The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
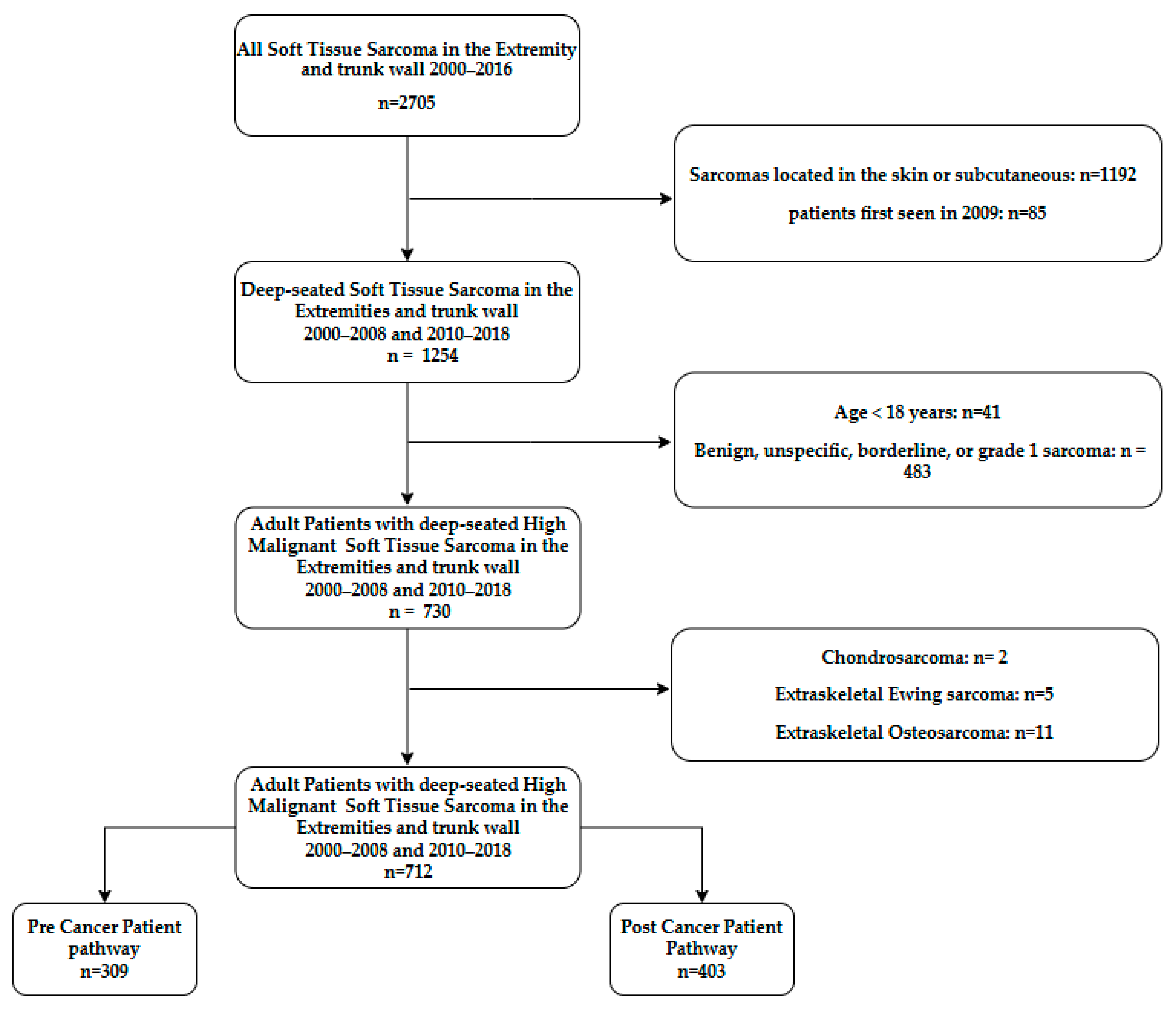
2.2. Patients
2.3. Variables
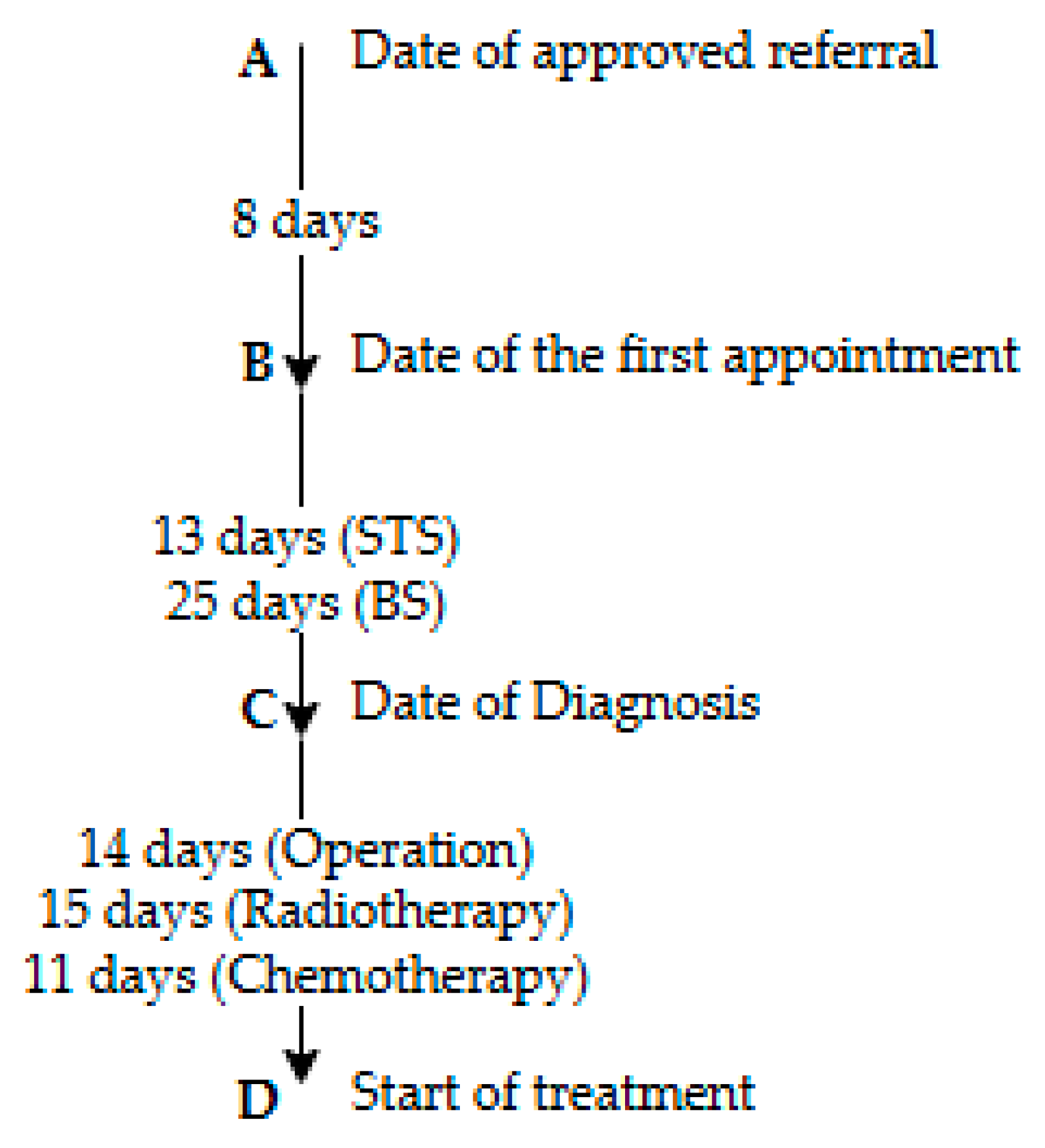
2.4. The Danish Cancer Patient Pathway Timeframe
2.5. Statistical Analysis
3. Results
3.1. Patients and Tumor Characteristics
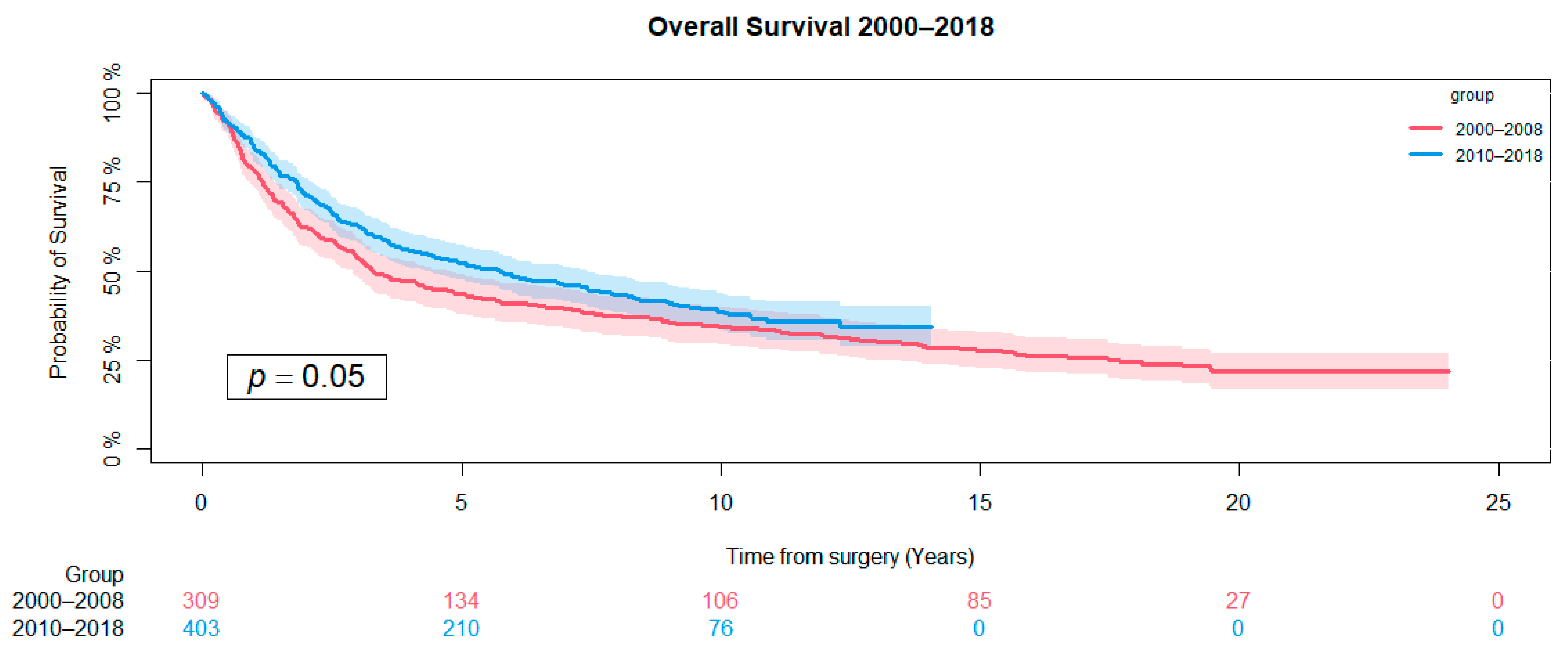
3.2. Overall Survival
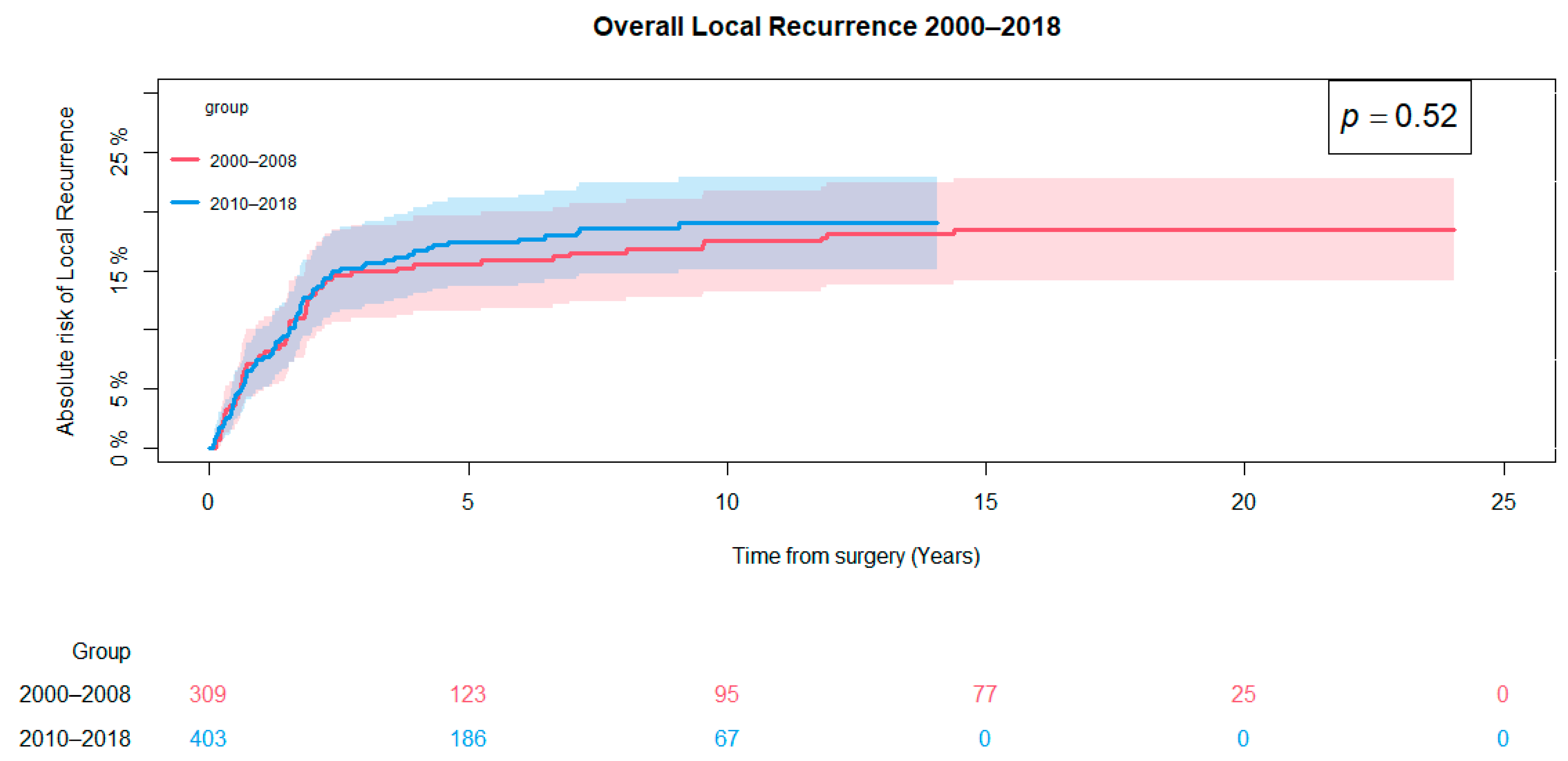
3.3. Local Recurrence
3.4. Diagnostic Time Intervals
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, C.D.M.; WHO. WHO Classification of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2013. [Google Scholar]
- Jørgensen, P.H.; Lausten, G.S.; Pedersen, A.B. The Danish Sarcoma Database. Clin. Epidemiol. 2016, 8, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Ducimetière, F.; Lurkin, A.; Ranchère-Vince, D.; Decouvelaere, A.V.; Péoc’h, M.; Istier, L.; Chalabreysse, P.; Muller, C.; Alberti, L.; Bringuier, P.P.; et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS ONE 2011, 6, e20294. [Google Scholar] [CrossRef] [PubMed]
- Coleman, M.P.P.; Forman, D.P.; Bryant, H.P.; Butler, J.M.; Rachet, B.P.; Maringe, C.M.; Nur, U.P.; Tracey, E.M.; Coory, M.P.; Hatcher, J.P.; et al. Cancer survival in Australia, Canada, Denmark, Norway, Sweden, and the UK, 1995–2007 (the International Cancer Benchmarking Partnership): An analysis of population-based cancer registry data. Lancet 2011, 377, 127–138. [Google Scholar] [CrossRef]
- Pakkeforløb for Sarkomer i Knogle og Bløddele, Version: 1,1; Sundhedsstyrelsen: København, Denmark, 2009.
- Jensen, H.; Torring, M.L.; Olesen, F.; Overgaard, J.; Vedsted, P. Cancer suspicion in general practice, urgent referral and time to diagnosis: A population-based GP survey and registry study. BMC Cancer 2014, 14, 636. [Google Scholar] [CrossRef]
- Probst, H.B.; Hussain, Z.B.; Andersen, O. Cancer patient pathways in Denmark as a joint effort between bureaucrats, health professionals and politicians--a national Danish project. Health Policy 2012, 105, 65–70. [Google Scholar] [CrossRef]
- Dyrop, H.B.; Safwat, A.; Vedsted, P.; Maretty-Nielsen, K.; Hansen, B.H.; Jørgensen, P.H.; Baad-Hansen, T.; Bünger, C.; Keller, J. Cancer Patient Pathways shortens waiting times and accelerates the diagnostic process of suspected sarcoma patients in Denmark. Health Policy 2013, 113, 110–117. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Keller, J.; Safwat, A.; Baerentzen, S.; Pedersen, A.B. Population-based Aarhus Sarcoma Registry: Validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin. Epidemiol. 2013, 5, 45–56. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; De Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef]
- van Praag, V.M.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.K.; Pollock, R.; Aston, W.; van der Hage, J.A.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised sarcoma care (PERSARC). Eur. J. Cancer 2017, 83, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Tichanek, F.; Försti, A.; Hemminki, O.; Hemminki, A.; Hemminki, K. Steady survival improvements in soft tissue and bone sarcoma in the Nordic countries through 50 years. Cancer Epidemiol. 2024, 92, 102449. [Google Scholar] [CrossRef] [PubMed]
- Abarca, T.; Gao, Y.; Monga, V.; Tanas, M.R.; Milhem, M.M.; Miller, B.J. Improved survival for extremity soft tissue sarcoma treated in high-volume facilities. J. Surg. Oncol. 2018, 117, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.J. Size matters for sarcomas. Ann. R. Coll. Surg. Engl. 2006, 88, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Traub, F.; Griffin, A.M.; Wunder, J.S.; Ferguson, P.C. Influence of unplanned excisions on the outcomes of patients with stage III extremity soft-tissue sarcoma. Cancer 2018, 124, 3868–3875. [Google Scholar] [CrossRef]
- Ferguson, P.C.; Deheshi, B.M.; Chung, P.; Catton, C.N.; O’Sullivan, B.; Gupta, A.; Griffin, A.M.; Wunder, J.S. Soft Tissue Sarcoma Presenting with Metastatic Disease: Outcome with Primary Surgical Resection. Cancer 2011, 117, 372–379. [Google Scholar] [CrossRef]
- Outani, H.; Hamada, K.; Yasuda, N.; Ueda, T.; Myoui, A.; Yoshikawa, H.; Okada, S. Impact of surgical resection on survival in patients with metastatic soft tissue sarcoma and comparison between synchronous and metachronous metastases. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2022, 27, 892–898. [Google Scholar] [CrossRef]
- Thorn, A.; Iljazi, A.; Engelmann, B.E.; Aggerholm-Pedersen, N.; Baad-Hansen, T.; Petersen, M.M. Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall. Cancers 2024, 16, 3423. [Google Scholar] [CrossRef]
- Willeumier, J.; Fiocco, M.; Nout, R.; Dijkstra, S.; Aston, W.; Pollock, R.; Hartgrink, H.; Bovée, J.; van de Sande, M. High-grade soft tissue sarcomas of the extremities: Surgical margins influence only local recurrence not overall survival. Int. Orthop. 2015, 39, 935–941. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, X.; Feng, Y.; Yang, Z.; Chen, X.; Wand, J.; Ma, S.; Zhang, Z.; Guo, X. Local recurrence is correlated with decreased overall survival in patients with intermediate high-grade localized primary soft tissue sarcoma of extremity and abdominothoracic wall. Asia-Pac. J. Clin. Oncol. 2018, 14, e109–e115. [Google Scholar] [CrossRef]
- Demetri, G.D.; von Mehren, M.; Jones, R.L.; Hensley, M.L.; Schuetze, S.M.; Staddon, A.; Milhem, M.; Elias, A.; Ganjoo, K.; Tawbi, H.; et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J. Clin. Oncol. 2016, 34, 786–793. [Google Scholar] [CrossRef] [PubMed]
- van der Graaf, W.T.A.P.; Blay, J.-Y.P.; Chawla, S.P.M.D.; Kim, D.-W.M.D.; Bui-Nguyen, B.M.D.; Casali, P.G.M.D.; Schöffski, P.P.; Aglietta, M.M.D.; Staddon, A.P.M.D.; Beppu, Y.M.D.; et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012, 379, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Dei Tos, A.P. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Matushansky, I.; Charytonowicz, E.; Mills, J.; Siddiqi, S.; Hricik, T.; Cordon-Cardo, C. MFH classification: Differentiating undifferentiated pleomorphic sarcoma in the 21st Century. Expert Rev. Anticancer. Ther. 2009, 9, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Dyrop, H.B.; Vedsted, P.; Safwat, A.; Maretty-Nielsen, K.; Hansen, B.H.; Jørgensen, P.H.; Baad-Hansen, T.; Keller, J. Alarm symptoms of soft-tissue and bone sarcoma in patients referred to a specialist center. Acta Orthop. 2014, 85, 657–662. [Google Scholar] [CrossRef]
- George, A.; Grimer, R. Early symptoms of bone and soft tissue sarcomas: Could they be diagnosed earlier? Ann. R. Coll. Surg. Engl. 2012, 94, 261–266. [Google Scholar] [CrossRef]
- Grignol, V.P.; Lopez-Aguiar, A.G. The Implications of an Unplanned Sarcoma Excision (the “Whoops” Operation). Surg. Clin. N. Am. 2022, 102, 529–538. [Google Scholar] [CrossRef]
- Funovics, P.T.; Vaselic, S.; Panotopoulos, J.; Kotz, R.I.; Dominkus, M. The impact of re-excision of inadequately resected soft tissue sarcomas on surgical therapy, results, and prognosis: A single institution experience with 682 patients. J. Surg. Oncol. 2010, 102, 626–633. [Google Scholar] [CrossRef]
- Schärer, M.; Heesen, P.; Bode-Lesniewska, B.; Studer, G.; Fuchs, B. Benchmarking Time-to-Treatment Initiation in Sarcoma Care Using Real-World-Time Data. Cancers 2023, 15, 5849. [Google Scholar] [CrossRef]
- Derbel, O.; Heudel, P.E.; Cropet, C.; Meeus, P.; Vaz, G.; Biron, P.; Cassier, P.; Decouvelaere, A.-V.; Ranchere-Vince, D.; Collard, O.; et al. Survival impact of centralization and clinical guidelines for soft tissue sarcoma (A prospective and exhaustive population-based cohort). PLoS ONE 2017, 12, e0158406. [Google Scholar] [CrossRef]
- Fujiwara, T.; Ogura, K.; Healey, J. Greater travel distance to specialized facilities is associated with higher survival for patients with soft-tissue sarcoma: US nationwide patterns. PLoS ONE 2021, 16, e0252381. [Google Scholar] [CrossRef]
- Middellevetid. Available online: https://www.dst.dk/da/Statistik/emner/borgere/befolkning/middellevetid (accessed on 17 October 2024).
| Treatment group | Overall | 2000–2008 | 2010–2018 |
|---|---|---|---|
| (n = 712 1) | (n = 309 1) | (n = 403 1) | |
| Histological diagnosis | |||
| Sarcoma NOS | 145 (20%) | 64 (21%) | 81 (20%) |
| Undifferentiated pleomorphic sarcoma | 70 (10%) | 0 (0%) | 70 (17%) |
| Leiomyosarcoma | 68 (10%) | 20 (9%) | 39 (10%) |
| Myxoid liposarcoma | 62 (9%) | 27 (9%) | 35 (9%) |
| Malignant fibrous histiocytoma | 59 (8%) | 54 (17%) | 5 (1%) |
| Myxofibrosarcoma | 57 (8%) | 4 (2%) | 53 (13%) |
| Other | 251 (35%) | 131 (42%) | 120 (30%) |
| Treatment Group | Overall | 2000–2008 | 2010–2018 p-Value 3 | |
|---|---|---|---|---|
| Overall | (n = 712 1) | (n = 309 1) | (n = 403 1) | |
| Sex | 0.045 | |||
| Female | 337 (47%) | 133 (43%) | 204 (51%) | |
| Male | 375 (52%) | 176 (57%) | 199 (49%) | |
| Age (Years 2) | 62 (19–96) | 60 (19–91) | 63 (19–96) | 0.052 |
| Location | 0.7 | |||
| Lower Extremity | 499 (70%) | 220 (71%) | 279 (69%) | |
| Truncal | 70 (10%) | 27 (9%) | 43 (11%) | |
| Upper Extremity | 143 (20%) | 62 (20%) | 81 (20%) | |
| Tumor Size (cm) | 10.7 (1–40) | 10.8 (1–30) | 10.6 (1–40) | 0.4 |
| Tumor Size | 0.2 | |||
| <5 cm | 86 (12%) | 32 (10%) | 54 (13%) | |
| >=5 cm | 626 (88%) | 277 (90%) | 349 (87%) | |
| Tumor Size | 0.3 | |||
| <15 cm | 541 (76%) | 229 (74%) | 312 (77%) | |
| >=15 cm | 171 (24%) | 80 (26%) | 91 (23%) | |
| Treatment Group | Overall | 2000–2008 | 2010–2018 p-Value 2 | |
|---|---|---|---|---|
| Overall | (n = 712 1) | (n = 309 1) | (n = 403 1) | |
| Surgical Margin | 0.7 | |||
| Intralesional | 76 (11%) | 28 (9%) | 48 (12%) | |
| Marginal Wide No Surgery | 272 (38%) 288 (40%) 76 (11%) | 120 (39%) 128 (41%) 33 (11%) | 152 (38%) 160 (40%) 43 (11%) | |
| Amputation (Only Extremities) | 64 (10%) | 35 (12%) | 29 (8%) | 0.070 |
| Whoops Procedures | 47 (7%) | 32 (10%) | 15 (4%) | <0.001 |
| Local Recurrence | 133 (19%) | 57 (18%) | 76 (19%) | >0.9 |
| Chemotherapy | 0.002 | |||
| <3 months of Surgery | 22 (3%) | 5 (2%) | 17 (4%) | |
| >3 months of Surgery Before Surgery Chemo, No Surgery No Chemotherapy | 161 (23%) 30 (3%) 40 (6%) 459 (64%) | 61 (20%) 9 (3%) 11 (4%) 223 (72%) | 100 (25%) 21 (5%) 29 (7%) 236 (59%) | |
| Radiotherapy | <0.001 | |||
| <3 months of Surgery | 374 (52%) | 146 (47%) | 228 (57%) | |
| >3 months of Surgery | 79 (11%) | 50 (16%) | 29 (7%) | |
| Before Surgery Radiation, No Surgery No Radiation | 8 (1%) 39 (6%) 212 (30%) | 5 (2%) 11 (4%) 97 (31%) | 3 (1%) 28 (7%) 115 (29%) | |
| Metastasis | 0.9 | |||
| No Metastatic Disease | 349 (49%) | 150 (49%) | 199 (49%) | |
| At Diagnosis <3 months of Diagnosis >3 months of Diagnosis | 31 (4%) 63 (9%) 269 (38%) | 15 (5%) 27 (9%) 117 (38%) | 16 (4%) 36 (9%) 152 (38%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorn, A.; Seem, K.M.; Talman, M.-L.; Engelmann, B.E.; Sørensen, M.S.; Aggerholm-Pedersen, N.; Baad-Hansen, T.; Petersen, M.M. The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study. Cancers 2024, 16, 4077. https://doi.org/10.3390/cancers16234077
Thorn A, Seem KM, Talman M-L, Engelmann BE, Sørensen MS, Aggerholm-Pedersen N, Baad-Hansen T, Petersen MM. The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study. Cancers. 2024; 16(23):4077. https://doi.org/10.3390/cancers16234077
Chicago/Turabian StyleThorn, Andrea, Kristoffer Michael Seem, Maj-Lis Talman, Bodil E. Engelmann, Michala Skovlund Sørensen, Ninna Aggerholm-Pedersen, Thomas Baad-Hansen, and Michael Mørk Petersen. 2024. "The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study" Cancers 16, no. 23: 4077. https://doi.org/10.3390/cancers16234077
APA StyleThorn, A., Seem, K. M., Talman, M.-L., Engelmann, B. E., Sørensen, M. S., Aggerholm-Pedersen, N., Baad-Hansen, T., & Petersen, M. M. (2024). The Influence of Danish Cancer Patient Pathways on Survival in Deep-Seated, High-Grade Soft-Tissue Sarcomas in the Extremities and Trunk Wall: A Retrospective Observational Study. Cancers, 16(23), 4077. https://doi.org/10.3390/cancers16234077







