skin cancer in patients with non-Hodgkin lymphoma: A Rochester Epidemiology Project population-based study in Minnesota. J. Am. Acad. Dermatol. 2015, 72, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Zilinska, Z.; Sersenova, M.; Chrastina, M.; Breza, J.S.; Bena, L.; Baltesova, T.; Jurcina, A.; Roland, R.; Lackova, E.; Cellar, M.; et al. Occurrence of malignancies after kidney transplantation in adults: Slovak multicenter experience. Neoplasma 2017, 64, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Hock, B.D.; McIntosh, N.D.; McKenzie, J.L.; Pearson, J.F.; Simcock, J.W.; MacPherson, S.A. Incidence of cutaneous squamous cell carcinoma in a New Zealand population of chronic lymphocytic leukaemia patients. Intern. Med. J. 2016, 46, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Cardella, C.J.; Haberman, H.F. Cutaneous malignant neoplasms in patients with renal transplants. Arch. Dermatol. 1986, 122, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xiao, W.; He, Z.; Kang, D.; Chen, A.; Qi, Z. Target splitting non-coplanar RapidArc radiation therapy for a diffuse sebaceous carcinoma of the scalp: A novel delivery technique. Radiat. Oncol. 2014, 9, 204. [Google Scholar] [CrossRef][Green Version]
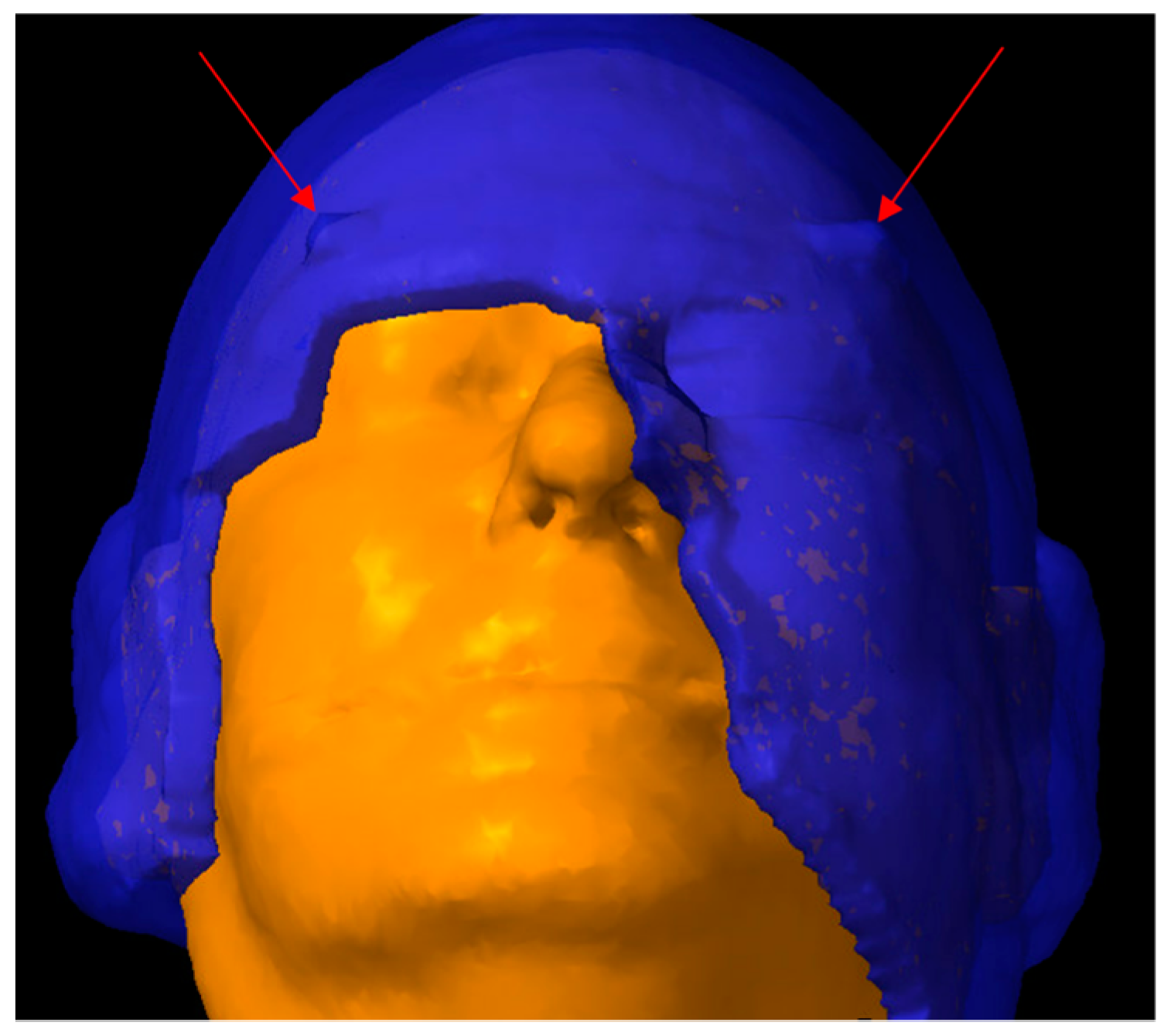
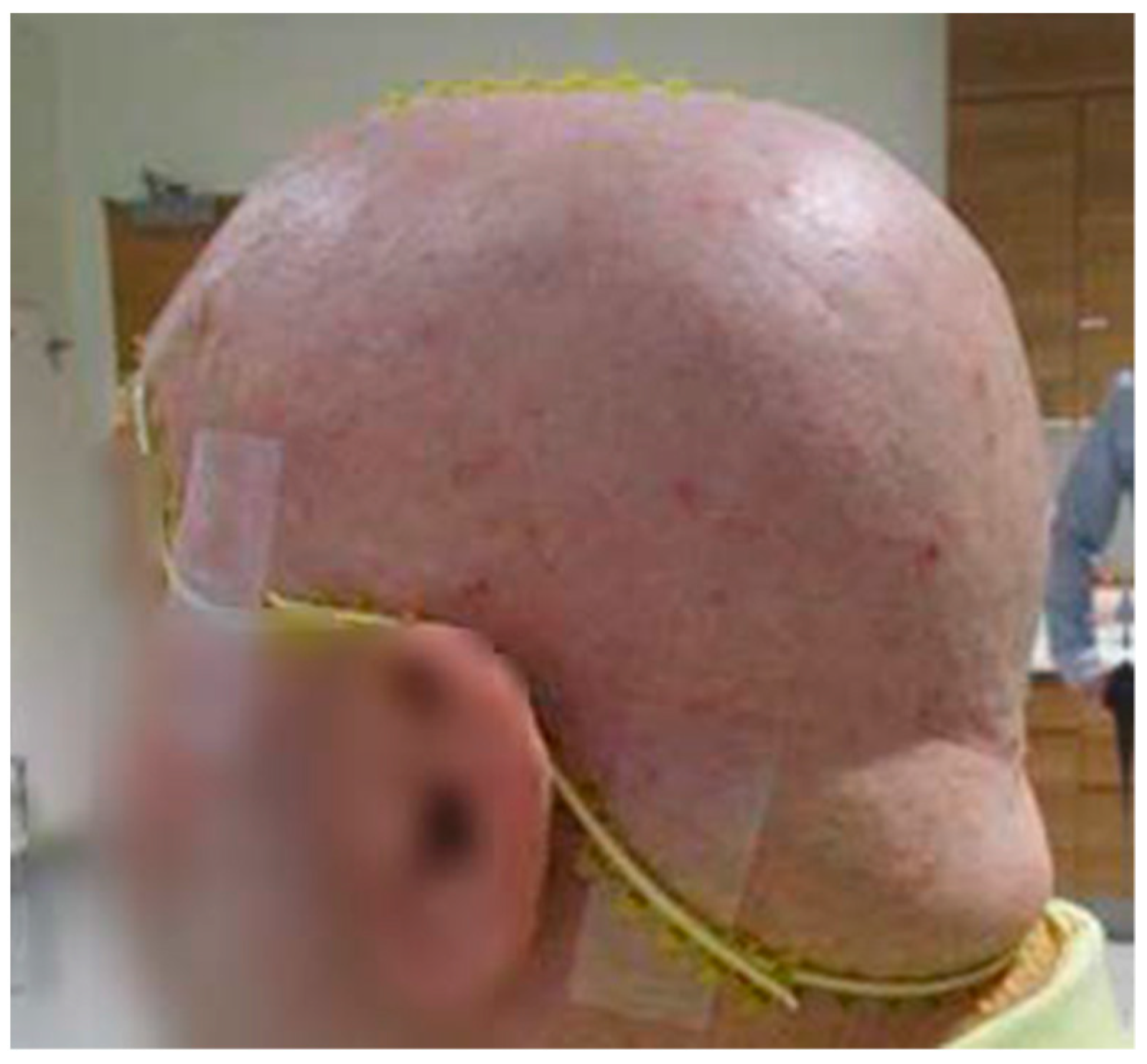
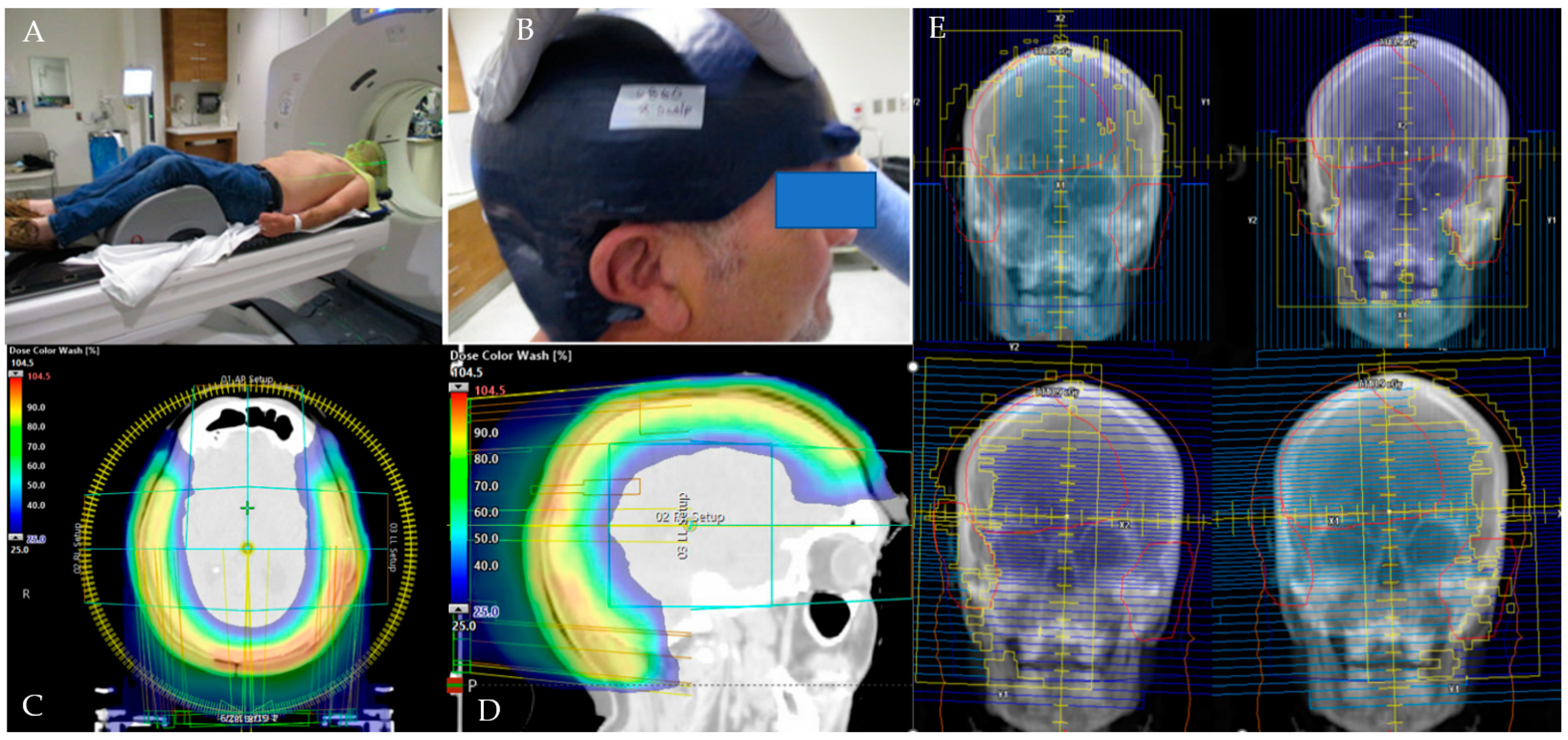
| Variable (n = 22) | n (%) | |||
|---|---|---|---|---|
| Follow-up (mo): median (range) | 21.4 (4.0–75.4) | |||
| Age (yr): median (range) | 74 (46–85) | |||
| Sex | ||||
| Male | 20 (91) | |||
| Female | 2 (9) | |||
| ECOG performance status | ||||
| 0 | 16 (73) | |||
| 1 | 3 (14) | |||
| 2 | 3 (14) | |||
| Immune compromised | ||||
| Yes | 8 (36) | |||
| No | 14 (64) | |||
| Histopathology | ||||
| Squamous cell carcinoma | 14 (64) | |||
| Angiosarcoma | 8 (36) | |||
| Stage | ||||
| Squamous cell carcinoma | ||||
| T-stage | ||||
| T1 | 0 (0) | |||
| T2 | 1 (7) | |||
| T3 | 8 (57) | |||
| T4a | 5 (36) | |||
| N-stage | ||||
| N0 | 12 (86) | |||
| N1 | 0 (0) | |||
| N2 | 2 (14) | |||
| Angiosarcoma | ||||
| T-stage | ||||
| T1 | 0 (0) | |||
| T2 | 4 (50) | |||
| T3 | 3 (37) | |||
| T4a | 1 (13) | |||
| N-stage | ||||
| N0 | 7 (87) | |||
| N1 | 1 (13) | |||
| N2 | N/A | |||
| Concurrent systemic therapy | ||||
| None | 13 (59) | |||
| Paclitaxel | 5 (23) | |||
| Cemiplimab | 3 (14) | |||
| Nivolumab | 1 (5) | |||
| Variable | Median (Range) | |
|---|---|---|
| Extent of radiation: n (%) | ||
| Whole scalp | 8 (36) | |
| Partial scalp | 14 (64) | |
| Scalp + neck | 5 (23) | |
| Prescription dose (Gy) | 66.0 (60.0–69.96) | |
| PTV54.0–54.12 (n = 3) (cc) | 538 (121–624) | |
| PTV59.4–60.0 (n = 21) (cc) | 355 (40–1352) | |
| PTV66.0 (n = 11) (cc) | 130 (62–789) | |
| PTV69.96 (n = 3) (cc) | 107 (6–827) | |
| Gross disease (Gy) | 66.0 (66.0–69.96) | |
| Adjuvant (Gy) | 66.0 (60.0–66.0) | |
| Number of fractions: n (%) | ||
| 30 fractions | 10 (45) | |
| 33 fractions | 12 (55) | |
| Skin V60 (cc) | 268 (19–598) | |
| Skin V64 (cc) | 112 (1–580) | |
| Brain Dmax * (Gy) | 65.0 (52.5–71.9) | |
| Brain Dmean (Gy) | 13.0 (1.4–38.4) | |
| Eye Dmax * (Gy) | 10.0 (0.2–49.0) | |
| Cochlea Dmean (Gy) | 8.0 (0.1–20.4) | |
| Lacrimal gland Dmean (Gy) | 6.4 (0.1–38.0) | |
| Hippocampus Dmin (Gy) | 5.7 (0.1–14.9) | |
| Hippocampus Dmax * (Gy) | 10.0 (0.3–23.0) | |
| PTV Dmax * | ||
| Dose (Gy) | 72.3 (66.2–79.6) | |
| Percentage (%) | 111.3 (105.0–118.0) | |
| Dose homogeneity index | 1.07 (1.03–1.15) | |
| D2% (Gy) | 70.3 (63.4–76.9) | |
| D98% (Gy) | 65.1 (57.6–69.5) | |
| Toxicity | n (%) | |
|---|---|---|
| Acute side effects | ||
| Fatigue | ||
| Grade 0 | 0 (0) | |
| Grade 1 | 18 (82) | |
| Grade 2 | 4 (18) | |
| Pain | ||
| Grade 0 | 5 (23) | |
| Grade 1 | 14 (64) | |
| Grade 2 | 3 (14) | |
| Dysgeusia | ||
| Grade 0 | 17 (77) | |
| Grade 1 | 5 (23) | |
| Dermatitis | ||
| Grade 0 | 0 (0) | |
| Grade 1 | 5 (23) | |
| Grade 2 | 11 (50) | |
| Grade 3 | 6 (27) | |
| Late side effects | ||
| Osteoradionecrosis | ||
| Grade 0 | 21 (95) | |
| Grade 1 | 0 (0) | |
| Grade 2 | 1 (5) | |
| Skin ulceration | ||
| Grade 0 | 20 (90) | |
| Grade 1 | 0 (0) | |
| Grade 2 | 1 (5) | |
| Grade 3 | 1 (5) | |
| Memory impairment | ||
| Grade 0 | 18 (82) | |
| Grade 1 | 4 (18) | |
| Eye dryness | ||
| Grade 0 | 18 (82) | |
| Grade 1 | 4 (18) | |
| Variable (n = 22) | LRC (%) | p-Value | DMFS (%) | p-Value | OS (%) | p-Value | |
|---|---|---|---|---|---|---|---|
| Overall cohort | 75 | 62 | 79 | ||||
| ECOG performance status | 0.647 | 0.656 | 0.269 | ||||
| 0 | 72 | 62 | 86 | ||||
| 1 | 100 | 50 | 50 | ||||
| 2 | 67 | 66 | 66 | ||||
| Immune compromised | 0.006 * | 0.527 | 0.106 | ||||
| Yes | 42 | 56 | 56 | ||||
| No | 92 | 67 | 92 | ||||
| Histopathology | 0.058 | 0.781 | 0.397 | ||||
| Squamous cell carcinoma | 60 | 66 | 66 | ||||
| Angiosarcoma | 100 | 57 | 100 | ||||
| T-stage | 0.780 | 0.921 | 0.791 | ||||
| T2 | 80 | 80 | 100 | ||||
| T3 | 70 | 62 | 75 | ||||
| T4a | 83 | 52 | 62 | ||||
| N-stage | 0.028 * | <0.001 * | <0.001 * | ||||
| N0 | 82 | 74 | 85 | ||||
| N ≥ 1 | 34 | 0 | 34 | ||||
| Treatment intent | 0.560 | 0.020 * | 0.092 | ||||
| Intact/unresected disease | 72 | 24 | 74 | ||||
| Adjuvant/postoperative | 78 | 77 | 84 | ||||
| Surgical margin | 0.971 | 0.297 | 0.089 | ||||
| Negative | 75 | 88 | 100 | ||||
| Positive | 80 | 60 | 60 | ||||
| PNI | 0.884 | 0.321 | 0.557 | ||||
| Negative | 75 | 60 | 82 | ||||
| Positive | 75 | 75 | 75 | ||||
| Systemic therapy | 0.970 | 0.057 | 0.060 | ||||
| Yes | 78 | 39 | 64 | ||||
| No | 72 | 81 | 90 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dibs, K.; Gogineni, E.; Jhawar, S.M.; Baliga, S.; Grecula, J.C.; Mitchell, D.L.; Palmer, J.; Haglund, K.; Andraos, T.Y.; Zoller, W.; et al. Scalp Irradiation with 3D-Milled Bolus: Initial Dosimetric and Clinical Experience. Cancers 2024, 16, 688. https://doi.org/10.3390/cancers16040688
Dibs K, Gogineni E, Jhawar SM, Baliga S, Grecula JC, Mitchell DL, Palmer J, Haglund K, Andraos TY, Zoller W, et al. Scalp Irradiation with 3D-Milled Bolus: Initial Dosimetric and Clinical Experience. Cancers. 2024; 16(4):688. https://doi.org/10.3390/cancers16040688
Chicago/Turabian StyleDibs, Khaled, Emile Gogineni, Sachin M. Jhawar, Sujith Baliga, John C. Grecula, Darrion L. Mitchell, Joshua Palmer, Karl Haglund, Therese Youssef Andraos, Wesley Zoller, and et al. 2024. "Scalp Irradiation with 3D-Milled Bolus: Initial Dosimetric and Clinical Experience" Cancers 16, no. 4: 688. https://doi.org/10.3390/cancers16040688
APA StyleDibs, K., Gogineni, E., Jhawar, S. M., Baliga, S., Grecula, J. C., Mitchell, D. L., Palmer, J., Haglund, K., Andraos, T. Y., Zoller, W., Ewing, A., Bonomi, M., Bhateja, P., Tinoco, G., Liebner, D., Rocco, J. W., Old, M., Gamez, M. E., Chakravarti, A., ... Blakaj, D. M. (2024). Scalp Irradiation with 3D-Milled Bolus: Initial Dosimetric and Clinical Experience. Cancers, 16(4), 688. https://doi.org/10.3390/cancers16040688







