Impact of Patient’s Age and Physician’s Professional Background on the Number Needed to Treat in Malignant Melanoma Detection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
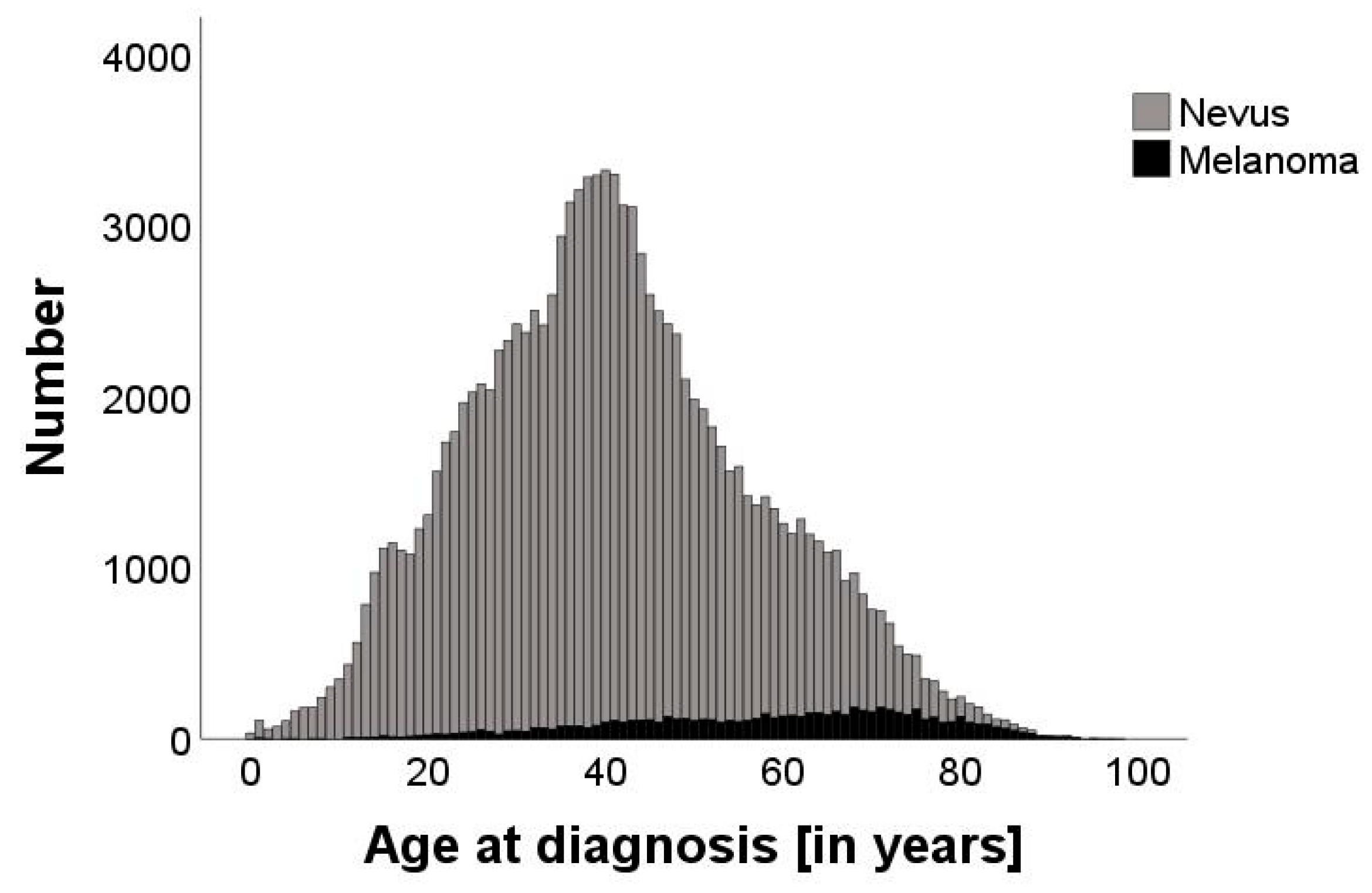
3.1. Demographics of the Study Population
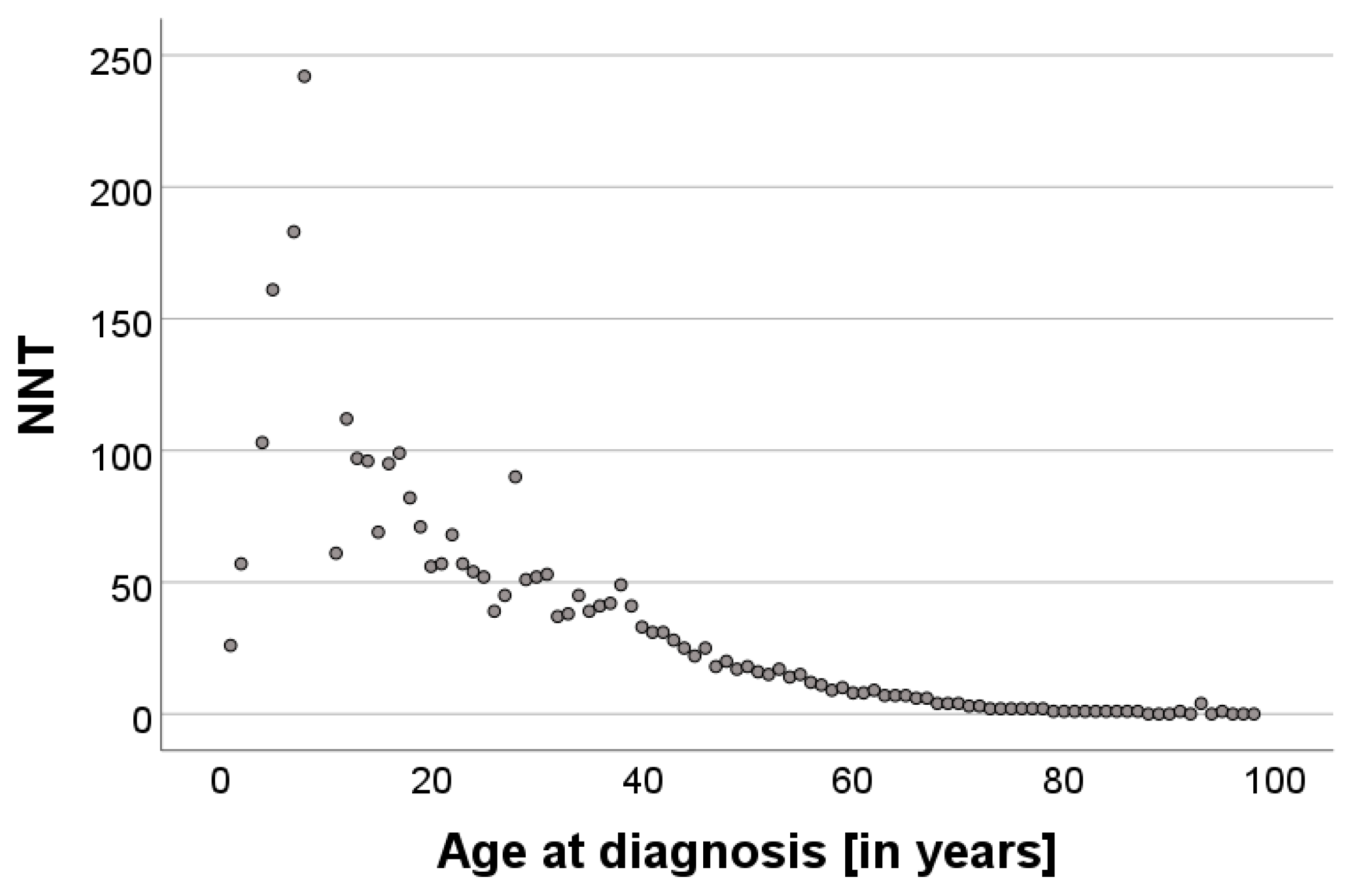
3.2. Correlation of the NNT with Age
3.3. Variability in the NNT Based on the Professional Background of the Referring Physician
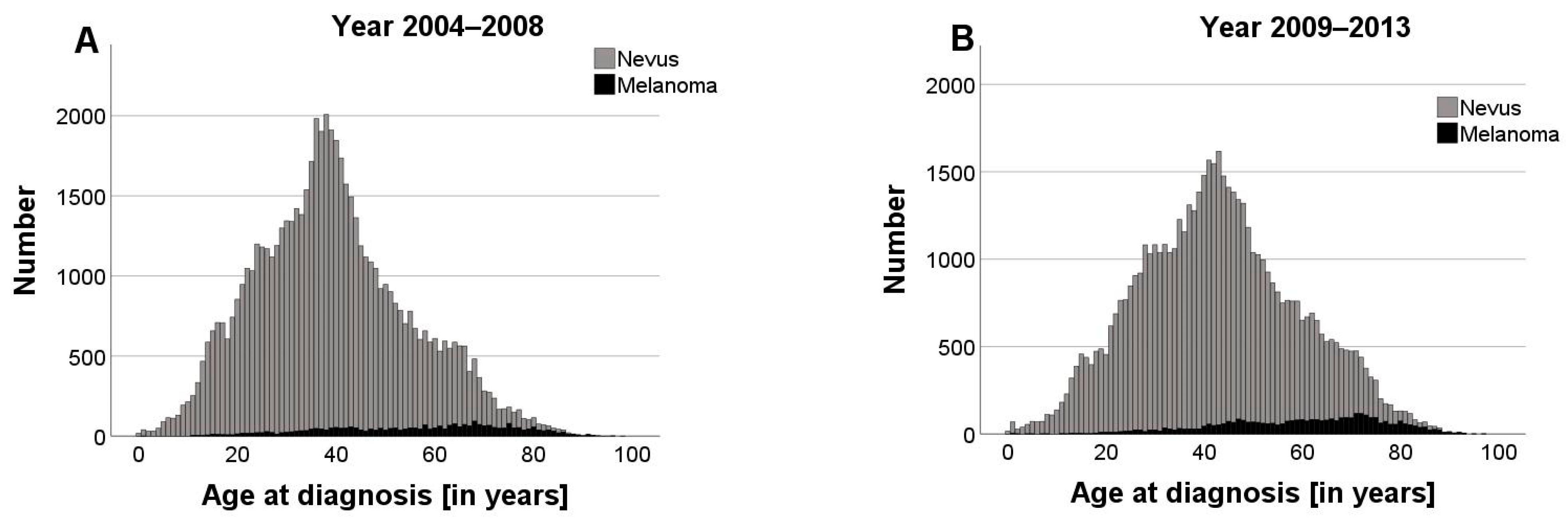
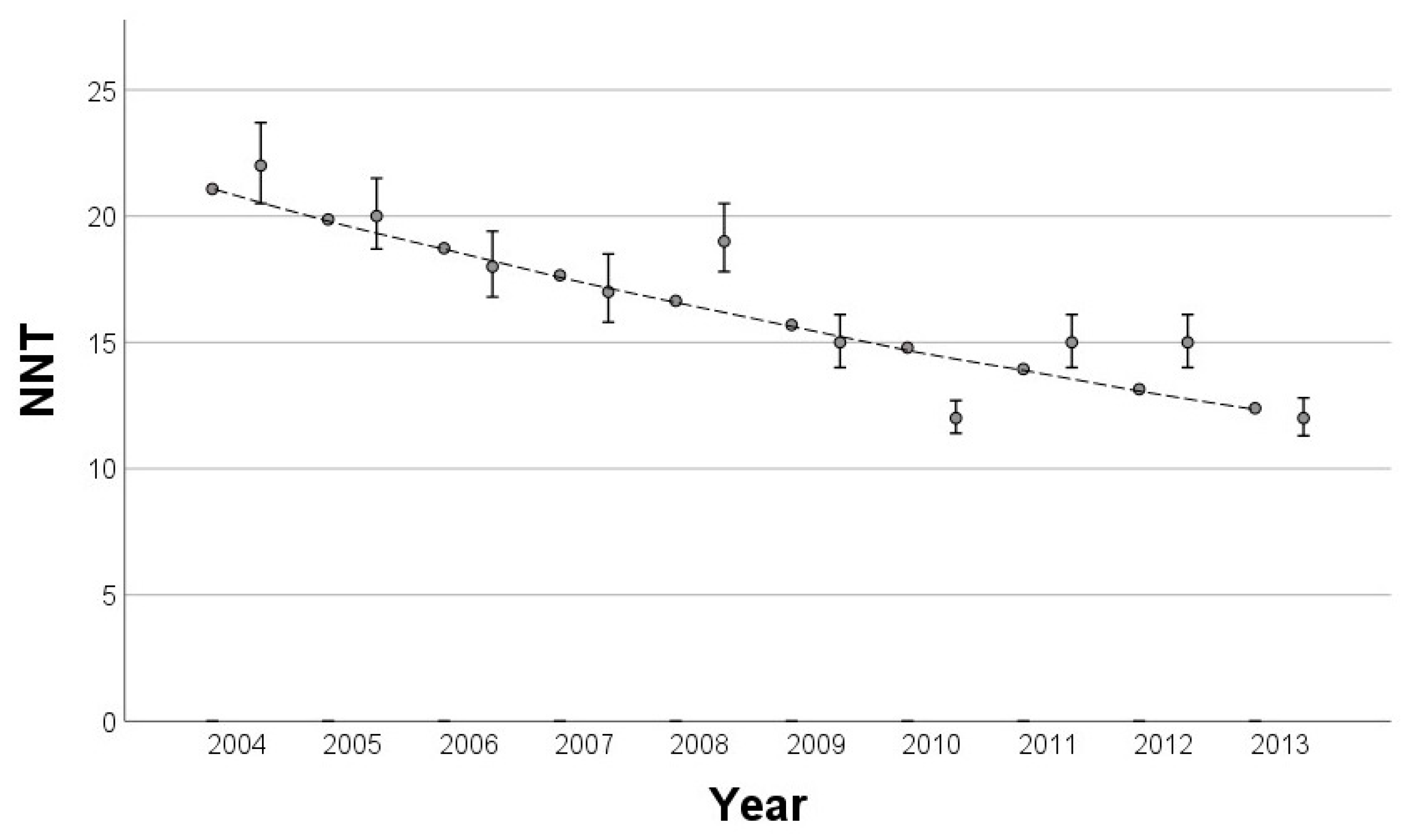
3.4. Impact of Statutory Skin Cancer Screening on the NNT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laupacis, A.; Sackett, D.L.; Roberts, R.S. An assessment of clinically useful measures of the consequences of treatment. N. Engl. J. Med. 1988, 318, 1728–1733. [Google Scholar] [CrossRef] [PubMed]
- Esdaile, B.; Mahmud, I.; Palmer, A.; Bowling, J. Diagnosing melanoma: How do we assess how good we are? Clin. Exp. Dermatol. 2014, 39, 129–134. [Google Scholar] [CrossRef] [PubMed]
- English, D.; Del Mar, C.; Burton, R.C. Factors influencing the number needed to excise: Excision rates of pigmented lesions by general practitioners. Med. J. Aust. 2004, 180, 16–19. [Google Scholar] [CrossRef] [PubMed]
- Mylle, S.; Verhaeghe, E.; van Coile, L.; van de Maele, B.; Hoorens, I.; Brochez, L. Lesion-directed screening to optimize skin cancer detection in dermatology practice: An observational study. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Richards, E.; Brown, A.; Wernham, A. A Review of Infection and Bleeding Complications in Excisional Skin Surgery. Clin. Exp. Dermatol. 2024, 49, 111–120. [Google Scholar] [CrossRef]
- Abhishek, K.; Khunger, N. Complications of skin biopsy. J. Cutan. Aesthet. Surg. 2015, 8, 239–241. [Google Scholar] [CrossRef]
- Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Prävention von Hautkrebs, Kurzversion 2.1, 2021, AWMF Registernummer: 032/052OL. Available online: https://www.leitlinienprogrammonkologie.de/leitlinien/hautkrebs-praevention/ (accessed on 24 November 2024).
- Eisemann, N.; Waldmann, A.; Katalinic, A. Inzidenz des malignen Melanoms und Veränderung der stadienspezifischen Inzidenz nach Einführung eines Hautkrebsscreenings in Schleswig-Holstein. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2014, 57, 77–83. [Google Scholar] [CrossRef]
- Aitken, J.; Elwood, M.; Baade, P.D.; Youl, P.; English, D. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int. J. Cancer 2010, 126, 450–458. [Google Scholar] [CrossRef]
- Swetter, S.M.; Pollitt, R.A.; Johnson, T.M.; Brooks, D.R.; Geller, A.C. Behavioral determinants of successful early melanoma detection: Role of self and physician skin examination. Cancer 2012, 118, 3725–3734. [Google Scholar] [CrossRef]
- Grange, F.; Woronoff, A.S.; Bera, R.; Colomb, M.; Lavole, B.; Fournier, E.; Arnold, F.; Barbe, C. Efficacy of a general practitioner training campaign for early detection of melanoma in France. Br. J. Dermatol. 2014, 170, 123–129. [Google Scholar] [CrossRef]
- Schuldt, K.; Trocchi, P.; Lax, H.; Nonnemacher, M.; Günster, C.; Pahmeier, K.; Speckemeier, C.; Neusser, S.; Stang, A. Hautkrebsscreening und medizinische Behandlungsintensität bei malignem Melanom und nichtmelanozytärem Hautkrebs. Dtsch. Ärzteblatt 2023, 120, 33–39. [Google Scholar]
- Ji, Q.; Tang, J.; Li, S.; Chen, J. Prognostic model for predicting overall and cancer-specific survival among patients with superficial spreading melanoma: A SEER based study. Medicine 2022, 101, 3252. [Google Scholar] [CrossRef] [PubMed]
- Lideikaitė, A.; Mozūraitienė, J.; Letautienė, S. Analysis of prognostic factors for melanoma patients. Acta Med. Litu 2017, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, N.; Schumann, L.; Baltus, H.; Labohm, L.; Kraywinkel, K.; Katalinic, A. Longer Survival From Melanoma in Germany. Dtsch. Arztebl. Int. 2024, 121, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Bundesministerium für Gesundheit. Krebsfrüherkennungs-Richtlinien (Hautkrebs-Screening). BAnz 2008, 37, 871. [Google Scholar]
- Drexler, K.; Zenderowski, V.; Schreieder, L.; Koschitzki, K.; Karrer, S.; Berneburg, M.; Haferkamp, S.; Niebel, D. Subtypes of Melanomas Associated with Different Degrees of Actinic Elastosis in Conventional Histology, Irrespective of Age and Body Site, Suggesting Chronic Ultraviolet Light Exposure as Driver for Lentigo Maligna Melanoma and Nodular Melanoma. Cancers 2024, 16, 1. [Google Scholar] [CrossRef]
- Waldmann, A.; Nolte, S.; Geller, A.C.; Katalinic, A.; Weinstock, M.A.; Volkmer, B.; Greinert, R.; Breitbart, E.W. Frequency of excisions and yields of malignant skin tumors in a population-based screening intervention of 360,288 whole-body examinations. Arch. Dermatol. 2012, 148, 903–910. [Google Scholar] [CrossRef]
- Argenziano, G.; Cerroni, L.; Zalaudek, I.; Staibano, S.; Hofmann-Wellenhof, R.; Arpaia, N.; Bakos, R.M.; Balme, B.; Bandic, J.; Bandelloni, R.; et al. Accuracy in melanoma detection: A 10-year multicenter survey. J. Am. Acad. Dermatol. 2011, 67, 54–59. [Google Scholar] [CrossRef]
- Hansen, C.; Wilkinson, D.; Hansen, M.; Argenziano, G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J. Am. Acad. Dermatol. 2009, 61, 599–604. [Google Scholar] [CrossRef]
- Chia, A.L.K.; Simonova, G.; Dutta, B.; Lim, A.; Shumack, S. Melanoma diagnosis: Australian dermatologists’ number needed to treat. Australas. J. Dermatol. 2008, 49, 12–15. [Google Scholar] [CrossRef]
- Privalle, A.; Havighurst, T.; Kim, K.; Bennett, D.D.; Xu, Y.G. Number of skin biopsies needed per malignancy: Comparing the use of skin biopsies among dermatologists and nondermatologist clinicians. J. Am. Acad. Dermatol. 2020, 82, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.L.; Yentzer, B.A.; Isom, S.P.; Feldman, S.R.; Fleischer, A.B. How good are US dermatologists at discriminating skin cancers? A number-needed-to-treat analysis. J. Dermatolog. Treat. 2012, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Baade, P.D.; Youl, P.H.; Janda, M.; Whiteman, D.C.; Del Mar, C.B.; Aitken, J.F. Factors Associated With the Number of Lesions Excised for Each Skin Cancer: A Study of Primary Care Physicians in Queensland, Australia. Arch. Dermatol. 2008, 144, 1468–1476. [Google Scholar] [CrossRef]
- Rolfe, H.M. Accuracy in skin cancer diagnosis: A retrospective study of an Australian public hospital dermatology department. Australas. J. Dermatol. 2012, 53, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Lund HZ, S.G. The natural history of the pigmented nevus; factors of age and anatomic location. Am. J. Pathol. 1949, 25, 1117–1155. [Google Scholar] [PubMed]
- Memon, A.; Bannister, P.; Rogers, I.; Sundin, J.; Al-Ayadhy, B.; James, P.W.; McNally, R.J.Q. Changing epidemiology and age-specific incidence of cutaneous malignant melanoma in England: An analysis of the national cancer registration data by age, gender and anatomical site, 1981–2018. Lancet Reg. Health—Eur. 2021, 2, 100024–100037. [Google Scholar] [CrossRef]
- Paulson, K.G.; Gupta, D.; Kim, T.S.; Veatch, J.R.; Byrd, D.R.; Bhatia, S.; Wojcik, K.; Chapuis, A.G.; Thompson, J.A.; Madeleine, M.M.; et al. Age-Specific Incidence of Melanoma in the United States. JAMA Dermatol. 2020, 156, 57–64. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Elgart, G.W.; Federman, D.G. Malignancy ratio and number needed to biopsy: Quality measures. J. Am. Acad. Dermatol. 2020, 83, e19. [Google Scholar] [CrossRef]
- Bundesinstitut für Bevölkerungsforschung. Durchschnittsalter der Bevölkerung in Deutschland (1871–2021). Available online: https://www.bib.bund.de/Permalink.html?cms_permaid=1217910 (accessed on 24 November 2024).
- Eissing, L.; Schäfer, I.; Strömer, K.; Kaufmann, R.; Enk, A.; Reusch, M.; Augustin, M. Die Wahrnehmung des gesetzlichen Hautkrebsscreenings in der Allgemeinbevölkerung: Aktuelle Erkenntnisse über Teilnahmequote, Kenntnisstand und Beurteilung. Der Hautarzt 2017, 68, 371–376. [Google Scholar] [CrossRef]
- Starker, A.; Saß, A.-C. Inanspruchnahme von Krebsfrüherkennungsuntersuchungen: Ergebnisse der Studie zur Gesundheit Erwachsener in Deutschland (DEGS1). Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2013, 56, 858–867. [Google Scholar] [CrossRef]
- Hoorens, I.; Vossaert, K.; Pil, L.; Boone, B.; De Schepper, S.; Ongenae, K.; Annemans, L.; Chevolet, I.; Brochez, L. Total-Body Examination vs Lesion-Directed Skin Cancer Screening. J. Am. Acad. Dermatol. 2016, 152, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Zalaudek, I.; Kittler, H.; Marghoob, A.A.; Balato, A.; Blum, A.; Dalle, S.; Ferrara, G.; Fink-Puches, R.; Giorgio, C.M.; Hofmann-Wellenhof, R.; et al. Time Required for a Complete Skin Examination With and Without Dermoscopy: A Prospective, Randomized Multicenter Study. Arch. Dermatol. 2008, 144, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Argenziano, G.; Zalaudek, I.; Hofmann-Wellenhof, R.; Bakos, R.M.; Bergman, W.; Blum, A.; Broganelli, P.; Cabo, H.; Caltagirone, F.; Catricalà, C.; et al. Total body skin examination for skin cancer screening in patients with focused symptoms. J. Am. Acad. Dermatol. 2012, 66, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Krensel, M.; Andrees, V.; Mohr, N.; Hischke, S. Costs of routine skin cancer screening in Germany: A claims data analysis. Clin. Exp. Dermatol. 2021, 46, 842–850. [Google Scholar] [CrossRef]
- Li, Z.; Koban, K.C.; Schenck, T.L.; Giunta, R.E.; Li, Q.; Sun, Y. Artificial Intelligence in Dermatology Image Analysis: Current Developments and Future Trends. J. Clin. Med. 2022, 11, 6826. [Google Scholar] [CrossRef]
- Swetter, S.M.; Boldrick, J.C.; Jung, S.Y.; Egbert, B.M.; Harvell, J.D. Increasing incidence of lentigo maligna melanoma subtypes: Northern California and national trends 1990–2000. J. Investig. Dermatol. 2005, 125, 685–691. [Google Scholar] [CrossRef]
- Greveling, K.; Wakkee, M.; Nijsten, T.; van den Bos, R.R.; Hollestein, L.M. Epidemiology of Lentigo Maligna and Lentigo Maligna Melanoma in the Netherlands, 1989–2013. J. Investig. Dermatol. 2016, 136, 1955–1960. [Google Scholar] [CrossRef]
- Patel, V.R.; Roberson, M.L.; Pignone, M.P.; Adamson, A.S. Risk of Mortality After a Diagnosis of Melanoma In Situ. JAMA Dermatol. 2023, 159, 703–710. [Google Scholar] [CrossRef]
- Schumann, L.; Eisemann, N.; Augustin, J.; Kieschke, J.; Meyer, M.; Kajüter, H.; Katalinic, A. Zusammenhang zwischen der Inzidenz früher Stadien und der Mortalität beim malignen Melanom—eine bevölkerungsbasierte ökologische Studie. J. Dtsch Dermatol. Ges. 2013, 33–41, 33–41. [Google Scholar] [CrossRef]
- Brunssen, A.; Waldmann, A.; Eisemann, N.; Katalinic, A. Impact of skin cancer screening and secondary prevention campaigns on skin cancer incidence and mortality: A systematic review. J. Am. Acad. Dermatol. 2017, 76, 129–139. [Google Scholar] [CrossRef]
- Katalinic, A.; Eisemann, N.; Waldmann, A. Skin Cancer Screening in Germany. Documenting Melanoma Incidence and Mortality From 2008 to 2013. Dtsch. Arztebl. Int. 2015, 112, 629–634. [Google Scholar] [PubMed]
- Wolf, S.; Augustin, M.; Hagenström, K.; Garbe, C.; Baltus, H.; Eisemann, N.; Hübner, J.; Katalinic, A.; Augustin, J. Evaluation der Hautkrebsfrüherkennung in Deutschland—Raumzeitliche Assoziationen zwischen Hautkrebsfrüherkennung und Hautkrebsmortalität auf Grundlage ambulanter Abrechnungsdaten. J. Dtsch. Dermatol. Ges. 2013, 21, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Drexler, K.; Drexler, H.; Geissler, E.K.; Berneburg, M.; Haferkamp, S.; Apfelbacher, C. Incidence and Mortality of Malignant Melanoma in Relation to Dermatologist Density in Bavaria. Adv. Ther. 2021, 38, 5548–5556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreieder, L.; Zenderowski, V.; Berneburg, M.; Haferkamp, S.; Drexler, K.; Niebel, D. Impact of Patient’s Age and Physician’s Professional Background on the Number Needed to Treat in Malignant Melanoma Detection. Cancers 2024, 16, 4014. https://doi.org/10.3390/cancers16234014
Schreieder L, Zenderowski V, Berneburg M, Haferkamp S, Drexler K, Niebel D. Impact of Patient’s Age and Physician’s Professional Background on the Number Needed to Treat in Malignant Melanoma Detection. Cancers. 2024; 16(23):4014. https://doi.org/10.3390/cancers16234014
Chicago/Turabian StyleSchreieder, Laura, Veronika Zenderowski, Mark Berneburg, Sebastian Haferkamp, Konstantin Drexler, and Dennis Niebel. 2024. "Impact of Patient’s Age and Physician’s Professional Background on the Number Needed to Treat in Malignant Melanoma Detection" Cancers 16, no. 23: 4014. https://doi.org/10.3390/cancers16234014
APA StyleSchreieder, L., Zenderowski, V., Berneburg, M., Haferkamp, S., Drexler, K., & Niebel, D. (2024). Impact of Patient’s Age and Physician’s Professional Background on the Number Needed to Treat in Malignant Melanoma Detection. Cancers, 16(23), 4014. https://doi.org/10.3390/cancers16234014






