Addressing COVID-19 Screening Delays: The Impact of HPV Self-Sampling on Non-Attenders in a Cervical Cancer Screening Program
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Design
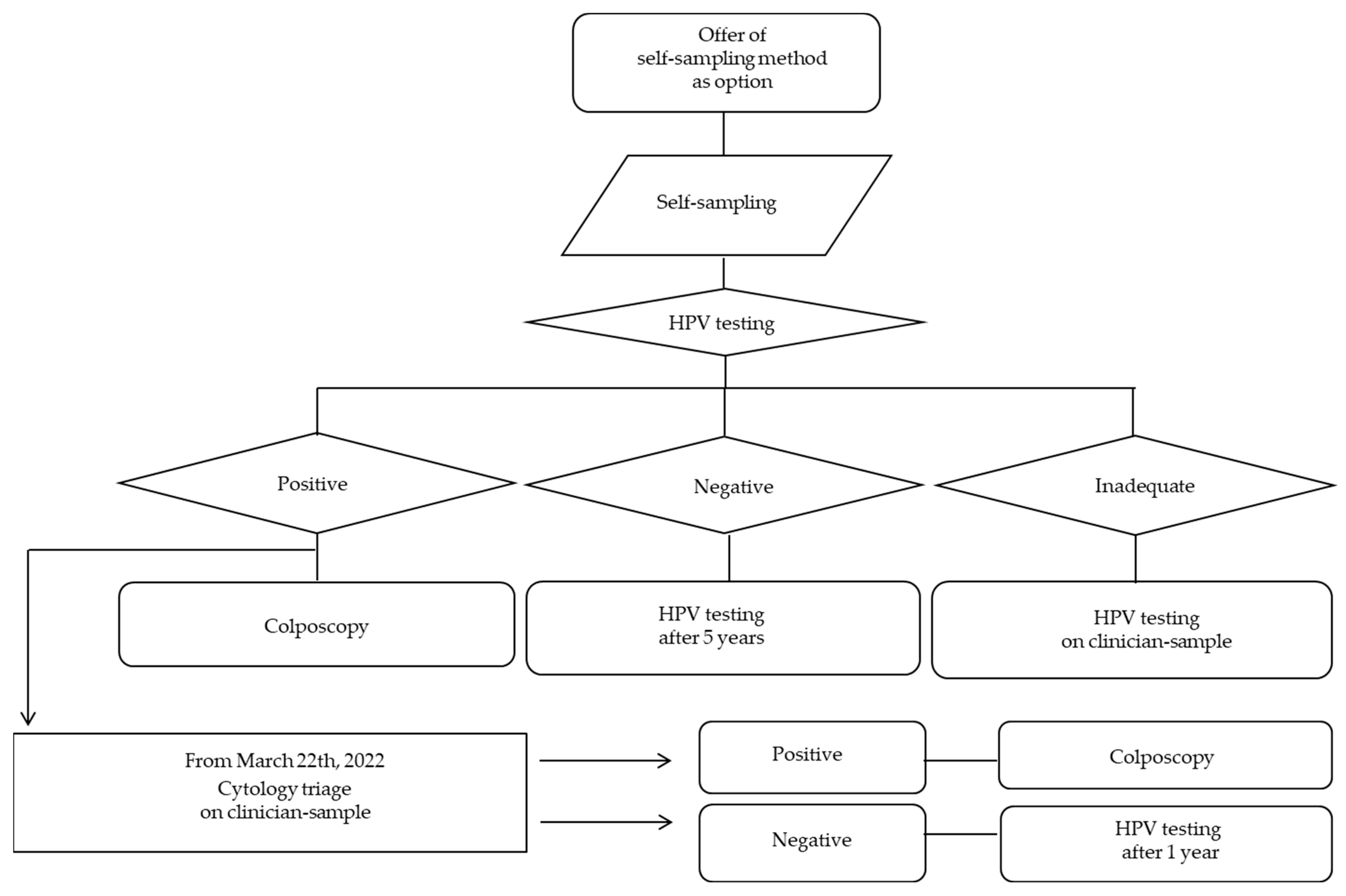
2.3. Local Procedures for Offering HPV Self-Sampling
2.4. Self-Collected Vaginal Swabs and hrHPV-DNA Testing
2.5. Management of Women with hrHPV-DNA Positive Results
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Invited Women
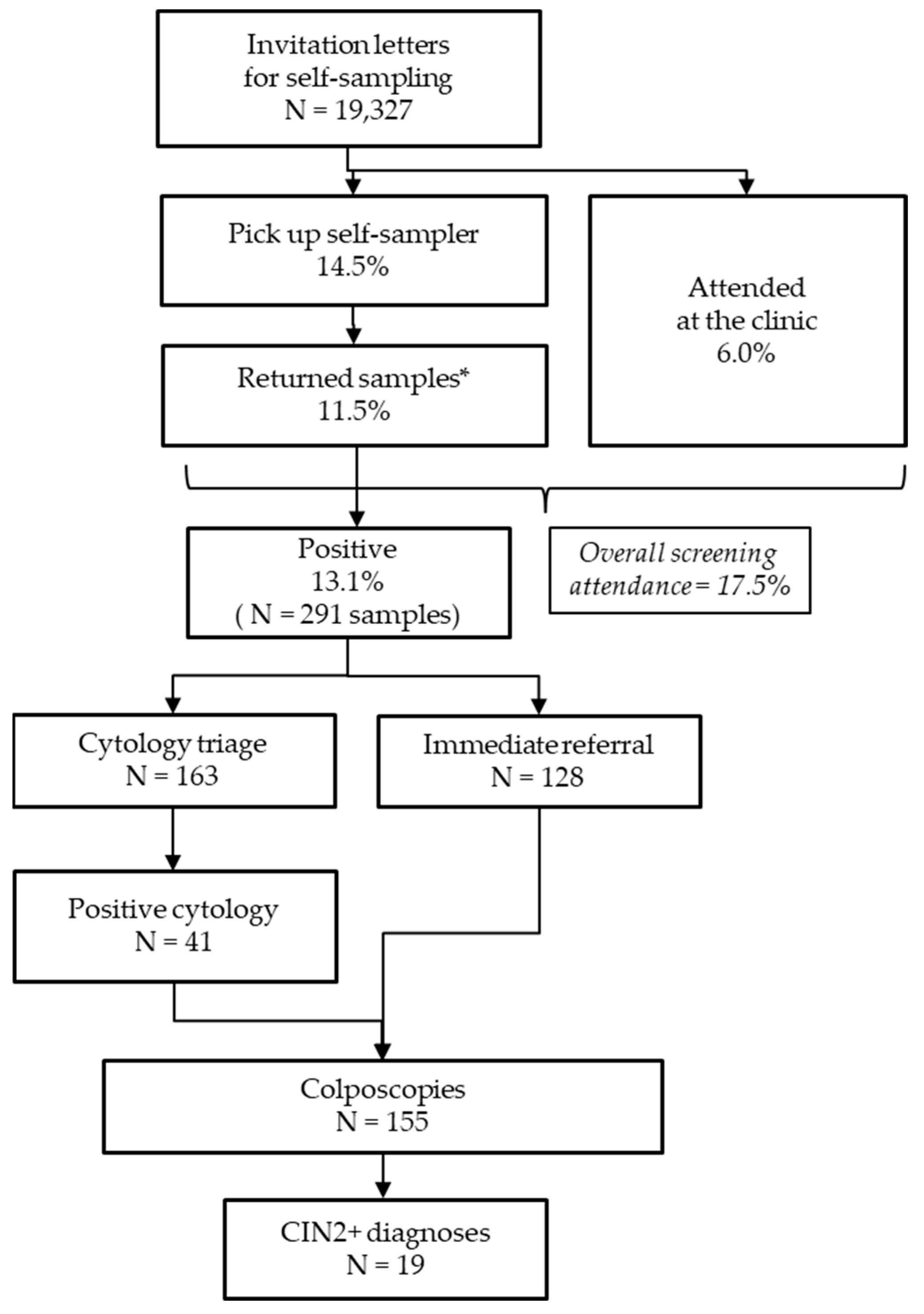
3.2. Response to the Offer of Self-Sampling Method
3.3. Results of hrHPV Testing on Self-Collected Samples
3.4. Compliance with a Follow-Up Examination After a Positive hrHPV Test and Histological Outcomes
4. Discussion
Study Limits
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Castanon, A.; Rebolj, M.; Pesola, F.; Pearmain, P.; Stubbs, R. COVID-19 disruption to cervical cancer screening in England. J. Med. Screen. 2022, 29, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Puricelli Perin, D.M.; Elfström, K.M.; Bulliard, J.L.; Burón, A.; Campbell, C.; Flugelman, A.A.; Giordano, L.; Kamineni, A.; Ponti, A.; Rabeneck, L.; et al. International Cancer Screening Network. Early assessment of the first wave of the COVID-19 pandemic on cancer screening services: The International Cancer Screening Network COVID-19 survey. Prev. Med. 2021, 151, 106642. [Google Scholar] [CrossRef] [PubMed]
- Rapporto sui Ritardi Accumulati dai Programmi di Screening Italiani in Seguito alla Pandemia da COVID 19. Terzo Rapporto Aggiornato al 31 Dicembre 2020. Available online: https://www.osservatorionazionalescreening.it/sites/default/files/allegati/Rapporto%20ripartenza-12_20.pdf (accessed on 22 October 2024).
- Castanon, A.; Rebolj, M.; Pesola, F.; Sasieni, P. Recovery strategies following COVID-19 disruption to cervical cancer screening and their impact on excess diagnoses. Br. J. Cancer 2021, 124, 1361–1365. [Google Scholar] [CrossRef]
- Castanon, A.; Rebolj, M.; Burger, E.A.; de Kok, I.M.C.M.; Smith, M.A.; Hanley, S.J.B.; Carozzi, F.M.; Peacock, S.; O’Mahony, J.F. Cervical screening during the COVID-19 pandemic: Optimising recovery strategies. Lancet Public Health 2021, 6, e522–e527. [Google Scholar] [CrossRef]
- Nishimura, H.; Yeh, P.T.; Oguntade, H.; Kennedy, C.E.; Narasimhan, M. HPV self-sampling for cervical cancer screening: A systematic review of values and preferences. BMJ Glob. Health 2021, 6, e003743. [Google Scholar] [CrossRef]
- Yeh, P.T.; Kennedy, C.E.; de Vuyst, H.; de Vuyst, H.; Narasimhan, M. Self-sampling for human papillomavirus (HPV) testing: A systematic review and meta-analysis. BMJ Glob. Health 2019, 4, e001351. [Google Scholar] [CrossRef]
- WHO. Recommendations on Self-Care Interventions. Human Papillomavirus (HPV) Self-Sampling as Part of Cervical Cancer Screening and Treatment, 2022 Update. Available online: https://iris.who.int/bitstream/handle/10665/366868/WHO-SRH-23.1-eng.pdf (accessed on 22 October 2024).
- Inturrisi, F.; Aitken, C.A.; Melchers, W.J.G.; van den Brule, A.J.C.; Molijn, A.; Hinrichs, J.W.J.; Niesters, H.G.M.; Siebers, A.G.; Schuurman, R.; Heideman, D.A.M.; et al. Clinical performance of high-risk HPV testing on self-samples versus clinician samples in routine primary HPV screening in the Netherlands: An observational study. Lancet Reg. Health Eur. 2021, 11, 100235. [Google Scholar] [CrossRef]
- Economist Impact. A Global Blueprint for Cervical Cancer Elimination: Learnings from Sweden. Available online: https://impact.economist.com/perspectives/sites/default/files/msd_ccp_fullreport_final.pdf (accessed on 22 October 2024).
- Giorgi Rossi, P.; Marsili, L.M.; Camilloni, L.; Iossa, A.; Lattanzi, A.; Sani, C.; Di Pierro, C.; Grazzini, G.; Angeloni, C.; Capparucci, P.; et al. The effect of self sampled HPV testing on participation to cervical cancer screening in Italy: A randomised controlled trial (ISRCTN96071600). Br. J. Cancer 2011, 104, 248–254. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Fortunato, C.; Barbarino, P.; Boveri, S.; Caroli, S.; Del Mistro, A.; Ferro, A.; Giammaria, C.; Manfredi, M.; Moretto, T.; et al. Self-sampling to increase participation in cervical cancer screening: An RCT comparing home mailing, distribution in pharmacies, and recall letter. Br. J. Cancer 2015, 112, 667–675. [Google Scholar] [CrossRef]
- Feltri, G.; Valenti, G.; Isidoro, E.; Kaur, J.; Treleani, M.; Bartelloni, A.; Mauro, C.; Spiga, F.; Ticich, G.; Di Napoli, M.; et al. Evaluation of self-sampling-based cervical cancer screening strategy using HPV Selfy CE-IVD test coupled with home-collection kit: A clinical study in Italy. Eur. J. Med. Res. 2023, 28, 582. [Google Scholar] [CrossRef] [PubMed]
- Sechi, I.; Muresu, N.; Puci, M.V.; Saderi, L.; Del Rio, A.; Cossu, A.; Muroni, M.R.; Castriciano, S.; Martinelli, M.; Cocuzza, C.E.; et al. Preliminary results of feasibility and acceptability of self-collection for cervical screening in Italian women. Pathogens 2023, 12, 1169. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, M.; Giubbi, C.; Sechi, I.; Bottari, F.; Iacobone, A.D.; Musumeci, R.; Perdoni, F.; Muresu, N.; Piana, A.; Fruscio, R.; et al. Evaluation of BD Onclarity™ HPV Assay on self-collected vaginal and first-void urine samples as compared to clinician-collected cervical samples: A pilot study. Diagnostics 2022, 12, 3075. [Google Scholar] [CrossRef]
- Gazzetta Ufficiale della Repubblica Italiana. Ministero della Sanità. Comunicato. Linee di guida, in Applicazione di Quanto Previsto nel Piano Sanitario Nazionale per il Triennio 1994–1996, Relativo All’azione Programmata “Prevenzione e cura delle Malattie Oncologiche” Concernente L’organizzazione della Prevenzione e Dell’assistenza in Oncologia. (GU Serie Generale n. 42 del 20-02-1996). Available online: https://www.gazzettaufficiale.it/eli/id/1996/02/20/096A1067/sg (accessed on 22 October 2024).
- Gazzetta Ufficiale della Repubblica Italiana. Decreto del Presidente del Consiglio dei Ministri 29 Novembre 2001. Definizione dei Livelli Essenziali di Assistenza. Supplemento Ordinario alla Gazzetta Ufficiale n. 33 dell’8 Febbraio 2002—Serie Generale. Available online: https://www.gazzettaufficiale.it/eli/gu/2002/02/08/33/so/26/sg/pdf (accessed on 22 October 2024).
- Chiereghin, A.; Squillace, L.; Pizzi, L.; Bazzani, C.; Roti, L.; Mezzetti, F. Applying the healthcare failure mode and effects analysis approach to improve the quality of an organised colorectal cancer screening programme. J. Med. Screen. 2024, 31, 70–77. [Google Scholar] [CrossRef]
- Pelullo, C.P.; Cantore, F.; Lisciotto, A.; Di Giuseppe, G.; Pavia, M. Organized breast and cervical cancer screening: Attendance and determinants in southern Italy. Cancers 2021, 13, 1578. [Google Scholar] [CrossRef]
- Bechini, A.; Cosma, C.; Di Pisa, G.; Fanfani, A.; Ionita, G.; Liedl, D.; Lunetta, C.; Martorella, L.; Mele, S.; Stacchini, L.; et al. Human papilloma virus vaccination and cervical screening in the Italian regions: An overview of the current state of the art. Vaccines 2024, 12, 504. [Google Scholar] [CrossRef]
- Regione Emilia-Romagna. Atti Amministrativi. Giunta Regionale. Delibera n. 1887 del 07/11/2022. Rimodulazione del Programma di Screening Regionale del Tumore Della Cervice Uterina per le Donne Venticinquenni Vaccinate Contro L’HPV nelle Campagne Vaccinali delle Dodicenni. Available online: https://servizissiir.regione.emilia-romagna.it/deliberegiunta/servlet/AdapterHTTP?action_name=ACTIONRICERCADELIBERE&operation=downloadTesto&codProtocollo=GPG/2022/1960&ENTE=1 (accessed on 22 October 2024).
- Regione Emilia-Romagna. Assessorato Politiche per la Salute. Circolare n. 8 del 17/07/2015. Indicazioni per la Riconversione del Programma di Screening per la Prevenzione e Diagnosi Precoce dei Tumori del Collo Dell’utero con HPV DNA Test: Criteri di Ammissibilità e Modalità di Erogazione del Test. Available online: https://salute.regione.emilia-romagna.it/screening/documentazione/delibere-e-circolari/circolare-8-2015-indicazioni-per-la-riconversione-del-programma-di-screening-per-la-prevenzione-e-diagnosi-precoce-dei-tumorid-el-collo-dellutero-con-hpv-dna-test-criteri-di-ammissibilita-e-modalita-di-erogazione-del-test/@@download/file/Circolare_n_8.pdf (accessed on 22 October 2024).
- Chiereghin, A.; Squillace, L.; Pizzi, L.; Sanna, T.; Bazzani, C.; Roti, L.; Mezzetti, F. Management choices for better outcomes in oncologic screening programmes. Epidemiol. Prev. 2024, 48, 158–164. [Google Scholar]
- Chiereghin, A.; Pizzi, L.; Squillace, L.; Bazzani, C.; Roti, L.; Mezzetti, F. The positive effect of an online appointment portal on a breast cancer screening program. Appl. Clin. Inform. 2023, 14, 609–619. [Google Scholar] [CrossRef]
- Saville, M.; Hawkes, D.; Keung, M.H.T.; Ip, E.L.O.; Silvers, J.; Sultana, F.; Malloy, M.J.; Velentzis, L.S.; Canfel, K.; Wrede, C.D.; et al. Analytical performance of HPV assays on vaginal self-collected vs practitioner-collected cervical samples: The SCoPE study. J. Clin. Virol. 2020, 127, 104375. [Google Scholar] [CrossRef]
- Nayar, R.; Wilbur, D.C. The Pap test and Bethesda 2014. Cancer Cytopathol. 2015, 123, 271–281. [Google Scholar] [CrossRef]
- WHO. Classification of Tumours Editorial Board. In Female Genital Tumours: WHO Classification of Tumours, 5th ed.; IARC: Lyon, France, 2020; Volume 4. [Google Scholar]
- Ivanus, U.; Jerman, T.; Fokter, A.R.; Takac, I.; Prevodnik, V.K.; Marcec, M.; Gajsek, U.S.; Pakiz, M.; Koren, J.; Celik, S.H.; et al. Randomised trial of HPV self-sampling among non-attenders in the Slovenian cervical screening programme ZORA: Comparing three different screening approaches. Radiol. Oncol. 2018, 52, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Serrano, B.; Ibáñez, R.; Robles, C.; Peremiquel-Trillas, P.; de Sanjosé, S.; Bruni, L. Worldwide use of HPV self-sampling for cervical cancer screening. Prev. Med. 2022, 154, 106900. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Smith, S.B.; Temin, S.; Sultana, F.; Castle, P. Collaboration on self-sampling and HPV testing. Detecting cervical precancer and reaching underscreened women by using HPV testing on self samples: Updated meta-analyses. BMJ 2018, 363, k4823. [Google Scholar] [CrossRef]
- Chiereghin, A.; Pizzi, L.; Sanna, T.; Squillace, L.; Bazzani, C.; Roti, L.; Mezzetti, F. Integration of community pharmacies in an Italian colorectal cancer screening program: Insights from the Local Health Authority of Bologna. Cancer Metastasis Treat. 2024, 10, 10. [Google Scholar] [CrossRef]
- Ploysawang, P.; Pitakkarnkul, S.; Kolaka, W.; Ratanasrithong, P.; Khomphaiboonkij, U.; Tipmed, C.; Seeda, K.; Pangmuang, P.; Sangrajrang, S. Acceptability and preference for human papilloma virus self-sampling among Thai women attending National Cancer Institute. Asian Pac. J. Cancer Prev. 2023, 24, 607–612. [Google Scholar] [CrossRef]
- Darlin, L.; Borgfeldt, C.; Forslund, O.; Hénic, E.; Hortlund, M.; Dillner, J.; Kannisto, P. Comparison of use of vaginal HPV self-sampling and offering flexible appointments as strategies to reach long-term non-attending women in organized cervical screening. J. Clin. Virol. 2013, 58, 155–160. [Google Scholar] [CrossRef]
- Gibert, M.J.; Sánchez-Contador, C.; Artigues, G. Validity and acceptance of self vs conventional sampling for the analysis of human papillomavirus and Pap smear. Sci. Rep. 2023, 13, 2809. [Google Scholar]
- Aasbø, G.; Tropè, A.; Nygård, M.; Christiansen, I.K.; Baasland, I.; Iversen, G.A.; Munk, A.C.; Christiansen, M.H.; Presthus, G.K.; Undem, K.; et al. HPV self-sampling among long-term non-attenders to cervical cancer screening in Norway: A pragmatic randomised controlled trial. Br. J. Cancer 2022, 127, 1816–1826. [Google Scholar] [CrossRef]
- Presser, B.E.; Katz, M.L.; Shoben, A.B.; Moore, D.; Ruffin, M.T.; Paskett, E.D.; Reiter, P.L. Effects of an education intervention about HPV self-testing for healthcare providers and staff. J. Cancer Educ. 2018, 33, 954–959. [Google Scholar] [CrossRef]
- Winer, R.L.; Hughes, J.P.; Feng, Q.; Stern, J.E.; Xi, L.F.; Koutsky, L.A. Incident detection of high-risk human papillomavirus infections in a cohort of high-risk women aged 25–65 years. J. Infect. Dis. 2016, 214, 665–675. [Google Scholar] [CrossRef]
- Indicatori per il Monitoraggio dei Programmi di Screening con Test HPV Primario. Available online: https://www.gisci.it/documenti/documenti_gisci/HPV-indicatori-GISCi-2016.pdf (accessed on 22 October 2024).
- Gök, M.; Heideman, D.A.; van Kemenade, F.J.; de Vries, A.L.; Berkhof, J.; Rozendaal, L.; Beliën, J.A.; Overbeek, L.; Babović, M.; Snijders, P.J.; et al. Offering self-sampling for human papillomavirus testing to non-attendees of the cervical screening programme: Characteristics of the responders. Eur. J. Cancer 2012, 48, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Istituto Superiore di Sanità. Aspetti Epidemiologici Dell’infezione da HPV in Italia. Available online: https://www.epicentro.iss.it/hpv/epidemiologia-italia (accessed on 23 November 2024).
- Presidenza del Consiglio dei Ministri. Intesa tra il Governo, le Regioni e le Provincie Autonome di Trento e Bolzano Concernente “Strategie per L’offerta Attiva del Vaccino Contro L’infezione da HPV in Italia” del 20 Dicembre 2007. Available online: http://archivio.statoregioni.it/Documenti/DOC_016696_264%20csr.pdf (accessed on 23 November 2024).
- Jansen, E.E.L.; Zielonke, N.; Gini, A.; Anttila, A.; Segnan, N.; Vokó, Z.; Ivanuš, U.; McKee, M.; de Koning, H.J.; de Kok, I.M.C.M.; et al. Effect of organised cervical cancer screening on cervical cancer mortality in Europe: A systematic review. Eur. J. Cancer 2020, 127, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Eun, T.J.; Perkins, R.B. Screening for cervical cancer. Med. Clin. N. Am. 2020, 104, 1063–1078. [Google Scholar] [CrossRef]
- Fowler, J.R.; Maani, E.V.; Dunton, C.J.; Gasalberti, D.P.; Jack, B.V. Cervical Cancer. [Updated 12 November 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK431093/ (accessed on 23 November 2024).

| Variable | Number | Percentage | Average Age in Years (Standard Deviation) |
|---|---|---|---|
| Age group in year | |||
| 30–39 | 8971 | 46.4% | 34.8 (±2.8) |
| 40–49 | 5352 | 27.7% | 42.7 (±2.5) |
| 50–64 | 5004 | 25.9% | 59.4 (±3.0) |
| Nationality | |||
| Italian | 14,534 | 75.2% | 43.9 (±10.6) |
| Other | 4793 | 24.8% | 41.8 (±9.6) |
| District of residence | |||
| Pianura Ovest | 1438 | 7.4% | 41.5 (±9.0) |
| Città di Bologna | 11,186 | 57.9% | 42 (±9.9) |
| Pianura Est | 2654 | 13.7% | 42.7 (±10.0) |
| Reno, Lavino e Samoggia | 1680 | 8.7% | 49.2 (±11.3) |
| Savena Idice | 1820 | 9.4% | 45.9 (±11) |
| Appenino Bolognese | 549 | 2.8% | 52 (±10.9) |
| Time since last screening test | |||
| Never screened (no protection) | 12,280 | 63.5% | 41.4 (±9.9) |
| ≥10 years (low protection) | 3393 | 17.6% | 51.7 (±9.5) |
| <10 years (medium protection) | 3654 | 18.9% | 42.4 (±9.1) |
| Total | 19,327 | 100.0% | 43.4 (±10.4) |
| Variable | No. | Screening Participation by Using Self-Sampling Method No. (%) | p-Value | hrHPV-DNA Positivity No. (%) | p-Value | Inadequate Self-Collected Samples No. (%) | p-Value |
|---|---|---|---|---|---|---|---|
| Age group in years | |||||||
| 30–39 | 8971 | 1091 (12.2%) | p = 0.010 | 173 (15.9%) | p = 0.000 | 11 (1.0%) | p = 0.021 |
| 40–49 | 5352 | 612 (11.4%) | 74 (12.1%) | 2 (0.3%) | |||
| 50–64 | 5004 | 523 (10.54%) | 44 (8.4%) | 11 (2.1%) | |||
| Nationality | |||||||
| Italian | 14,534 | 1818 (12.5%) | p = 0.000 | 244 (13.4%) | p = 0.289 | 21 (1.2%) | p = 0.436 |
| Other | 4793 | 408 (8.5%) | 47 (11.5%) | 3 (0.7%) | |||
| District of residence | |||||||
| Pianura Ovest | 1438 | 187 (13.0%) | p = 0.009 | 27 (14.4%) | p = 0.719 | 0 (0.0%) | p = 0.590 |
| Città di Bologna | 11,186 | 1252 (11.2%) | 170 (13.6%) | 13 (1.0%) | |||
| Pianura Est | 2654 | 349 (13.2%) | 47 (13.5%) | 6 (1.7%) | |||
| Reno, Lavino e Samoggia | 1680 | 172 (10.2%) | 17 (9.9%) | 2 (1.2%) | |||
| Savena Idice | 1820 | 212 (11.7%) | 24 (11.3%) | 2 (0.9%) | |||
| Appenino Bolognese | 549 | 54 (9.8%) | 6 (11.1%) | 1 (1.9%) | |||
| Time since last screening test | |||||||
| Never screened (no protection) | 12,280 | 1194 (9.7%) | p = 0.000 | 176 (14.7%) | p = 0.027 | 15 (1.3%) | p = 0.309 |
| ≥10 years (low protection) | 3393 | 460 (13.6%) | 56 (12.2%) | 2 (0.4%) | |||
| <10 years (medium protection) | 3654 | 572 (15.7%) | 59 (10.3%) | 7 (1.2%) | |||
| Total | 19,327 | 2226 (11.5%) | 291 (13.1%) | 24 (1.1%) |
| Variable | Odds Ratio | Standard Error | 95% CI | p-Value |
|---|---|---|---|---|
| Log likelihood = −6799.80, χ2 = 207.66 (10df), p < 0.00001, No. of obs = 19,327 | ||||
| Age group in years | ||||
| 30–39 | 1.00 * | |||
| 40–49 | 0.84 | 0.05 | 0.76–0.94 | 0.002 |
| 50–64 | 0.72 | 0.05 | 0.64–0.82 | <0.001 |
| Nationality | ||||
| Italian | 1.00 * | |||
| Other | 0.64 | 0.04 | 0.57–0.72 | <0.001 |
| District of residence | ||||
| Città di Bologna | 1.00 * | |||
| Pianura Ovest | 1.07 | 0.09 | 0.91–1.26 | 0.424 |
| Pianura Est | 1.12 | 0.07 | 0.99–1.28 | 0.078 |
| Reno, Lavino e Samoggia | 0.92 | 0.08 | 0.78–1.09 | 0.324 |
| Savena Idice | 1.01 | 0.08 | 0.87–1.19 | 0.849 |
| Appenino Bolognese | 0.87 | 0.13 | 0.65–1.16 | 0.340 |
| Time since last screening test | ||||
| Never screened (no protection) | 1.00 * | |||
| ≥10 years (low protection) | 1.61 | 0.10 | 1.42–1.82 | <0.001 |
| <10 years (medium protection) | 1.74 | 0.10 | 1.56–1.95 | <0.001 |
| HPV Genotype | Number of HPV Genotype (%) | YES HPV Vaccine | Total Vaccine YES (%) | |||
|---|---|---|---|---|---|---|
| NO HPV Vaccine (%) | Bivalent Against HPV 16, 18 | Tetravalent Against HPV 16, 18, 6, 11 | Nonavalent Against HPV 16, 18, 6, 11, 31, 33, 45, 52, 58 | |||
| HPV 16 | 50 (17.2) | 48 (17.2) | 0 | 1 | 1 | 2 (16.7) |
| HPV 16 and 18 | 3 (1.0) | 3 (1.1) | 0 | 0 | 0 | 0 (0.0) |
| HPV 16 and 18 and OHR * | 2 (0.7) | 2 (0.7) | 0 | 0 | 0 | 0 (0.0) |
| HPV 16 and OHR | 21 (7.2) | 20 (7.2) | 0 | 0 | 1 | 1 (8.3) |
| HPV 18 | 17 (5.8) | 16 (5.7) | 0 | 1 | 0 | 1 (8.3) |
| HPV 18 and OHR | 7 (2.4) | 7 (2.5) | 0 | 0 | 0 | 0 (0.0) |
| OHR HPV | 191 (65.7) | 183 (65.6) | 1 | 2 | 5 | 8 (66.7) |
| Total | 291 | 279 (95.9%) | 1 | 4 | 7 | 12 (4.1%) |
| (a) Immediate Referral to Colposcopy | |||||||
| Time Since Last Screening Test | No. of Colposcopies | No. of Biopsies | CIN 2 * | CIN 3 # | Cervical Adenocarcinoma ¥ | CIN2+ | PPV |
| Never-screened women (no protection) ≥10 years (low protection) | 95 | 11 | 5 | 3 | 1 | 9 | 9.5% |
| <10 years (medium protection) | 23 | 48 | 3 | 1 | 0 | 4 | 17.4% |
| Total | 118 | 59 (50.0%) | 8 | 4 | 1 | 13 | 11.0% |
| (b) Cytology Triage on Clinician-Sample | |||||||
| Time Since Last Screening Test | No. of Colposcopies | No. of Biopsies | CIN 2 * | CIN 3 # | Cervical Adenocarcinoma ¥ | CIN2+ | PPV |
| Never-screened women (no protection) ≥10 years (low protection) | 28 | 4 | 4 | 1 | 1 | 6 | 21.4% |
| <10 years (medium protection) | 9 | 10 | 0 | 0 | 0 | 0 | 0.0% |
| Total | 37 | 14 (37.8%) | 4 | 1 | 1 | 6 | 16.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiereghin, A.; Pizzi, L.; Buriani, C.; Sanna, T.; Amico, A.; Squillace, L.; Molinari, E.; Florean, M.S.; Lanza, G.; Mezzetti, F. Addressing COVID-19 Screening Delays: The Impact of HPV Self-Sampling on Non-Attenders in a Cervical Cancer Screening Program. Cancers 2024, 16, 4071. https://doi.org/10.3390/cancers16234071
Chiereghin A, Pizzi L, Buriani C, Sanna T, Amico A, Squillace L, Molinari E, Florean MS, Lanza G, Mezzetti F. Addressing COVID-19 Screening Delays: The Impact of HPV Self-Sampling on Non-Attenders in a Cervical Cancer Screening Program. Cancers. 2024; 16(23):4071. https://doi.org/10.3390/cancers16234071
Chicago/Turabian StyleChiereghin, Angela, Lorenzo Pizzi, Carolina Buriani, Tiziana Sanna, Andrea Amico, Lorena Squillace, Elena Molinari, Maria Siponta Florean, Giovanni Lanza, and Francesca Mezzetti. 2024. "Addressing COVID-19 Screening Delays: The Impact of HPV Self-Sampling on Non-Attenders in a Cervical Cancer Screening Program" Cancers 16, no. 23: 4071. https://doi.org/10.3390/cancers16234071
APA StyleChiereghin, A., Pizzi, L., Buriani, C., Sanna, T., Amico, A., Squillace, L., Molinari, E., Florean, M. S., Lanza, G., & Mezzetti, F. (2024). Addressing COVID-19 Screening Delays: The Impact of HPV Self-Sampling on Non-Attenders in a Cervical Cancer Screening Program. Cancers, 16(23), 4071. https://doi.org/10.3390/cancers16234071








