The Novel HDAC Inhibitor OBP-801 Promotes MHC Class I Presentation Through LMP2 Upregulation, Enhancing the PD-1-Targeting Therapy in Clear Cell Renal Cell Carcinoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Clinically Available Transcriptomes and Tumor Immune Microenvironment
2.2. Cell Lines and Reagents
2.3. Western Blot Analysis
2.4. Reverse Transcription-Quantitative PCR (RT-qPCR) Analysis
2.5. Analysis for the Cell Surface MHC Class I Expression
2.6. Transfection Experiments
2.7. Cell-Line-Derived Syngraft Mouse Study
2.8. Western Blot and Flow Cytometry Analyses of Tumor Samples
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results
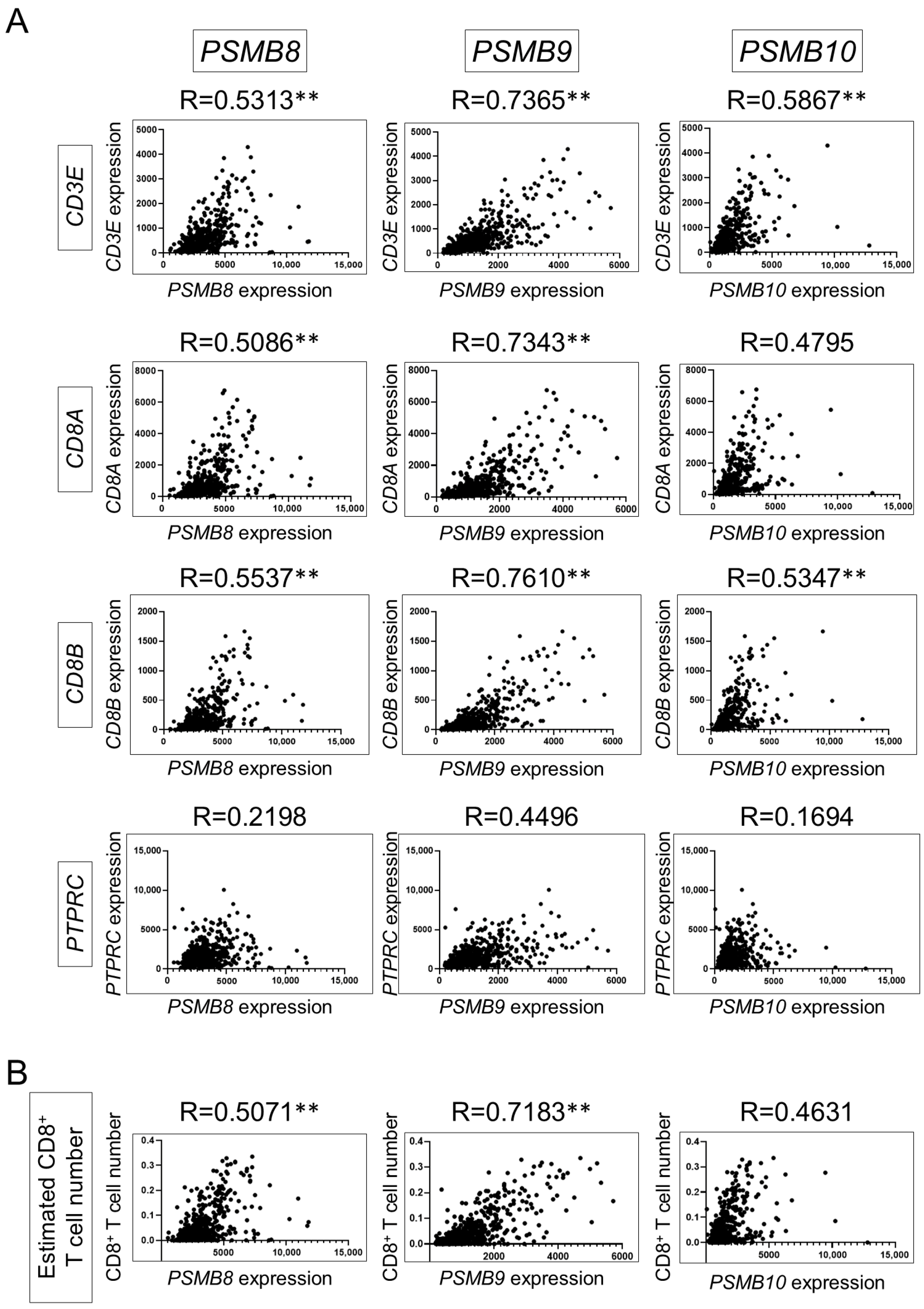
3.1. Gene Expression of Immunoproteasome Subunits Is Associated with Tumor-Infiltrating CD8+ T Cells
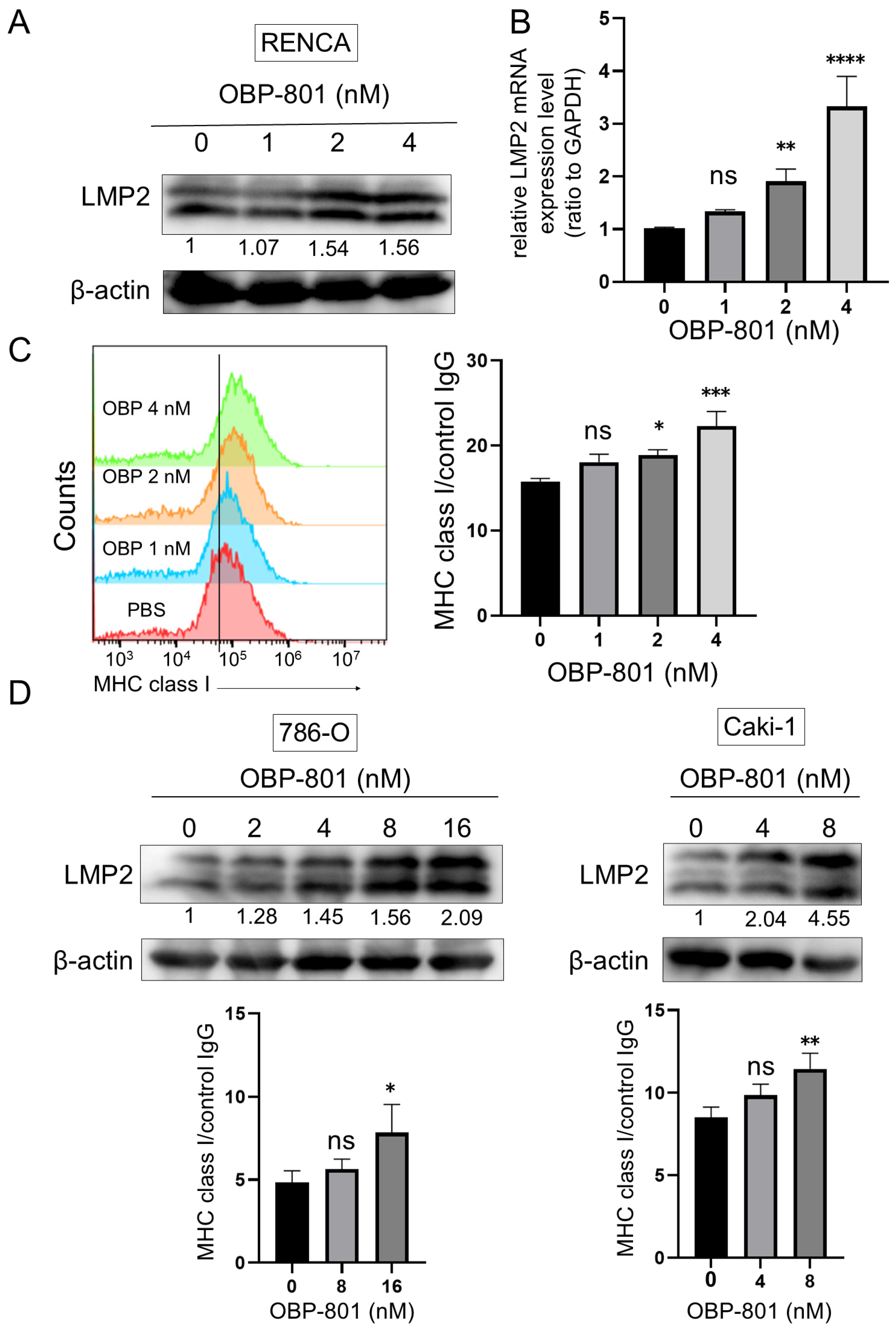
3.2. Novel HDAC Inhibitor OBP-801 Upregulates LMP2 Expression and MHC Class I Presentation in ccRCC Cell Lines
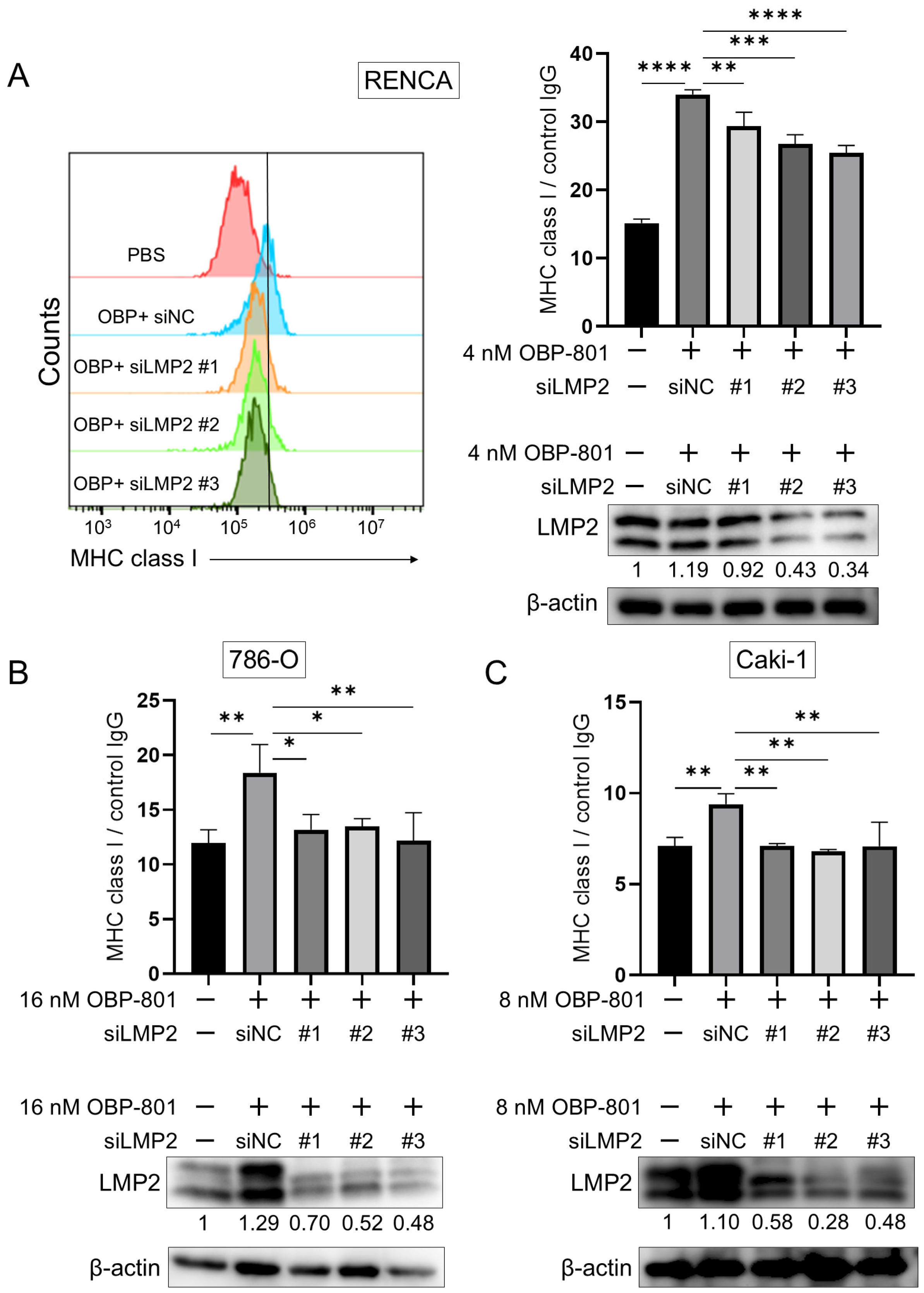
3.3. Knockdown of LMP2 Gene Downregulates MHC Class I Presentation in ccRCC Cell Lines
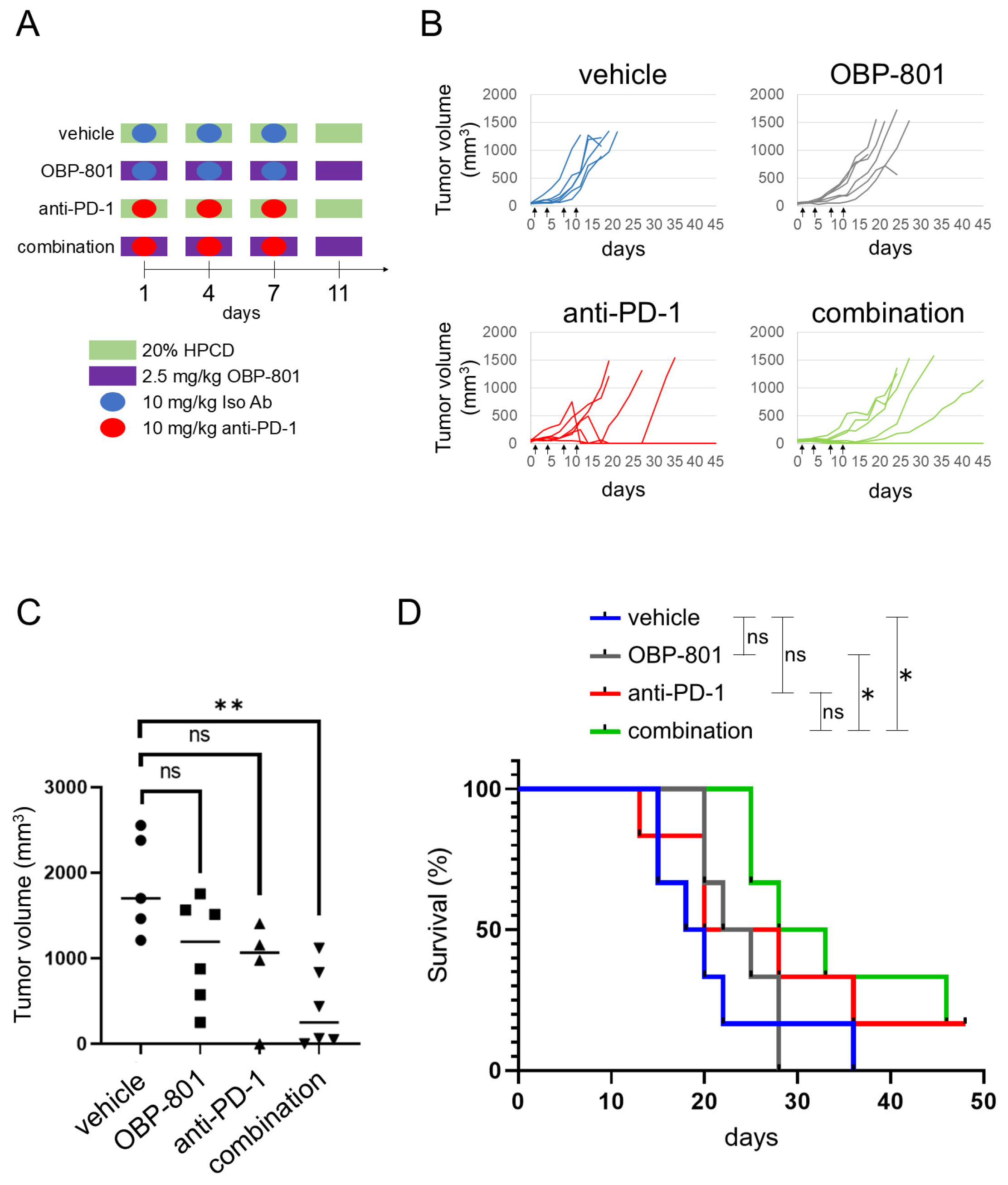
3.4. OBP-801 Enhances the Anti-Tumor Activity of PD-1 Inhibition in RENCA Allograft Model
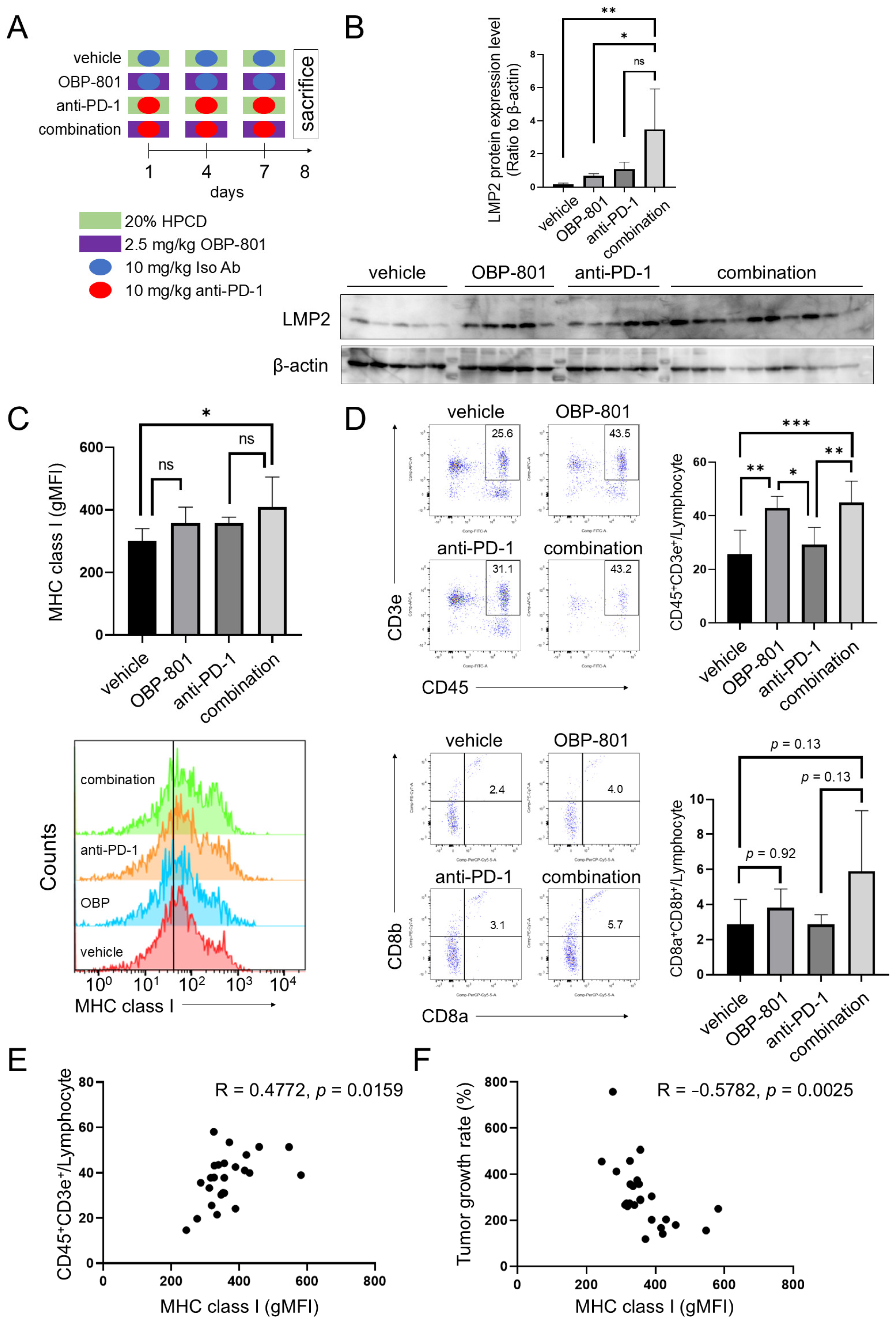
3.5. Combination of OBP-801 and Anti-PD-1 Antibody Treatment Upregulates MHC Class I Expression on Tumor Cells and Is Correlated with Intratumoral T Cell Abundance with a Reduction in Tumor Growth
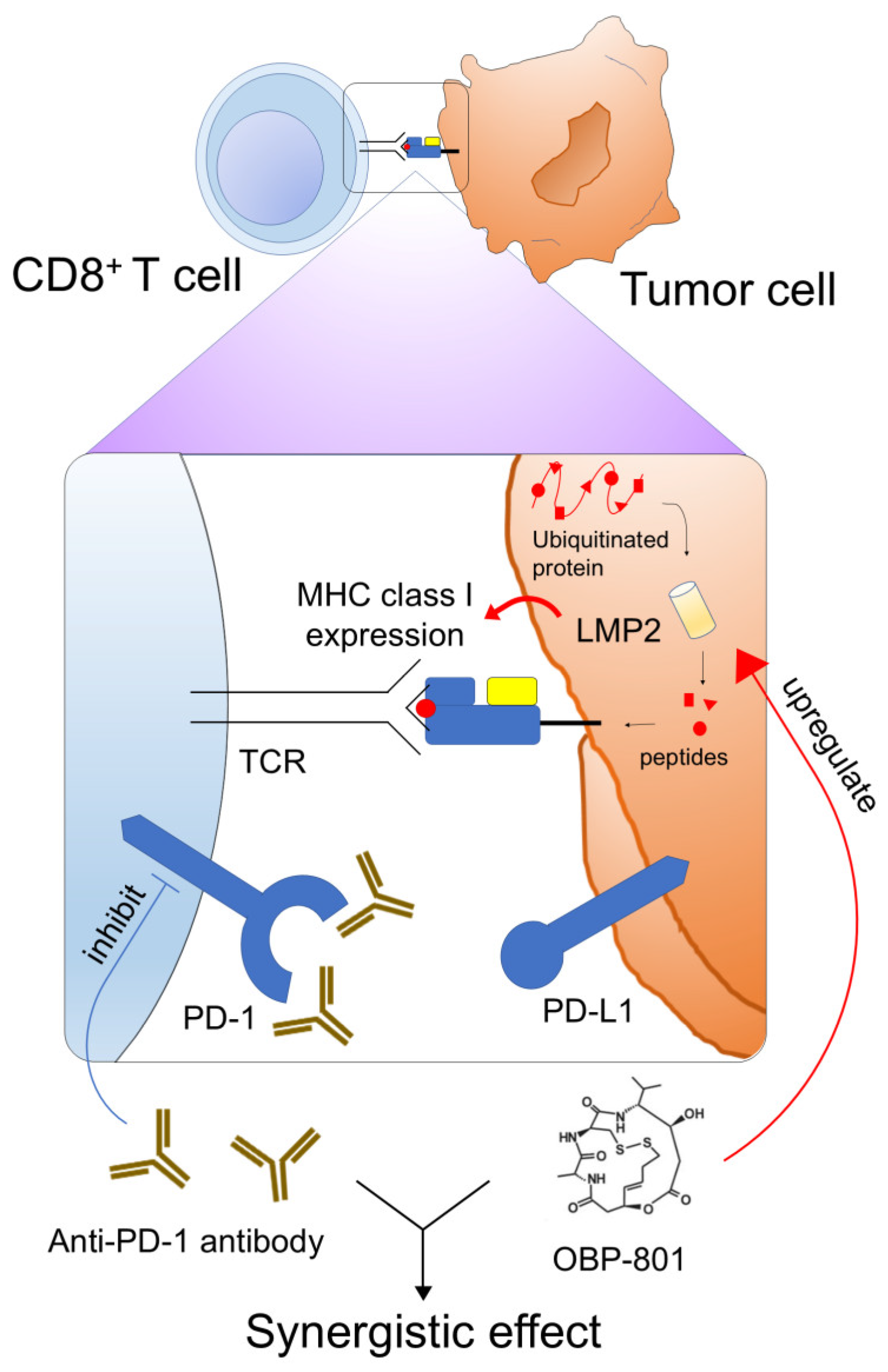
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Drake, C.G.; Sznol, M.; Choueiri, T.K.; Powderly, J.D.; Smith, D.C.; Brahmer, J.R.; Carvajal, R.D.; Hammers, H.J.; Puzanov, I.; et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J. Clin. Oncol. 2015, 33, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, Y.; Ohta, S.; Sayem, M.A.; Tsukamoto, N.; Yaguchi, T. Immune-resistant mechanisms in cancer immunotherapy. Int. J. Clin. Oncol. 2020, 25, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Sah, V.R.; Karlsson, J.; Jespersen, H.; Lindberg, M.F.; Nilsson, L.M.; Ny, L.; Nilsson, J.A. Epigenetic therapy to enhance therapeutic effects of PD-1 inhibition in therapy-resistant melanoma. Melanoma Res. 2022, 32, 241–248. [Google Scholar] [CrossRef]
- Yan, W.; Qiu, L.; Yang, M.; Xu, A.; Ma, M.; Yuan, Q.; Ma, X.; Liang, W.; Li, X.; Lu, Y. CXCL10 mediates CD8+ T cells to facilitate vessel normalization and improve the efficacy of cetuximab combined with PD-1 checkpoint inhibitors in colorectal cancer. Cancer Lett. 2023, 567, 216263. [Google Scholar] [CrossRef]
- Zarrabi, K.K.; Lanade, O.; Geynisman, D.M. Determining front-line therapeutic strategy for metastatic clear cell renal cell carcinoma. Cancers 2022, 14, 4607. [Google Scholar] [CrossRef]
- Leone, P.; Shin, E.C.; Perosa, F.; Vacca, A.; Dammacco, F.; Racanelli, V. MHC class I antigen processing and presenting machinery: Organization, function, and defects in tumor cells. J. Natl. Cancer Inst. 2013, 105, 1172–1187. [Google Scholar] [CrossRef]
- Manning, J.; Indrova, M.; Lubyova, B.; Pribylova, H.; Bieblova, J.; Hejnar, J.; Simova, J.; Jandlova, T.; Bubenik, J.; Reinis, M. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology 2008, 123, 218–227. [Google Scholar] [CrossRef]
- Dhatchinamoorthy, K.; Colbert, J.D.; Rock, K.L. Cancer immune evasion through loss of MHC class I antigen presentation. Front. Immunol. 2021, 12, 636568. [Google Scholar] [CrossRef]
- Seliger, B.; Atkins, D.; Bock, M.; Ritz, U.; Ferrone, S.; Huber, C.; Storkel, S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transporter-associated with antigen-processing down-regulation. Clin. Cancer Res. 2003, 9, 1721–1727. [Google Scholar]
- Madhusoodanan, M.; Shilong, Z.; Tien, L.H.; Guangdi, W. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules 2015, 20, 3898–3941. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.J.; Doss, S.; Hay, N.; Sutcliffe, F.; Stevens, A. NICE guidance on panobinostat for patients with multiple myeloma after at least two previous treatments. Lancet Oncol. 2016, 17, 279–280. [Google Scholar] [CrossRef]
- Coiffier, B.; Pro, B.; Prince, H.M.; Foss, F.; Sokol, L.; Greenwood, M.; Caballero, D.; Morschhauser, F.; Wilhelm, M.; Pinter-Brown, L.; et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: Pivotal study update demonstrates durable responses. J. Hematol. Oncol. 2014, 7, 11. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone deacetylase inhibitors as anticancer drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed]
- Magner, W.J.; Kazim, A.L.; Stewart, C.; Romano, M.A.; Catalano, G.; Grande, C.; Keiser, N.; Santaniello, F.; Tomasi, T.B. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J. Immun. 2000, 165, 7017–7024. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Gregorie, C.J.; Tomasi, T.B. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol. Immunother. 2008, 57, 647–654. [Google Scholar] [CrossRef]
- Van Kaer, L.; Ashton-Rickardt, P.G.; Eichelberger, M.; Gaczynska, M.; Nagashima, K.; Rock, K.L.; Goldberg, A.L.; Doherty, P.C.; Tonegawa, S. Altered peptidase and viral-specific T cell response in LMP2 mutant mice. Immunity 1994, 1, 533–541. [Google Scholar] [CrossRef]
- Leister, H.; Luu, M.; Staudenraus, D.; Lopez Krol, A.; Mollenkopf, H.J.; Sharma, A.; Schmerer, N.; Schulte, L.N.; Bertrams, W.; Schmerck, B.; et al. Pro- and antitumorigenic capacity of immunoproteasomes in shaping the tumor microenvironment. Cancer Immunol. Res. 2021, 9, 682–692. [Google Scholar] [CrossRef]
- Shindoh, N.; Mori, M.; Terada, Y.; Oda, K.; Amino, N.; Kita, A.; Taniguchi, M.; Sohda, K.; Nagai, K.; Sowa, Y.; et al. YM753, a novel histone deacetylase inhibitor, exhibits antitumor activity with selective, sustained accumulation of acetylated histones in tumors in the WiDr xenograft model. Int. J. Oncol. 2008, 32, 545–555. [Google Scholar] [CrossRef][Green Version]
- Kawarazaki, A.; Horinaka, M.; Yasuda, S.; Kawashima, H.; Numajiri, T.; Sakai, T. The HDAC inhibitor OBP-801 suppresses the growth of myxofibrosarcoma cells. J. Buon 2020, 25, 464–471. [Google Scholar]
- Sugimoto, Y.; Katsumi, Y.; Iehara, T.; Kaneda, D.; Tomoyasu, C.; Ouchi, K.; Yoshida, H.; Miyachi, M.; Yagyu, S.; Kikuchi, K.; et al. The novel histone deacetylase inhibitor, OBP-801, induces apoptosis in rhabdoid tumors by releasing the silencing of NOXA. Mol. Cancer Ther. 2020, 19, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, D.; Iehara, T.; Kikuchi, K.; Sugimoto, Y.; Nakagawa, N.; Yagyu, S.; Miyachi, M.; Konishi, E.; Sakai, T.; Hosoi, H. The histone deacetylase inhibitor OBP-801 has in vitro/in vivo anti-neuroblastoma activity. Pediatr. Int. 2022, 64, e15159. [Google Scholar] [CrossRef] [PubMed]
- Heath, E.I.; Weise, A.; Vaishampayan, U.; Danforth, D.; Ungerleider, R.S.; Urata, Y. Phase Ia dose escalation study of OBP-801, a cyclic depsipeptide class I histone deacetylase inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2022, 40, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Vigneron, N.; Abi Habib, J.; Van den Eynde, B.J. Learning from the proteasome how to fine-tune cancer immunotherapy. Trends Cancer 2017, 3, 726–741. [Google Scholar] [CrossRef]
- Groettrup, M.; Kirk, C.J.; Basler, M. Proteasomes in immune cells: More than peptide producers? Nat. Rev. Immunol. 2010, 10, 73–78. [Google Scholar] [CrossRef]
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef]
- Geynisman, D.M.; Maranchie, J.K.; Ball, M.W.; Bratslavsky, G.; Singer, E.A. A 25 year perspective on the evolution and advances in an understanding of the biology, evaluation and treatment of kidney cancer. Urol. Oncol. 2021, 39, 548–560. [Google Scholar] [CrossRef]
- Lombardi, P.; Filetti, M.; Falcone, R.; Di Bidino, R.; Iacovelli, R.; Ciccarese, C.; Bria, E.; Tortora, G.; Scambia, G.; Daniele, G. New first-line immunotherapy-based combinations for metastatic renal cell carcinoma: A systematic review and network meta-analysis. Cancer Treat. Rev. 2022, 106, 102377. [Google Scholar] [CrossRef]
- Bedke, J.; Albiges, L.; Capitanio, U.; Giles, R.H.; Hora, M.; Ljungberg, B.; Marconi, L.; Klatte, T.; Volpe, A.; Abu-Ghanem, Y.; et al. The 2022 updated European Association of Urology guidelines on the use of adjuvant Immune checkpoint inhibitor therapy for renal cell carcinoma. Eur. Urol. 2023, 83, 10–14. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthelemy, P.; Porta, C.; George, S.; et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulieres, D.; Melichar, B.; et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juarez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grunwald, V.; Huston, T.E.; Kopyltsov, E.; Mendez-Vidal, M.; et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Zaretsky, J.M.; Garcia-Diaz, A.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016, 375, 819–829. [Google Scholar] [CrossRef]
- Setiadi, A.F.; Omilusik, K.; David, M.D.; Seipp, R.P.; Hartikainen, J.; Gopaul, R.; Choi, K.B.; Jefferies, W.A. Epigenetic enhancement of antigen processing and presentation promotes immune recognition of tumors. Cancer Res. 2008, 68, 9601–9607. [Google Scholar] [CrossRef]
- Briere, D.; Sudhakar, N.; Woods, D.M.; Hallin, J.; Engstrom, L.D.; Aranda, R.; Chiang, H.; Sodre, A.L.; Olson, P.; Weber, J.S.; et al. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol. Immunother. 2018, 67, 381–392. [Google Scholar] [CrossRef]
- Bretz, A.C.; Parnitzke, U.; Kronthaler, K.; Dreker, T.; Bartz, R.; Hermann, F.; Ammendola, A.; Wulff, T.; Hamm, S. Domatinostat favors the immunotherapy response by modulating the tumor immune microenvironment (TIME). J. Immunother. Cancer 2019, 7, 294. [Google Scholar] [CrossRef]
- Llopiz, D.; Ruiz, M.; Villanueva, L.; Iglesias, T.; Silva, L.; Egea, J.; Lasarte, J.J.; Pivette, P.; Trochon-Joseph, V.; Vasseur, B.; et al. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol. Immunother. 2019, 68, 379–393. [Google Scholar] [CrossRef]
- Ugurel, S.; Spassova, I.; Wohlfarth, J.; Drusio, C.; Cherouny, A.; Melior, A.; Sucker, A.; Zimmer, L.; Ritter, C.; Schadendorf, D.; et al. MHC class-I downregulation in PD-1/PD-L1 inhibitor refractory Merkel cell carcinoma and its potential reversal by histone deacetylase inhibition: A case series. Cancer Immunol. Immunother. 2019, 68, 983–990. [Google Scholar] [CrossRef]
- Chen, X.; Pan, X.; Zhang, W.; Guo, H.; Cheng, S.; He, Q.; Yang, B.; Ding, L. Epigenetic strategies synergize with PD-L1/PD-1 targeted cancer immunotherapies to enhance antitumor responses. Acta Pharm. Sin. B 2020, 10, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Bissonnette, R.P.; Cesario, R.M.; Goodenow, B.; Shojaei, F.; Gillings, M. The epigenetic immunomodulator, HBI-8000, enhances the response and reverses resistance to checkpoint inhibitors. BMC Cancer 2021, 21, 969. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Hou, L.; Wang, S.; Yu, Y.; Zhang, Y.; Sun, L.; Wang, C.; Ma, Z.; Yang, F. Lysosome activable polymeric vorinostat encapsulating PD-L1KD for a combination of HDACi and immunotherapy. Drug Deliv. 2021, 28, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fu, Y.; Zhang, X.; Zhao, G.; Yao, Y.; Guo, Y.; Ma, G.; Bai, S.; Li, H. Romidepsin (FK228) regulates the expression of the immune checkpoint ligand PD-L1 and suppresses cellular immune functions in colon cancer. Cancer Immunol. Immunother. 2021, 70, 61–73. [Google Scholar] [CrossRef]
- Hayashi, T.; Horiuchi, A.; Sano, K.; Hiraoka, N.; Kasai, M.; Ichimura, T.; Sudo, T.; Tagawa, Y.; Nishimura, R.; Ishiko, O.; et al. Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy. Sci. Rep. 2011, 1, 180. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J. Systemic therapy for metastatic renal-cell carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Mie Lee, Y.; Kim, S.H.; Kim, H.S.; Jin Son, M.; Nakajima, H.; Jeong Kwon, H.; Kim, K.W. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1α activity. Biochem. Biophys. Res. Commun. 2003, 300, 241–246. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Ku, S.; Ciamporcero, E.; Miles, K.M.; Attwood, K.; Chintala, S.; Shen, L.; Ellis, L.; Sotomayor, P.; Swetzig, W.; et al. HDAC 1 and 6 modulate cell invasion and migration in clear cell renal cell carcinoma. BMC Cancer 2016, 16, 617. [Google Scholar] [CrossRef]
- Hu, X.; Lin, Z.; Wang, Z.; Zhou, Q. Emerging role of PD-L1 modification in cancer immunotherapy. Am. J. Cancer Res. 2021, 11, 3832–3840. [Google Scholar]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narukawa, T.; Yasuda, S.; Horinaka, M.; Taniguchi, K.; Tsujikawa, T.; Morita, M.; Ukimura, O.; Sakai, T. The Novel HDAC Inhibitor OBP-801 Promotes MHC Class I Presentation Through LMP2 Upregulation, Enhancing the PD-1-Targeting Therapy in Clear Cell Renal Cell Carcinoma. Cancers 2024, 16, 4058. https://doi.org/10.3390/cancers16234058
Narukawa T, Yasuda S, Horinaka M, Taniguchi K, Tsujikawa T, Morita M, Ukimura O, Sakai T. The Novel HDAC Inhibitor OBP-801 Promotes MHC Class I Presentation Through LMP2 Upregulation, Enhancing the PD-1-Targeting Therapy in Clear Cell Renal Cell Carcinoma. Cancers. 2024; 16(23):4058. https://doi.org/10.3390/cancers16234058
Chicago/Turabian StyleNarukawa, Tsukasa, Shusuke Yasuda, Mano Horinaka, Keiko Taniguchi, Takahiro Tsujikawa, Mie Morita, Osamu Ukimura, and Toshiyuki Sakai. 2024. "The Novel HDAC Inhibitor OBP-801 Promotes MHC Class I Presentation Through LMP2 Upregulation, Enhancing the PD-1-Targeting Therapy in Clear Cell Renal Cell Carcinoma" Cancers 16, no. 23: 4058. https://doi.org/10.3390/cancers16234058
APA StyleNarukawa, T., Yasuda, S., Horinaka, M., Taniguchi, K., Tsujikawa, T., Morita, M., Ukimura, O., & Sakai, T. (2024). The Novel HDAC Inhibitor OBP-801 Promotes MHC Class I Presentation Through LMP2 Upregulation, Enhancing the PD-1-Targeting Therapy in Clear Cell Renal Cell Carcinoma. Cancers, 16(23), 4058. https://doi.org/10.3390/cancers16234058





