A Bayesian Network Approach to Lung Cancer Screening: Assessing the Impact of Data Quantity, Quality, and the Combination of Data from Danish Electronic Health Records
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
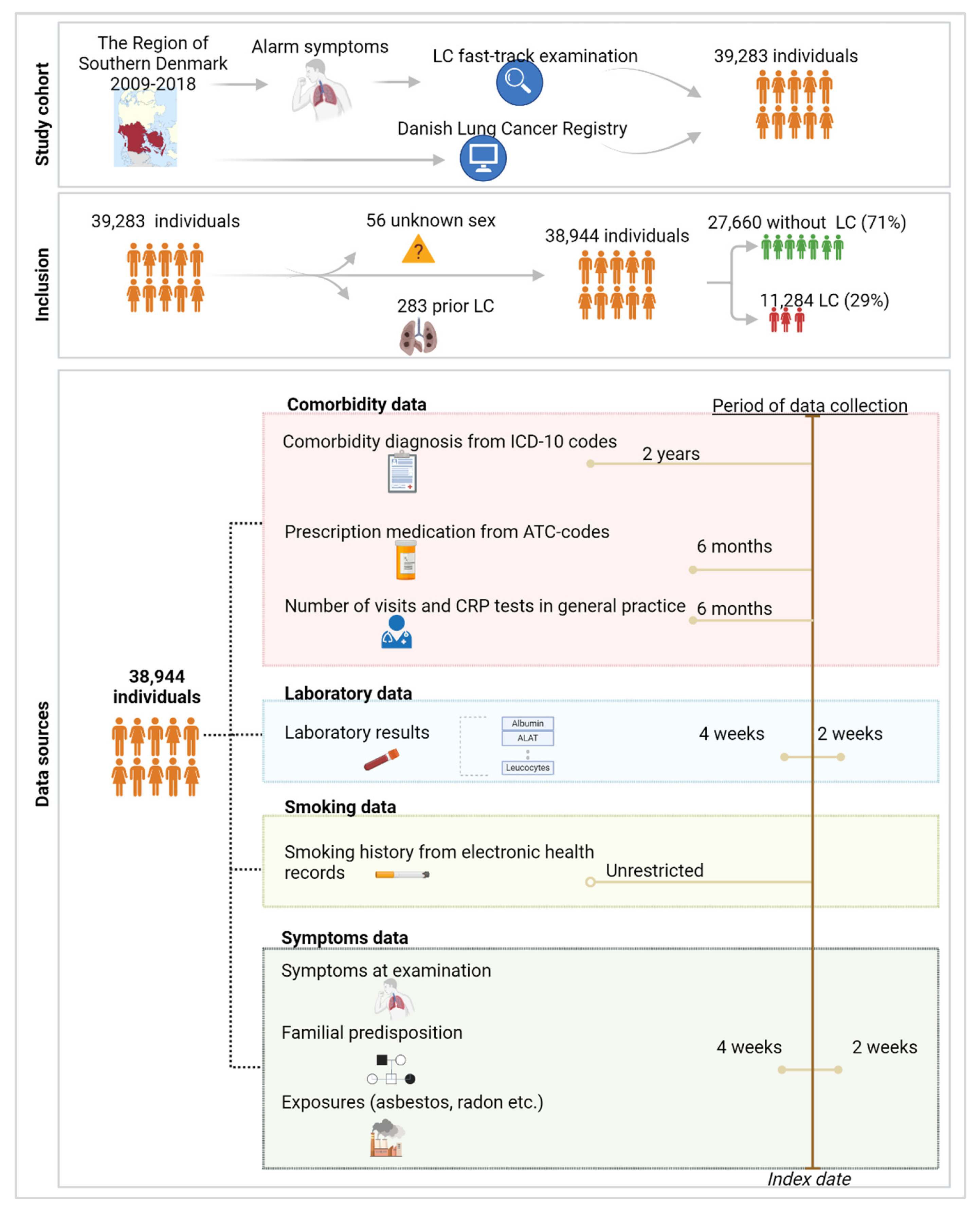
2.1. Study Cohort and Data Sources
2.2. Experimental Setup
2.2.1. Discretization
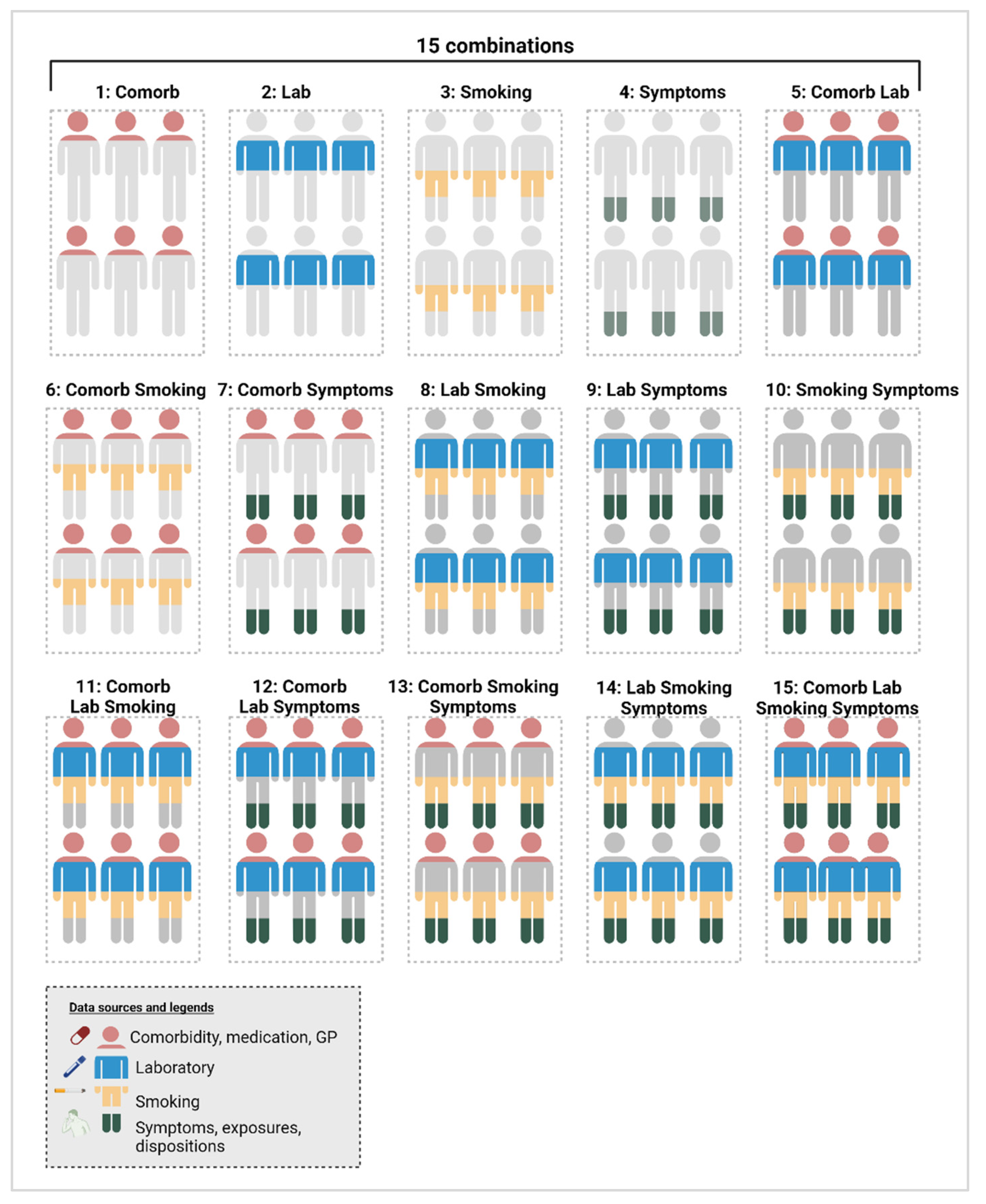
2.2.2. Model Development
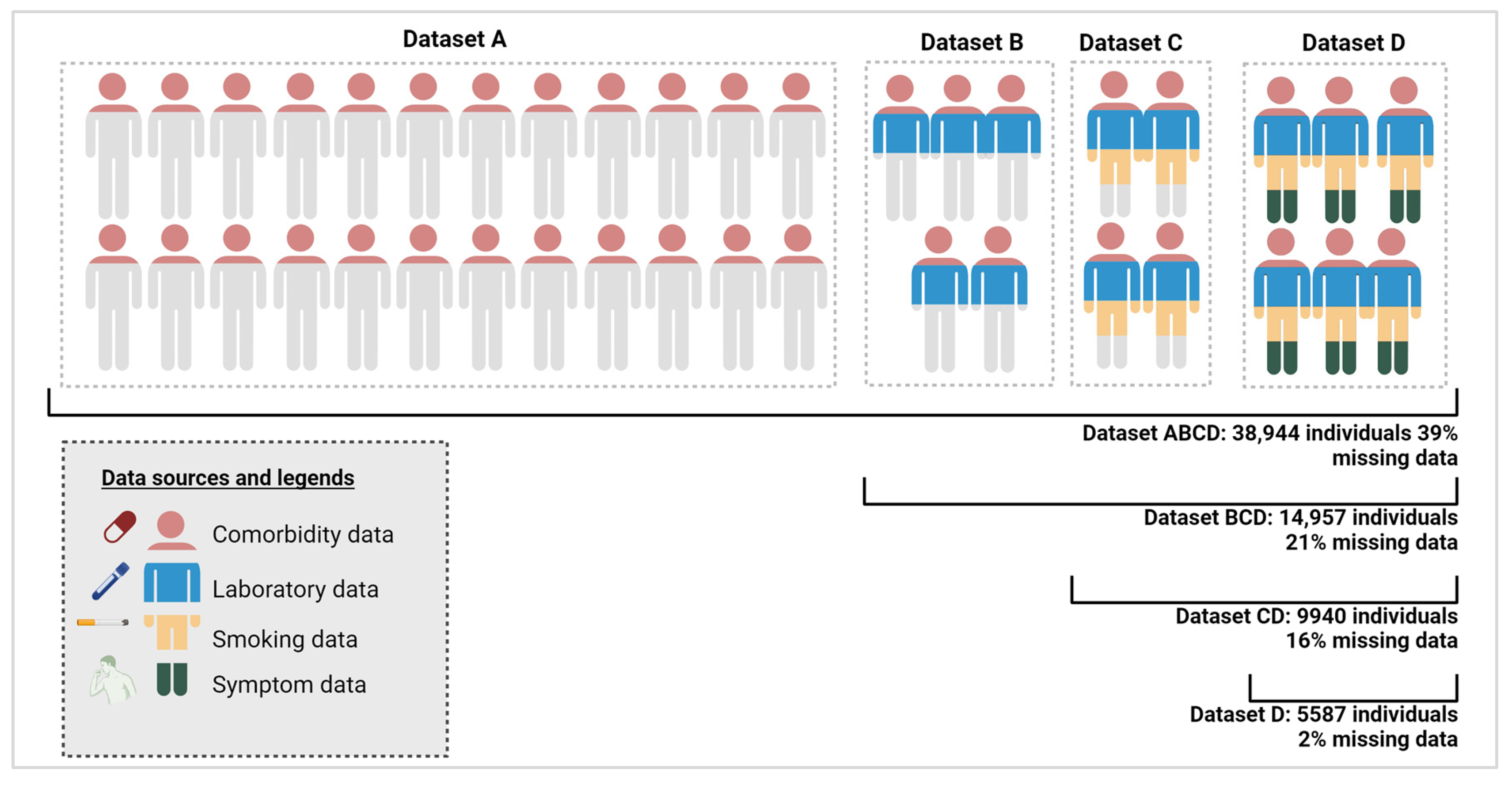
2.2.3. Division of Study Cohort
2.3. Evaluation Setup
2.3.1. Evaluation 1
2.3.2. Evaluation 2
2.4. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Performance Assessment
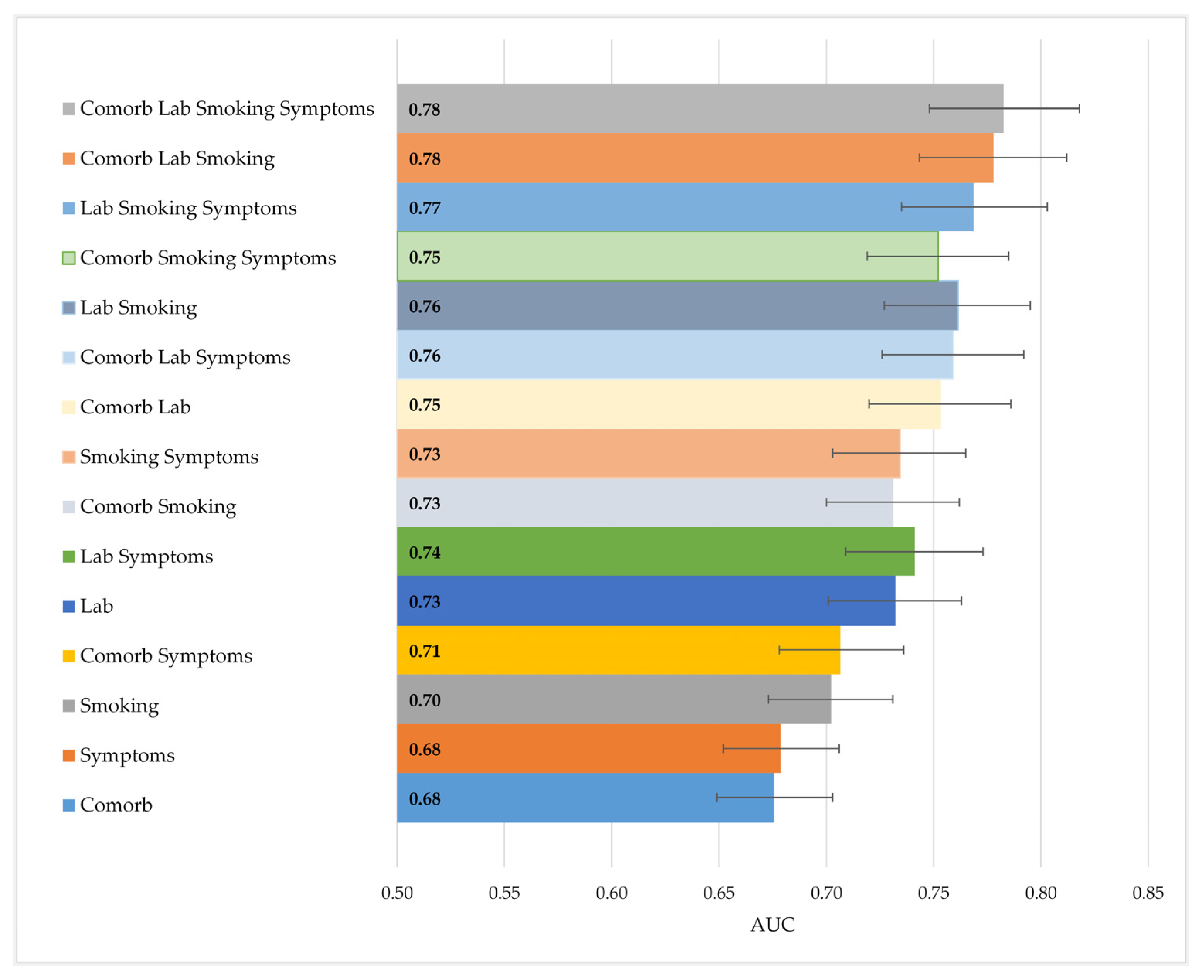
3.2.1. Evaluation 1
3.2.2. Evaluation 2
4. Discussion
4.1. Summary of Results
4.2. Interpretation and Comparison
4.3. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Lung Cancer [Internet]. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 13 December 2023).
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Aberle, D.; Adams, A.; Berg, C.; Black, W.; Clapp, J.; Fagerstrom, R.; Gareen, I.F.; Gatsonis, C.; Marcus, P.M.; Sicks, J.D. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Vulkan, D.; Davies, M.P.A.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health 2021, 10, 100179. [Google Scholar] [CrossRef]
- Paci, E.; Puliti, D.; Pegna, A.L.; Carrozzi, L.; Picozzi, G.; Falaschi, F.; Pistelli, F.; Aquilini, F.; Ocello, C.; Zappa, M.; et al. Mortality, survival and incidence rates in the ITALUNG randomised lung cancer screening trial. Thorax 2017, 72, 825–831. [Google Scholar] [CrossRef] [PubMed]
- van der Aalst, C.; Vonder, M.; Hubert, J.; Moldovanu, D.; Schmitz, A.; Delorme, S.; Kaaks, R.; ten Haaf, K.; Oudkerk, M.; de Koning, H. P1. 14-04 European lung cancer screening implementation: 4-IN-THE-LUNG-RUN trial. J. Thorac. Oncol. 2023, 18, S217. [Google Scholar] [CrossRef]
- McWilliams, A.M.; Mayo, J.R.; Im Ahn, M.; MacDonald, S.L.S.; Lam, S.C. Lung cancer screening using multi-slice thin-section computed tomography and autofluorescence bronchoscopy. J. Thorac. Oncol. 2006, 1, 61–68. [Google Scholar] [CrossRef]
- dos Santos, R.S.; Franceschini, J.P.; Chate, R.C.; Ghefter, M.C.; Kay, F.; Trajano, A.L.C.; Pereira, J.R.; Succi, J.E.; Fernando, H.C.; Júnior, R.S. Do current lung cancer screening guidelines apply for populations with high prevalence of granulomatous disease? Results from the First Brazilian Lung Cancer Screening Trial (BRELT1). Ann. Thorac. Surg. 2016, 101, 481–488. [Google Scholar] [CrossRef]
- Blanchon, T.; Bréchot, J.-M.; Grenier, P.A.; Ferretti, G.R.; Lemarié, E.; Milleron, B.; Chagué, D.; Laurent, F.; Martinet, Y.; Beigelman-Aubry, C.; et al. Baseline results of the Depiscan study: A French randomized pilot trial of lung cancer screening comparing low dose CT scan (LDCT) and chest X-ray (CXR). Lung Cancer 2007, 58, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Motsch, E.; Gross, M.-L.; Eigentopf, A.; Heussel, C.P.; Dienemann, H.; Schnabel, P.A.; Eichinger, M.; Optazaite, D.-E.; Puderbach, M.; et al. Randomized study on early detection of lung cancer with MSCT in Germany: Results of the first 3 years of follow-up after randomization. J. Thorac. Oncol. 2015, 10, 890–896. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kreftforeningen. Lung Cancer Screenings to Begin in Norway [Internet]. Available online: https://kreftforeningen.no/forebygging/screening-og-masseundersokelser/?gad_source=1&gclid=Cj0KCQiAo5u6BhDJARIsAAVoDWvb6pfV9_cs9bw5nBXUc5JvXYNlLAvWWByrea7eNuftzaq9aTj4-3oaAjlqEALw_wcB (accessed on 27 June 2024).
- Kaneko, M.; Eguchi, K.; Ohmatsu, H.; Kakinuma, R.; Naruke, T.; Suemasu, K.; Moriyama, N. Peripheral lung cancer: Screening and detection with low-dose spiral CT versus radiography. Radiology 1996, 201, 798–802. [Google Scholar] [CrossRef]
- Lu, L.; Tan, Y.; Schwartz, L.H.; Zhao, B. Hybrid detection of lung nodules on CT scan images. Med. Phys. 2015, 42, 5042–5054. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Dai, M.; Shi, J.; Ren, J.; Li, J.; Liao, X.; Du, L.; Liu, Y.; Chen, Z.; Wu, N.; et al. The feasibility study of a randomized cancer screening trial in China. Cancer Res. 2016, 76, 1795. [Google Scholar] [CrossRef]
- U.S. Preventive Services Task Force. Final Recommendation Statement. Lung Cancer: Screening [Internet]. 2021. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening (accessed on 22 May 2024).
- Pinsky, P.F.; Berg, C.D. Applying the National Lung Screening Trial eligibility criteria to the US population: What percent of the population and of incident lung cancers would be covered? J. Med. Screen. 2012, 19, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Myles, J.P.; van Tongeren, M.; Page, R.D.; Liloglou, T.; Duffy, S.W.; Field, J. The LLP risk model: An individual risk prediction model for lung cancer. Br. J. Cancer 2008, 98, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Tammemägi, M.C.; Katki, H.A.; Hocking, W.G.; Church, T.R.; Caporaso, N.; Kvale, P.A.; Chaturvedi, A.K.; Silvestri, G.A.; Riley, T.L.; Commins, J.; et al. Selection criteria for lung-cancer screening. N. Engl. J. Med. 2013, 368, 728–736. [Google Scholar] [CrossRef]
- Lebrett, M.B.; Balata, H.; Evison, M.; Colligan, D.; Duerden, R.; Elton, P.; Greaves, M.; Howells, J.; Irion, K.; Karunaratne, D.; et al. Analysis of lung cancer risk model (PLCO(M2012) and LLP(v2)) performance in a community-based lung cancer screening programme. Thorax 2020, 75, 661–668. [Google Scholar] [CrossRef]
- Tammemägi, M.C.; Church, T.R.; Hocking, W.G.; Silvestri, G.A.; Kvale, P.A.; Riley, T.L.; Commins, J.; Berg, C.D. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: Screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014, 11, e1001764. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chao, H.-S.; Chen, Y.-M. Application of Artificial Intelligence in Lung Cancer. Cancers 2022, 14, 1370. [Google Scholar] [CrossRef] [PubMed]
- Wüthrich, M.V.; Merz, M. Bayesian Methods, Regularization and Expectation-Maximization. In Statistical Foundations of Actuarial Learning and Its Applications; Springer: Cham, Switzerland, 2022; pp. 207–266. [Google Scholar]
- McLachlan, S.; Dube, K.; Hitman, G.A.; Fenton, N.E.; Kyrimi, E. Bayesian networks in healthcare: Distribution by medical condition. Artif. Intell. Med. 2020, 107, 101912. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, K.; Muñoz-Valencia, C.S.; Rabasa, A.; Quesada-Rico, J.A.; Orozco-Beltrán, D.; Barber, X. Bayesian Networks for the Diagnosis and Prognosis of Diseases: A Scoping Review. Mach. Learn. Knowl. Extr. 2024, 6, 1243–1262. [Google Scholar] [CrossRef]
- Henriksen, M.B.; Hansen, T.F.; Jensen, L.H.; Brasen, C.L.; Peimankar, A.; Ebrahimi, A.; Wii, U.K.; Hilberg, O. A Collection of Multiregistry Data on Patients at High Risk of Lung Cancer. Transl. Lung Cancer Res. 2023, 12, 2392–2411. [Google Scholar] [CrossRef]
- Flyckt, R.N.H.; Sjodsholm, L.; Henriksen, M.H.B.; Brasen, C.L.; Ebrahimi, A.; Hilberg, O.; Hansen, T.F.; Wiil, U.K.; Jensen, L.H.; Peimankar, A. Pulmonologists-Level lung cancer detection based on standard blood test results and smoking status using an explainable machine learning approach. Sci. Rep. 2024; Accepted for publication. [Google Scholar]
- Henriksen, M.B.; van Daalen, F.; Wee, L.; Hansen, T.F.; Jensen, L.H.; Brasen, C.L.; Hilberg, O.B.I. Lung cancer detection using Bayesian networks: A retrospective development and validation study on a Danish population of high-risk individuals. Cancer Med. 2024; Accepted for publication. [Google Scholar]
- Ebrahimi, A.; Henriksen, M.B.H.; Brasen, C.L.; Hilberg, O.; Hansen, T.F.; Jensen, L.H.; Peimankar, A.; Wiil, U.K. Identification of patients’ smoking status using an explainable AI approach: A Danish electronic health records case study. BMC Med. Res. Methodol. 2024, 24, 114. [Google Scholar] [CrossRef] [PubMed]
- Fayyad, U.M.; Irani, K.B. Multi-interval discretization of continuous-valued attributes for classification learning. IJCAI 1993, 93, 1022–1029. [Google Scholar]
- Cooper, G.F.; Herskovits, E. A Bayesian method for the induction of probabilistic networks from data. Mach. Learn. 1992, 9, 309–347. [Google Scholar] [CrossRef]
- Dempster, A.; Laird, N.; Rubin, D.; Dempster, A.P.; Laird, N.M.; Rubin, D. Likelihood from incomplete data via the em algorithm. JR Stat. Soc. B. 1977, 39, 1–38. [Google Scholar] [CrossRef]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- Ke, X.; Keenan, K.; Smith, V.A. Treatment of missing data in Bayesian network structure learning: An application to linked biomedical and social survey data. BMC Med. Res. Methodol. 2022, 22, 326. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-H.; Haug, P.J. Exploiting missing clinical data in Bayesian network modeling for predicting medical problems. J. Biomed. Inform. 2008, 41, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.; Geiger, D.; Goldszmidt, M. Bayesian network classifiers. Mach. Learn. 1997, 29, 131–163. [Google Scholar] [CrossRef]
- Tammemägi, M.C. Selecting lung cancer screenees using risk prediction models-where do we go from here. Transl. Lung Cancer Res. 2018, 7, 243–253. [Google Scholar] [CrossRef]
- Jantzen, R.; Ezer, N.; Camilleri-Broët, S.; Tammemägi, M.C.; Broët, P. Evaluation of the accuracy of the PLCO(m2012) 6-year lung cancer risk prediction model among smokers in the CARTaGENE population-based cohort. C. Open 2023, 11, E314–E322. [Google Scholar] [CrossRef]
- Robbins, H.A.; Alcala, K.; Swerdlow, A.J.; Schoemaker, M.J.; Wareham, N.; Travis, R.C.; Crosbie, P.A.J.; Callister, M.; Baldwin, D.R.; Landy, R.; et al. Comparative performance of lung cancer risk models to define lung screening eligibility in the United Kingdom. Br. J. Cancer 2021, 124, 2026–2034. [Google Scholar] [CrossRef]
| Demography | Non-LC | LC | p-Value |
|---|---|---|---|
| Total, no. (%) | 27,660 (100) | 11,284 (100) | |
| Age, median (IQR) | 67 (56–75) | 70 (63–77) | <0.001 |
| Females, no. (%) | 12,515 (45.3) | 5461 (48.4) | <0.001 |
| LC stage, no. (%) | |||
| I | 2.001 (17.7) | ||
| II | 914 (8.1) | ||
| III | 2242 (19.9) | ||
| IV | 5440 (48.2) | ||
| Unknown | 687 (6.1) | ||
| Comorbidity dataset | Non-LC | LC | p-value |
| Total, no. (%) | 27,660 (100) | 11,284 (100) | |
| Myocardial infarction | 454 (1.6) | 225 (2.0) | 0.02 |
| Congestive cardiac failure | 198 (0.7) | 69 (0.61) | 0.26 |
| Peripheral vascular disease | 828 (3.0) | 555 (4.9) | <0.001 |
| Cerebrovascular disease | 915 (3.3) | 525 (4.7) | <0.001 |
| Dementia | 200 (0.7) | 77 (0.7) | 0.67 |
| Chronic pulmonary disease | 3379 (12.2) | 1429 (12.7) | 0.22 |
| Rheumatological disease | 533 (1.9) | 228 (2.0) | 0.54 |
| Liver disease | 198 (0.7) | 65 (0.6) | 0.13 |
| Diabetes mellitus | 1245 (4.5) | 566 (5.0) | 0.03 |
| Hemiplegia or paraplegia | 30 (0.1) | 18 (0.2) | 0.19 |
| Renal disease | 522 (1.9) | 179 (1.6) | 0.05 |
| Metastatic solid tumor | 772 (2.8) | 6.5 (5.4) | <0.001 |
| AIDS/HIV infection | 19 (0.1) | 9 (0.1) | 0.94 |
| Pulmonary tuberculosis | 48 (0.2) | 8 (0.1) | 0.02 |
| Sarcoidosis | 79 (0.3) | 18 (0.2) | 0.02 |
| Interstitial lung disease | 194 (0.7) | 74 (0.7) | 0.62 |
| Abscess | 157 (0.6) | 43 (0.4) | 0.02 |
| Pleural disease | 725 (2.6) | 297 (2.6) | 0.95 |
| Pneumonia | 2944 (10.6) | 1132 (10.0) | 0.07 |
| SumCCI, sum (median) | 0 (50) | 0 (50) | <0.001 |
| Other malignancies | 3859 (14.0) | 1321 (11.7) | <0.001 |
| Antibiotics | 12,130 (43.9) | 4954 (43.9) | 0.93 |
| COPD inhalations | 7028 (25.4) | 3490 (30.9) | <0.001 |
| Antihypertensives | 9560 (34.6) | 4660 (41.3) | <0.001 |
| Glucocorticoids | 2770 (10.0) | 1534 (13.6) | <0.001 |
| Metformin | 1694 (6.1) | 837 (7.4) | <0.001 |
| Antidepressants | 3960 (14.3) | 1838 (16.3) | <0.001 |
| Consultations at GP | 3093 (11.2) | 932 (8.3) | <0.001 |
| CRP rapid tests at GP | 13,053 (47.2) | 5275 (46.8) | |
| Laboratory data | Non-LC | LC | p-value |
| Total, no. (%) | 10,503 (100) | 4454 (100) | |
| B-Hemoglobin, mmol/L | 8.7 (8.0–9.3) | 8.40 (7.7–9.0) | <0.001 |
| B-Leucocytes, 109/L | 7.64 (6.20–9.46) | 9.12 (7.43–11.20) | <0.001 |
| B-Neutrophils, 109/L | 4.70 (3.58–6.20) | 6.10 (4.71–7.95) | <0.001 |
| B-Lymphocytes, 109/L | 1.81 (1.39–2.33) | 1.74 (1.30–2.27) | <0.001 |
| NLR | 2.6 (1.8–3.8) | 3.4 (2.4–5.2) | <0.001 |
| B-Monocytes, 109/L | 0.65 (0.51–0.84) | 0.76 (0.59–0.97) | <0.001 |
| B-Basophils, 109/L | 0.04 (0.02–0.06) | 0.04 (0.02–0.06) | <0.001 |
| B-Eosinophils, 109/L | 0.17 (0.10–0.27) | 0.14 (0.07–0.25) | <0.001 |
| B-Platelets, 109/L | 272 (223–334) | 311 (250–391) | <0.001 |
| P-Albumin, g/L | 43 (41–45) | 42 (39–44) | <0.001 |
| Total Calcium, mmol/L | 2.34 (2.27–2.41) | 2.36 (2.29–2.43) | <0.001 |
| P-CRP, mg/L | 3.7 (1.4–10.0) | 9.9 (3.0–32.0) | <0.001 |
| P-ALAT, U/L | 22 (16–31) | 18 (13–26) | <0.001 |
| P-LDH, U/L | 192 (169–221) | 214 (182–257) | <0.001 |
| P-Alkaline phosphatase, U/L | 75 (62–92) | 83 (68–102) | <0.001 |
| P-Bilirubin-total, μmol/L | 7 (6–10) | 7 (5–9) | <0.001 |
| P-Amylase (pancreatic), U/L | 25 (19–34) | 25 (18–34) | 0.79 |
| P-INR | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | <0.001 |
| P-Creatinine, mmol/L | 76 (64–89) | 72 (60–87) | <0.001 |
| P-Sodium, mmol/L | 140 (138–142) | 139 (136–141) | <0.001 |
| P-Potassium, mmol/L | 4.0 (3.8–4.3) | 4.0 (3.8–4.3) | 0.08 |
| Smoking status | Non-LC | LC | p-value |
| Total | 7435 (100) | 2505 (100) | |
| Never smoker | 2288 (30.8) | 196 (1.8) | <0.001 |
| Former or current smoker | 5147 (69.2) | 2309 (92.2) | |
| Symptoms, familial predispositions and exposures, no. (%) | Non-LC | LC | p-value |
| Total | 3733 (100) | 1854 (100) | |
| Predispositions | 253 (6.8) | 167 (9.0) | 0.00 |
| Exposures | 785 (21.0) | 354 (19.1) | 0.09 |
| Hemoptysis | 694 (18.6) | 212 (11.4) | <0.001 |
| Pneumonia | 671 (18.0) | 303 (16.3) | 0.13 |
| Cough | 2012 (53.9) | 969 (52.3) | 0.25 |
| Dyspnoea | 1365 (36.6) | 663 (35.8) | 0.56 |
| Fever | 286 (7.2) | 81 (4.4) | <0.001 |
| Weight loss | 822 (22.0) | 584 (31.5) | <0.001 |
| Fatigue | 684 (18.3) | 428 (23.1) | <0.001 |
| Hot flash | 402 (10.8) | 177 (9.6) | 0.16 |
| Hoarseness | 174 (4.7) | 92 (5.0) | 0.62 |
| Back pain | 133 (3.6) | 129 (7.0) | <0.001 |
| Other pain | 340 (9.1) | 250 (13.5) | <0.001 |
| Angina | 428 (11.5) | 256 (13.8) | 0.01 |
| Headache | 144 (3.1) | 65 (3.5) | 0.37 |
| Dizziness | 161 (4.3) | 96 (5.2) | 0.15 |
| Edema | 196 (5.3) | 108 (5.8) | 0.37 |
| Val. Data | Training Data ABCD | Training Data BCD | Training Data CD | Training Data D | ||||
|---|---|---|---|---|---|---|---|---|
| Variables | AUC (95% CI) | Variables | AUC (95% CI) | Variables | AUC (95% CI) | Variables | AUC (95% CI) | |
| A | Comorb Symptoms | 0.63 (0.62–0.64) | Comorb Lab | 0.62 (0.60–0.63) | Comorb | 0.60 (0.59–0.61) | Comorb Smoking Symptoms | 0.60 (0.59–0.61) |
| B | Comorb Lab Symptoms | 0.78 (0.75–0.82) | Comorb Lab Smoking | 0.79 (0.75–0.83) | Comorb Lab | 0.78 (0.74–0.81) | Comorb Lab | 0.77 (0.73–0.80) |
| C | Lab Smoking | 0.72 (0.67–0.76) | Lab Smoking Symptoms | 0.72 (0.78–0.77) | Lab Smoking Symptoms | 0.73 (0.69–0.78) | Lab Smoking | 0.73 (0.58–0.80) |
| D | Comorb Lab Smoking Symptoms | 0.75 (0.72–0.79) | Comorb Lab Smoking Symptoms | 0.77 (0.73–0.80) | Comorb Lab Smoking | 0.77 (0.73–0.80) | Comorb Lab Smoking Symptoms | 0.78 (0.75–0.82) |
| CD | Comorb Lab Smoking Symptoms | 0.76 (0.73–0.79) | Comorb Lab Smoking Symptoms | 0.77 (0.74–0.80) | Comorb Lab Smoking Symptoms | 0.78 (0.75–0.81) | Comorb Lab Smoking Symptoms | 0.77 (0.74–0.80) |
| BCD | Comorb Lab Smoking Symptoms | 0.77 (0.75–0.79) | Comorb Lab Smoking Symptoms | 0.78 (0.76–0.80) | Comorb Lab Smoking | 0.78 (0.75–0.80) | Comorb Lab Smoking Symptoms | 0.78 (0.75–0.80) |
| ABCD | Comorb Lab Smoking Symptoms | 0.69 (0.68–0.70) | Comorb Lab Smoking | 0.68 (0.67–0.69) | Comorb Lab Smoking | 0.66 (0.65–0.67) | Comorb Lab Smoking Symptoms | 0.67 (0.66–0.68) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daalen, F.v.; Henriksen, M.H.B.; Hansen, T.F.; Jensen, L.H.; Brasen, C.L.; Hilberg, O.; Andersen, M.A.K.; Humerfelt, E.; Wee, L.; Bermejo, I. A Bayesian Network Approach to Lung Cancer Screening: Assessing the Impact of Data Quantity, Quality, and the Combination of Data from Danish Electronic Health Records. Cancers 2024, 16, 3989. https://doi.org/10.3390/cancers16233989
Daalen Fv, Henriksen MHB, Hansen TF, Jensen LH, Brasen CL, Hilberg O, Andersen MAK, Humerfelt E, Wee L, Bermejo I. A Bayesian Network Approach to Lung Cancer Screening: Assessing the Impact of Data Quantity, Quality, and the Combination of Data from Danish Electronic Health Records. Cancers. 2024; 16(23):3989. https://doi.org/10.3390/cancers16233989
Chicago/Turabian StyleDaalen, Florian van, Margrethe Høstgaard Bang Henriksen, Torben Frøstrup Hansen, Lars Henrik Jensen, Claus Lohman Brasen, Ole Hilberg, Martin Ask Klausholt Andersen, Elise Humerfelt, Leonard Wee, and Inigo Bermejo. 2024. "A Bayesian Network Approach to Lung Cancer Screening: Assessing the Impact of Data Quantity, Quality, and the Combination of Data from Danish Electronic Health Records" Cancers 16, no. 23: 3989. https://doi.org/10.3390/cancers16233989
APA StyleDaalen, F. v., Henriksen, M. H. B., Hansen, T. F., Jensen, L. H., Brasen, C. L., Hilberg, O., Andersen, M. A. K., Humerfelt, E., Wee, L., & Bermejo, I. (2024). A Bayesian Network Approach to Lung Cancer Screening: Assessing the Impact of Data Quantity, Quality, and the Combination of Data from Danish Electronic Health Records. Cancers, 16(23), 3989. https://doi.org/10.3390/cancers16233989







