Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
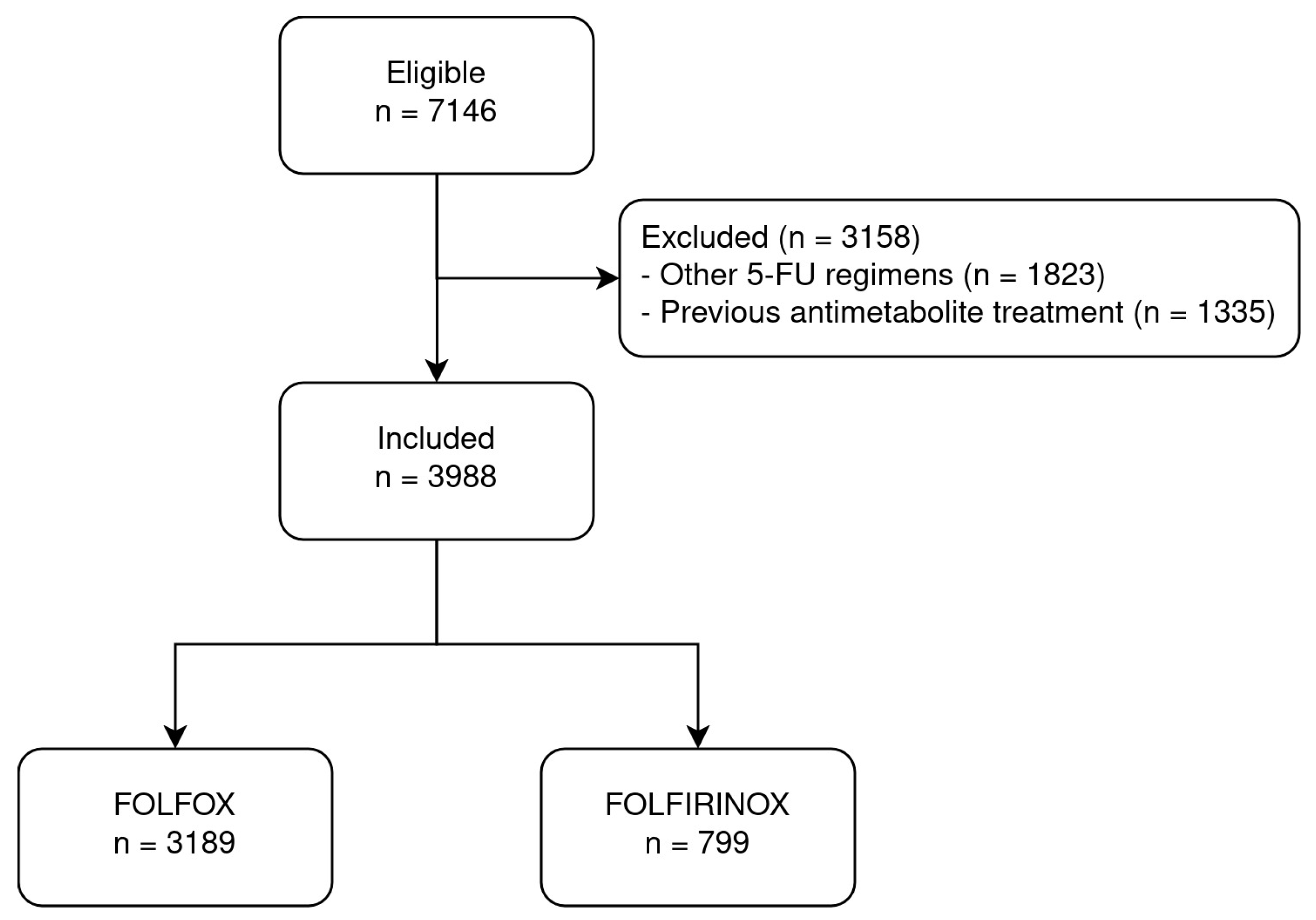
2.2. Patient Population
2.3. Explanatory Variable
2.4. Endpoints
2.5. Diagnosis, Baseline Characteristics, and Potential Confounders
2.6. Power Calculation
2.7. Statistical Methods
3. Results
3.1. Descriptive Statistics
3.2. Primary Outcome
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dean, L.; Kane, M. Fluorouracil Therapy and DPYD Genotype. In Medical Genetics Summaries; Pratt, V.M., Scott, S.A., Pirmohamed, M., Esquivel, B., Kattman, B.L., Malheiro, A.J., Eds.; Bethesda: Rockville, MD, USA, 2012. [Google Scholar]
- Kobuchi, S.; Ito, Y. Application of Pharmacometrics of 5-Fluorouracil to Personalized Medicine: A Tool for Predicting Pharma-cokinetic-Pharmacodynamic/Toxicodynamic Responses. Anticancer Res. 2020, 40, 6585–6597. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy for advanced biliary tract cancer—Authors’ reply. Lancet Oncol. 2021, 22, e288–e289. [Google Scholar] [CrossRef] [PubMed]
- Batra, S.; Jha, S.K.; Bahl, A.; Chartuvedi, H.; Taneja, S. Outcome of FOLFOX based chemotherapy in advanced pancreaticobiliary cancers presenting with hyperbilirubinemia. J. Clin. Oncol. 2020, 38 (Suppl. 15), e16605. [Google Scholar] [CrossRef]
- Guion-Dusserre, J.-F.; Bertaut, A.; Ghiringhelli, F.; Vincent, J.; Quipourt, V.; Marilier, S.; Tharin, Z.; Bengrine-Lefevre, L. Folfirinox in elderly patients with pancreatic or colorectal cancer-tolerance and efficacy. World J. Gastroenterol. 2016, 22, 9378–9386. [Google Scholar] [CrossRef]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef]
- Conroy, T.; Bosset, J.-F.; Etienne, P.-L.; Rio, E.; François, E.; Mesgouez-Nebout, N.; Vendrely, V.; Artignan, X.; Bouché, O.; Gargot, D.; et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 702–715. [Google Scholar] [CrossRef]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Abdelghani, M.B.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- More, L.A.; Lane, S.; Asnani, A. 5-FU Cardiotoxicity: Vasospasm, Myocarditis, and Sudden Death. Curr. Cardiol. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef]
- Raber, I.; Frazer, M.B.; Zerillo, J.A.; Asnani, A. Uridine Triacetate for Severe Fluoropyrimidine Cardiotoxicity in a Patient with Thymidylate Synthase Gene Variants. JACC CardioOncol. 2020, 2, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, W.; Takahashi, T.; Suto, K.; Sasaki, Y.; Hirayama, R. Orotate Phosphoribosyltransferase Gene Polymorphism Predicts Toxicity in Patients Treated with Bolus 5-Fluorouracil Regimen. Clin. Cancer Res. 2006, 12, 3928–3934. [Google Scholar] [CrossRef] [PubMed]
- Henricks, L.; Lunenburg, C.A.T.C.; de Man, F.; Meulendijks, D.; Frederix, G.W.J.; Kienhuis, E.; Creemers, G.-J.; Baars, A.; Dezentjé, V.O.; Imholz, A.L.T.; et al. DPYD genotype-guided dose individualisation of fluoropyrimidine therapy in patients with cancer: A prospective safety analysis. Lancet Oncol. 2018, 19, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- De Mattia, E.; Polesel, J.; Silvestri, M.; Roncato, R.; Scarabel, L.; Calza, S.; Spina, M.; Puglisi, F.; Toffoli, G.; Cecchin, E. The burden of rare variants in DPYS gene is a novel predictor of the risk of developing severe fluoropyrimidine-related toxicity. Hum. Genom. 2023, 17, 99. [Google Scholar] [CrossRef]
- Tsalic, M.; Bar-Sela, G.; Beny, A.; Visel, B.; Haim, N. Severe toxicity related to the 5-fluorouracil/leucovorin combination (the Mayo Clinic regimen): A prospective study in colorectal cancer patients. Am. J. Clin. Oncol. 2003, 26, 103–106. [Google Scholar] [CrossRef]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [CrossRef]
- Ison, G.; Beaver, J.A.; McGuinn, W.D., Jr.; Palmby, T.R.; Dinin, J.; Charlab, R.; Marathe, A.; Jin, R.; Liu, Q.; Chen, X.H.; et al. FDA Approval: Uridine Triacetate for the Treatment of Patients Following Fluor-ouracil or Capecitabine Overdose or Exhibiting Early-Onset Severe Toxicities Following Administration of These Drugs. Clin. Cancer Res. 2016, 22, 4545–4549. [Google Scholar] [CrossRef]
- Brutcher, E.; Christensen, D.; Smith, M.H.; Koutlas, J.B.; Sellers, J.B.; Timmons, T.; Thompson, J. 5-Fluorouracil and Capecitabine: Assessment and Treatment of Un-common Early-Onset Severe Toxicities Associated with Administration. Clin. J. Oncol. Nurs. 2018, 22, 627–634. [Google Scholar]
- Melas, M.; Subbiah, S.; Saadat, S.; Rajurkar, S.; McDonnell, K.J. The Community Oncology and Academic Medical Center Alliance in the Age of Precision Medicine: Cancer Genetics and Genomics Considerations. J. Clin. Med. 2020, 9, 2125. [Google Scholar] [CrossRef]
- Boyle, J.M.; Kuryba, A.; Cowling, T.E.; van der Meulen, J.; Fearnhead, N.S.; Walker, K.; Braun, M.S.; Aggarwal, A. Survival outcomes associated with completion of adjuvant oxaliplatin-based chemo-therapy for stage III colon cancer: A national population-based study. Int. J. Cancer 2022, 150, 335–346. [Google Scholar] [CrossRef]
- Shin, P.; Sharac, J.; Rosenbaum, S. Community Health Centers and Medicaid at 50: An Enduring Relationship Essential for Health System Transformation. Health Aff. 2015, 34, 1096–1104. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.J.; Lin, J.K.; Chen, W.S.; Jiang, J.K.; Teng, H.W.; Yen, C.C.; Lin, T.C.; Yang, S.H. Adjuvant FOLFOX treatment for stage III colon cancer: How many cycles are enough? SpringerPlus 2016, 5, 1318. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 1982, 69, 239–241. [Google Scholar] [CrossRef]
- R: A Language and Environment for Statistical Computing [Computer Program]; Version 4.4.0; R Foundation for Statistical Computing: Vienna, Austria, 2024.
- Diasio, R.B.; Offer, S.M. Testing for Dihydropyrimidine Dehydrogenase Deficiency to Individualize 5-Fluorouracil Therapy. Cancers 2022, 14, 3207. [Google Scholar] [CrossRef]
- Lunenburg, C.A.; van Staveren, M.C.; Gelderblom, H.; Guchelaar, H.-J.; Swen, J.J. Evaluation of Clinical Implementation of Prospective DPYD Genotyping in 5-Fluorouracil- or Capecitabine-Treated Patients. Pharmacogenomics 2016, 17, 721–729. [Google Scholar] [CrossRef]
- Chan, T.H.; Zhang, J.E.; Pirmohamed, M. DPYD genetic polymorphisms in non-European patients with severe fluoropyrimi-dine-related toxicity: A systematic review. Br. J. Cancer 2024, 131, 498–514. [Google Scholar] [CrossRef]

| Total Patients (N = 3988) | With Early-Onset Toxicity (N = 763) | Without Early-Onset Toxicity (N = 3225) | |
|---|---|---|---|
| Age, years (interquartile range) | 62.9 (54.7–70.4) | 61.9 (54.5–69.4) | 63.1 (54.7–70.6) |
| Male, n (%) | 2300 (57.7) | 386 (50.6) | 1914 (59.3) |
| Cancer diagnosis, n (%) | |||
| Colon-rectum | 2497 (63.2) | 415 (54.8) | 2082 (65.2) |
| Pancreas | 789 (20.0) | 201 (26.6) | 588 (18.4) |
| Stomach | 248 (6.3) | 56 (7.4) | 192 (6.0) |
| Esophagus | 241 (6.1) | 44 (5.8) | 197 (6.2) |
| Biliary tract | 101 (2.6) | 20 (2.6) | 81 (2.5) |
| Small intestine | 65 (1.6) | 15 (2.0) | 50 (1.6) |
| Other | 8 (0.2) | 6 (0.8) | 2 (0.1) |
| Treatment regimen, n (%) | |||
| FOLFOX | 3189 (80.0) | 559 (73.3) | 2630 (81.6) |
| FOLFIRINOX | 799 (20.0) | 204 (26.7) | 595 (18.4) |
| Bevacizumab, n (%) | 541 (13.6) | 80 (10.5) | 461 (14.3) |
| EGFR inhibitors, n (%) | 37 (0.9) | 5 (0.7) | 32 (1.0) |
| Immune checkpoint inhibitors, n (%) | 83 (2.1) | 12 (1.6) | 71 (2.2) |
| Hypertension, n (%) | 1616 (40.5) | 330 (43.3) | 1286 (39.9) |
| Ischemic heart disease, n (%) | 399 (10.0) | 72 (9.4) | 327 (10.1) |
| Heart failure, n (%) | 132 (3.3) | 33 (4.3) | 99 (3.1) |
| Chronic kidney disease, n (%) | 227 (5.7) | 68 (8.9) | 159 (4.9) |
| Cerebrovascular disease, n (%) | 195 (4.9) | 39 (5.1) | 156 (4.8) |
| Epilepsy, n (%) | 28 (0.7) | 11 (1.4) | 17 (0.5) |
| DPD test results, n (%) | |||
| Not tested | 3939 (98.8) | 754 (98.8) | 3185 (98.8) |
| Tested | 49 (1.2) | 9 (1.2) | 40 (1.2) |
| Normal/no deficiency | 39 (1.0) | 5 (0.7) | 34 (1.1) |
| Deficiency NOS | 5 (0.1) | 1 (0.1) | 4 (0.1) |
| Partial deficiency | 2 (0.05) | 0 | 2 (0.05) |
| Pending results | 3 (0.08) | 3 (0.4) | 0 |
| Total Patients (N = 3988) | FOLFOX (N = 3189) | FOLFIRINOX (N = 799) | |
|---|---|---|---|
| Any toxicity, n (%) | 763 (19.1) | 559 (17.5) | 204 (25.5) |
| Gastrointestinal, n (%) | |||
| Vomiting | 344 (8.6) | 258 (8.1) | 86 (10.8) |
| Diarrhea | 115 (2.9) | 60 (1.9) | 55 (6.9) |
| Mucositis | 23 (0.6) | 20 (0.6) | 3 (0.4) |
| Hematopoietic, n (%) | |||
| Thrombocytopenia | 124 (3.1) | 84 (2.6) | 40 (5.0) |
| Neutropenia | 19 (0.5) | 7 (0.2) | 12 (1.5) |
| Neurological, n (%) | 47 (1.2) | 33 (1.0) | 14 (1.8) |
| Cardiovascular, n (%) | 12.0 (0.3) | 12 (0.4) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tentoni, N.; Combs, R.; Hwang, M.; Ward, S.; McCracken, A.; Lowe, J.; Howard, S.C. Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study. Cancers 2024, 16, 4050. https://doi.org/10.3390/cancers16234050
Tentoni N, Combs R, Hwang M, Ward S, McCracken A, Lowe J, Howard SC. Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study. Cancers. 2024; 16(23):4050. https://doi.org/10.3390/cancers16234050
Chicago/Turabian StyleTentoni, Nicolás, Ryan Combs, Miriam Hwang, Suzanne Ward, Andrea McCracken, Jennifer Lowe, and Scott C. Howard. 2024. "Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study" Cancers 16, no. 23: 4050. https://doi.org/10.3390/cancers16234050
APA StyleTentoni, N., Combs, R., Hwang, M., Ward, S., McCracken, A., Lowe, J., & Howard, S. C. (2024). Long-Term Outcomes of 5-Fluorouracil-Related Early-Onset Toxicities: A Retrospective Cohort Study. Cancers, 16(23), 4050. https://doi.org/10.3390/cancers16234050






