The Association of the COVID-19 Pandemic with the Uptake of Colorectal Cancer Screening Varies by Socioeconomic Status in Flanders, Belgium
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. CRC Screening in Flanders
2.2. Interruption Period
2.3. Study Design and Data Sources
2.4. Study Population
2.5. Definitions
2.6. Statistical Analysis
3. Results
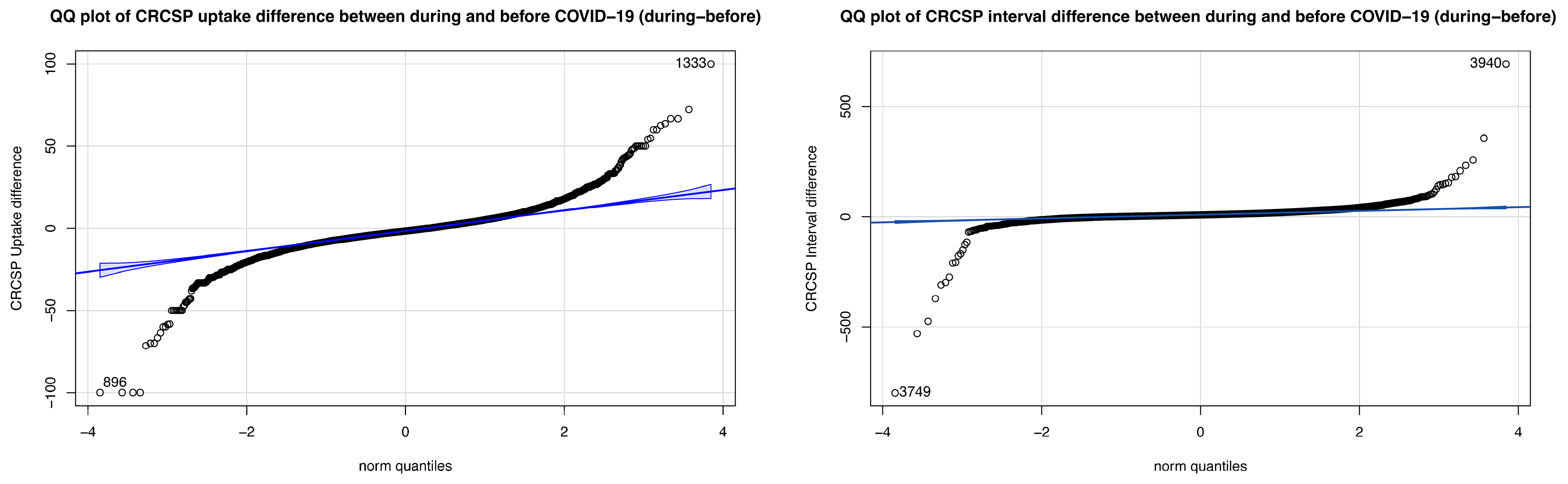
3.1. CRCSP Screening Uptake Rate and Screening Interval in 2018–2022
3.2. Description of SES Variables and Outcomes
3.3. Determinants of Differences in Screening Uptake Rate
3.4. Determinants of Difference in Screening Interval
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Bevan, R.; Rutter, M.D. Colorectal Cancer Screening-Who, How, and When? Clin. Endosc. 2018, 51, 37–49. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. Colorectal cancer screening. In IARC Handb Cancer Prev; International Agency for Research on Cancer: Lyon, France, 2019; Volume 17, p. 300. [Google Scholar]
- World Health Organization. Cancer Control: Module 3: Early Detection; World Health Organization, Ed.; WHO Press: Geneva, Switzerland, 2007; p. 50. [Google Scholar]
- Allemani, C.; Rachet, B.; Weir, H.K.; Richardson, L.C.; Lepage, C.; Faivre, J.; Gatta, G.; Capocaccia, R.; Sant, M.; Baili, P.; et al. Colorectal cancer survival in the USA and Europe: A CONCORD high-resolution study. BMJ Open 2013, 3, e003055. [Google Scholar] [CrossRef] [PubMed]
- Toes-Zoutendijk, E.; Kooyker, A.I.; Elferink, M.A.; Spaander, M.C.W.; Dekker, E.; Koning, H.J.; Lemmens, V.E.; van Leerdam, M.E.; Lansdorp-Vogelaar, I.; group, L.W. Stage distribution of screen-detected colorectal cancers in the Netherlands. Gut 2018, 67, 1745–1746. [Google Scholar] [CrossRef] [PubMed]
- Jidkova, S.; Hoeck, S.; Kellen, E.; le Cessie, S.; Goossens, M.C. Flemish population-based cancer screening programs: Impact of COVID-19 related shutdown on short-term key performance indicators. BMC Cancer 2022, 22, 183. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Comprehensive Cervical Cancer Control: A Guide to Essential Practice, 2nd ed.; World Health Organization, Ed.; WHO Press: Geneva, Switzerland, 2014; p. 364. [Google Scholar]
- Ponti, A.; Anttila, A.; Ronco, G.; Senore, C.; Basu, P.; Segnan, N.; Tomatis, M. Against Cancer. Cancer screening in the European Union. In Report on the Implementation of the Council Recommendation on Cancer Screening; European Commission: Brussels, Belgium, 2017; p. 333. [Google Scholar]
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 15 September 2024).
- Sud, A.; Jones, M.E.; Broggio, J.; Loveday, C.; Torr, B.; Garrett, A.; Nicol, D.L.; Jhanji, S.; Boyce, S.A.; Gronthoud, F.; et al. Collateral damage: The impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann. Oncol. 2020, 31, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Ricciardiello, L.; Ferrari, C.; Cameletti, M.; Gaianill, F.; Buttitta, F.; Bazzoli, F.; Luigi de’Angelis, G.; Malesci, A.; Laghi, L. Impact of SARS-CoV-2 Pandemic on Colorectal Cancer Screening Delay: Effect on Stage Shift and Increased Mortality. Clin. Gastroenterol. Hepatol. 2021, 19, 1410–1417.e9. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, V.; Lucidi, C.; Bruno, G.; Lisi, D.; Miglioresi, L.; Bazuro, M.E. Impact of COVID-19 Pandemic on Colorectal Cancer Screening Program. Clin. Color. Cancer 2021, 20, e5–e11. [Google Scholar] [CrossRef] [PubMed]
- Teglia, F.; Angelini, M.; Astolfi, L.; Casolari, G.; Boffetta, P. Global Association of COVID-19 Pandemic Measures With Cancer Screening: A Systematic Review and Meta-analysis. JAMA Oncol. 2022, 8, 1287–1293. [Google Scholar] [CrossRef]
- Lee, K.; Lee, Y.Y.; Suh, M.; Jun, J.K.; Park, B.; Kim, Y.; Choi, K.S. Impact of COVID-19 on cancer screening in South Korea. Sci. Rep. 2022, 12, 11380. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, S.A.; Star, J.; Bandi, P.; Minihan, A.; Han, X.; Yabroff, K.R.; Jemal, A. Changes in Cancer Screening in the US During the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e2215490. [Google Scholar] [CrossRef]
- Fedewa, S.A.; Cullati, S.; Bouchardy, C.; Welle, I.; Burton-Jeangros, C.; Manor, O.; Courvoisier, D.S.; Guessous, I. Colorectal Cancer Screening in Switzerland: Cross-Sectional Trends (2007–2012) in Socioeconomic Disparities. PLoS ONE 2015, 10, e0131205. [Google Scholar] [CrossRef]
- Hoeck, S.; van de Veerdonk, W.; De Brabander, I.; Kellen, E. Does the Flemish colorectal cancer screening programme reach equity in FIT uptake? Eur. J. Public Health 2019, 29, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.N.; Peeters, M.; Hoeck, S.; Van Hal, G.; Janssens, S.; De Schutter, H. Optimizing the colorectal cancer screening programme using faecal immunochemical test (FIT) in Flanders, Belgium from the “interval cancer” perspective. Br. J. Cancer 2022, 126, 1091–1099. [Google Scholar] [CrossRef] [PubMed]
- Hoeck, S.; Van Roy, K.; Willems, S. Barriers and facilitators to participate in the colorectal cancer screening programme in Flanders (Belgium): A focus group study. Acta Clin. Belg. 2022, 77, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Tran, T.N.; Hoeck, S.; Peeters, M.; Goossens, M.; van Hal, G. Differences and Similarities in Breast and Colorectal Cancer Screening Uptake among Municipalities in Flanders, Belgium. Gastrointest. Disord. 2022, 4, 84–96. [Google Scholar] [CrossRef]
- Centrum voor Kankeropsporing. Statistical Sectors Explained (Video). Available online: https://bevolkingsonderzoek.incijfers.be/jive (accessed on 27 September 2024).
- Chen, R.C.; Haynes, K.; Du, S.; Barron, J.; Katz, A.J. Association of Cancer Screening Deficit in the United States With the COVID-19 Pandemic. JAMA Oncol. 2021, 7, 878–884. [Google Scholar] [CrossRef]
- Miller, M.J.; Xu, L.; Qin, J.; Hahn, E.E.; Ngo-Metzger, Q.; Mittman, B.; Tewari, D.; Hodeib, M.; Wride, P.; Saraiya, M.; et al. Impact of COVID-19 on Cervical Cancer Screening Rates Among Women Aged 21–65 Years in a Large Integrated Health Care System—Southern California, 1 January–30 September 2019, and 1 January–30 September 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 109–113. [Google Scholar] [CrossRef]
- Wentzensen, N.; Clarke, M.A.; Perkins, R.B. Impact of COVID-19 on cervical cancer screening: Challenges and opportunities to improving resilience and reduce disparities. Prev. Med. 2021, 151, 106596. [Google Scholar] [CrossRef]
- Miller, M.M.; Meneveau, M.O.; Rochman, C.M.; Schroen, A.T.; Lattimore, C.M.; Gaspard, P.A.; Cubbage, R.S.; Showalter, S.L. Impact of the COVID-19 pandemic on breast cancer screening volumes and patient screening behaviors. Breast Cancer Res. Treat. 2021, 189, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Braveman, P.A.; Cubbin, C.; Egerter, S.; Williams, D.R.; Pamuk, E. Socioeconomic disparities in health in the United States: What the patterns tell us. Am. J. Public Health 2010, 100 (Suppl. S1), S186–S196. [Google Scholar] [CrossRef] [PubMed]
- Dickman, S.L.; Himmelstein, D.U.; Woolhandler, S. Inequality and the health-care system in the USA. Lancet 2017, 389, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Stormacq, C.; Van den Broucke, S.; Wosinski, J. Does health literacy mediate the relationship between socioeconomic status and health disparities? Integrative review. Health Promot. Int. 2019, 34, e1–e17. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Sharifi, A.; Damanbagh, S.; Nazarnia, H.; Nazarnia, M. Impacts of the COVID-19 pandemic on the social sphere and lessons for crisis management: A literature review. Nat. Hazards 2023, 117, 2139–2164. [Google Scholar] [CrossRef]
- Adler, N.E.; Newman, K. Socioeconomic disparities in health: Pathways and policies. Health Aff. 2002, 21, 60–76. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, S.A.; Lawrence, B.; Elder, R.; Mercer, S.L.; Wilson, K.M.; DeVinney, B.; Melillo, S.; Carvalho, M.; Taplin, S.; Bastani, R.; et al. Effectiveness of interventions to increase screening for breast, cervical, and colorectal cancers: Nine updated systematic reviews for the guide to community preventive services. Am. J. Prev. Med. 2012, 43, 97–118. [Google Scholar] [CrossRef]
- Zwirner, E.; Raihani, N. Neighbourhood wealth, not urbanicity, predicts prosociality towards strangers. Proc. Biol. Sci. 2020, 287, 20201359. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Eurostat Regional Yearbook, 2022nd ed.; Kotzeva, M., Ed.; Publicatios Office of the European Union: Luxembourg, 2022. [Google Scholar]
- Kadian, L.; Gulshan, G.; Sharma, S.; Kumari, I.; Yadav, C.; Nanda, S.; Yadav, R. A Study on Knowledge and Awareness of Cervical Cancer Among Females of Rural and Urban Areas of Haryana, North India. J. Cancer Educ. 2021, 36, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Wools, A.; Dapper, E.A.; de Leeuw, J.R. Colorectal cancer screening participation: A systematic review. Eur. J. Public Health 2016, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Equity, Social Determinants and Public Health Programmes; WHO Press: Geneva, Switzerland, 2019; p. 291. [Google Scholar]
- Ding, L.; Jidkova, S.; Greuter, M.J.W.; Van Herck, K.; Goossens, M.; De Schutter, H.; Martens, P.; Van Hal, G.; de Bock, G.H. The Role of Socio-Demographic Factors in the Coverage of Breast Cancer Screening: Insights From a Quantile Regression Analysis. Front. Public Health 2021, 9, 648278. [Google Scholar] [CrossRef]
- Fedewa, S.A.; Sauer, A.G.; Siegel, R.L.; Jemal, A. Prevalence of major risk factors and use of screening tests for cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2015, 24, 637–652. [Google Scholar] [CrossRef] [PubMed]
- Leung, D.Y.; Wong, E.M.; Chan, C.W. Determinants of participation in colorectal cancer screening among community-dwelling Chinese older people: Testing a comprehensive model using a descriptive correlational study. Eur. J. Oncol. Nurs. 2016, 21, 17–23. [Google Scholar] [CrossRef]
- Myong, J.P.; Shin, J.Y.; Kim, S.J. Factors associated with participation in colorectal cancer screening in Korea: The Fourth Korean National Health and Nutrition Examination Survey (KNHANES IV). Int. J. Color. Dis. 2012, 27, 1061–1069. [Google Scholar] [CrossRef]
- Wilson, H.F.D. Unemployment and Mental Health. Available online: https://www.health.org.uk/publications/long-reads/unemployment-and-mental-health (accessed on 5 June 2024).
- Falk, G.; Romero, P.D.; Nicchitta, I.A.; Nyhof, E.C. Unemployment Rates During the COVID-19 Pandemic; R46554; Congressional Research Service (CRS): Washington, DC, USA, 2021. [Google Scholar]
- de Klerk, C.M.; Gupta, S.; Dekker, E.; Essink-Bot, M.L.; Expert Working Group’ Coalition to Reduce Inequities in Colorectal Cancer Screening’ of the World Endoscopy Organization. Socioeconomic and ethnic inequities within organised colorectal cancer screening programmes worldwide. Gut 2018, 67, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Honein-AbouHaidar, G.N.; Kastner, M.; Vuong, V.; Perrier, L.; Daly, C.; Rabeneck, L.; Straus, S.; Baxter, N.N. Systematic Review and Meta-study Synthesis of Qualitative Studies Evaluating Facilitators and Barriers to Participation in Colorectal Cancer Screening. Cancer Epidemiol. Biomark. Prev. 2016, 25, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Mohnen, S.M.; Schneider, S.; Droomers, M. Neighborhood characteristics as determinants of healthcare utilization—A theoretical model. Health Econ. Rev. 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Putrik, P.; de Vries, N.K.; Mujakovic, S.; van Amelsvoort, L.; Kant, I.; Kunst, A.E.; van Oers, H.; Jansen, M. Living environment matters: Relationships between neighborhood characteristics and health of the residents in a Dutch municipality. J. Community Health 2015, 40, 47–56. [Google Scholar] [CrossRef]
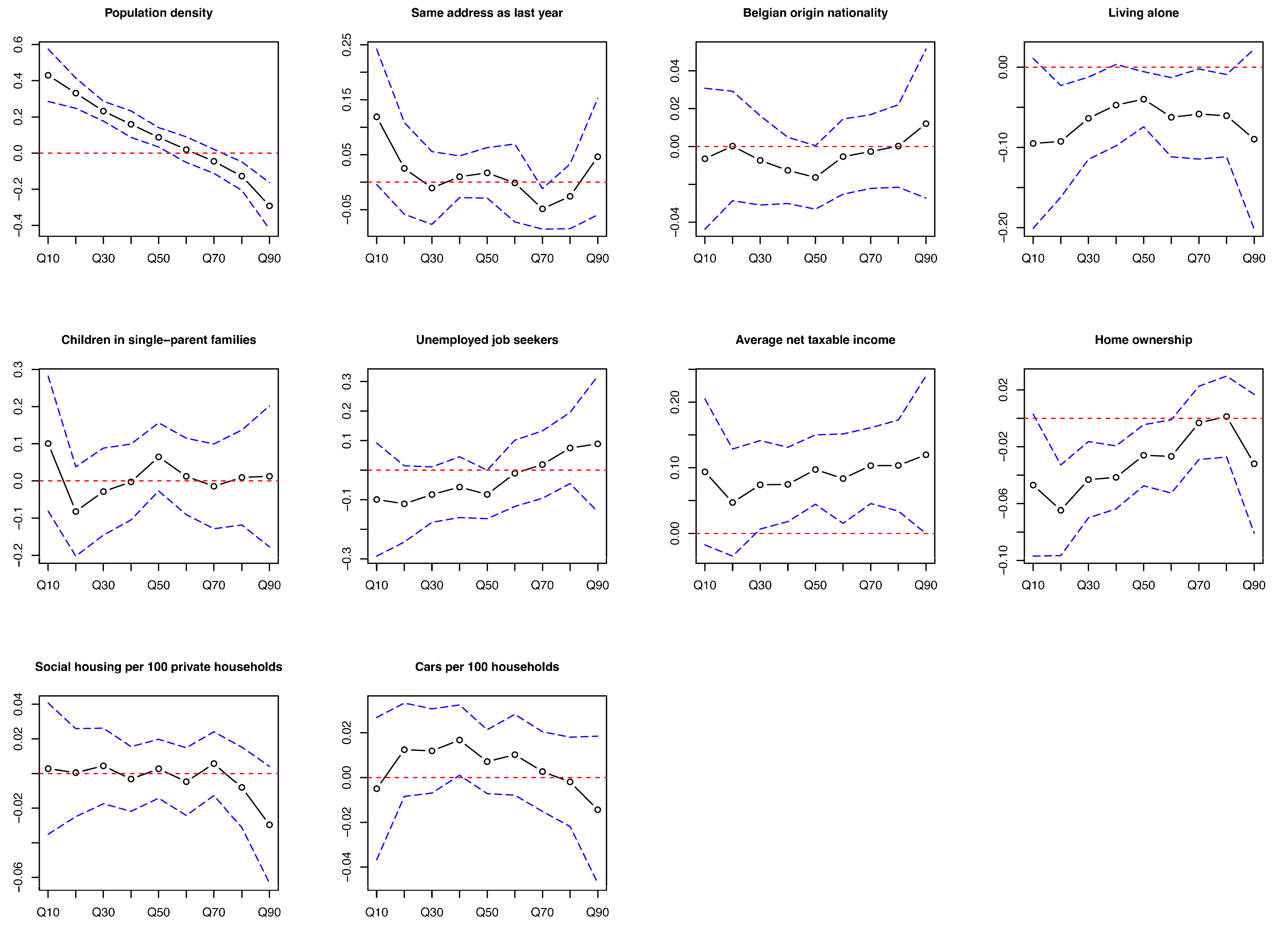
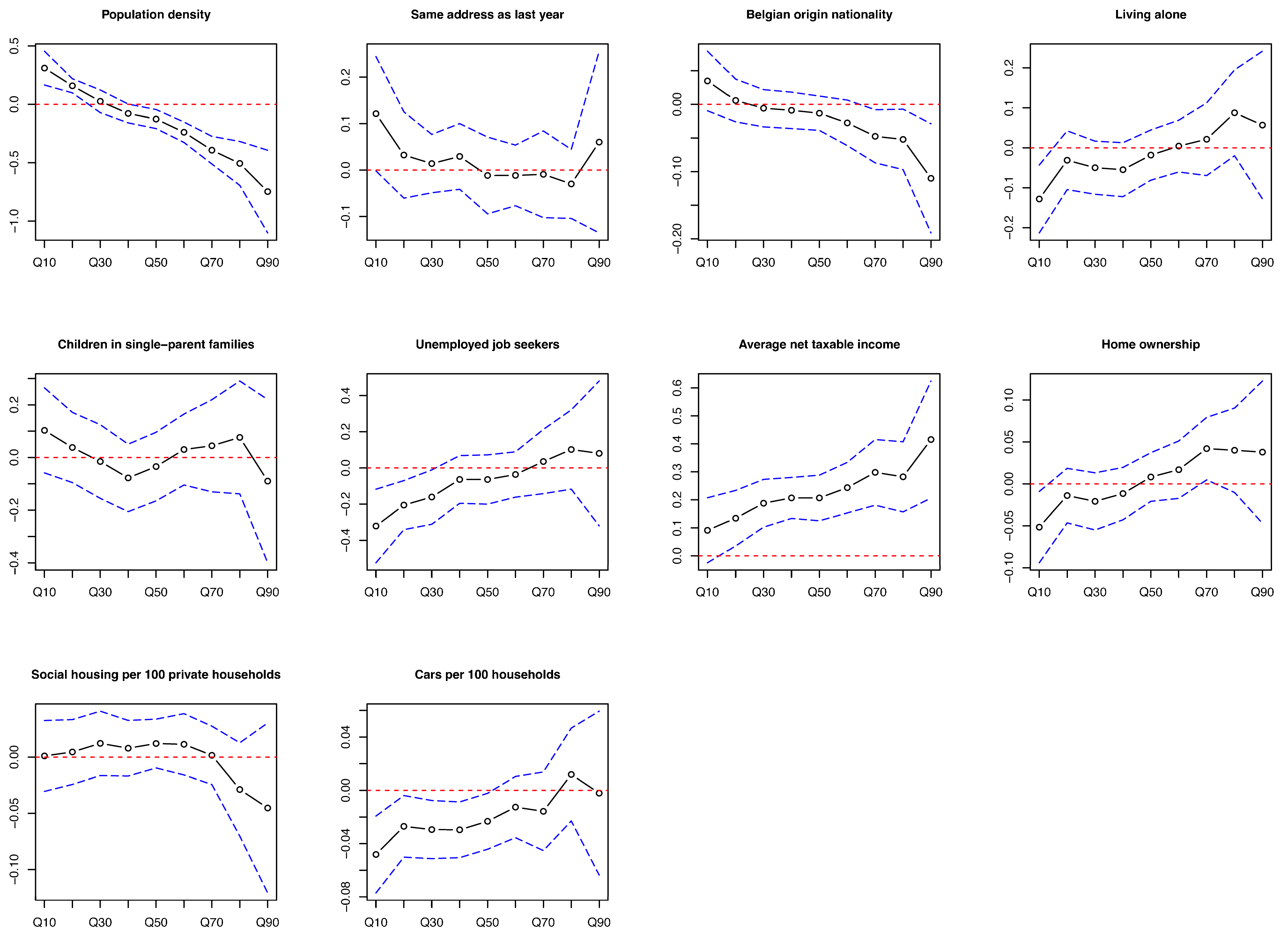
| Variables/Outcome | Q10 | Q20 | Q30 | Q40 | Q50 | Q60 | Q70 | Q80 | Q90 |
|---|---|---|---|---|---|---|---|---|---|
| Median uptake difference, % | −15.1 | −8.4 | −5.7 | −3.8 | −2.3 | −0.9 | 0.6 | 2.7 | 5.5 |
| Uptake difference range, % | ≤−10.8 | (−10.8, −7.0) | (−7.0, −4.8) | (−4.8, −3.1) | (−3.1, −1.7) | (−1.7, −0.1) | (−0.1, 1.7) | (1.7, 3.8) | (3.8, 7.6) |
| Male percentage, % | 51.2 | 51.3 | 51.0 | 50.5 | 50.7 | 50.6 | 50.4 | 50.7 | 51.0 |
| ≥60 years old percentage, % | 47.1 | 48.1 | 49.0 | 49.4 | 49.3 | 49.0 | 48.7 | 48.6 | 49.2 |
| Participation history rate, % | 55.6 | 54.7 | 54.8 | 53.6 | 54.4 | 53.9 | 54.1 | 53.9 | 54.6 |
| Population density, /km2 | 310.0 | 1100.0 | 1260.0 | 1530.0 | 1440.0 | 1590.0 | 1490.0 | 1290.0 | 1100.0 |
| Same address as last year, % | 93.2 | 93.1 | 92.7 | 92.4 | 92.4 | 92.1 | 92.2 | 92.3 | 92.7 |
| Belgian origin nationality, % | 93.1 | 92.5 | 91.7 | 91.0 | 91.0 | 90.2 | 90.6 | 91.7 | 92.5 |
| Living alone, % | 9.9 | 10.2 | 10.3 | 11.1 | 10.5 | 11.1 | 11.0 | 10.5 | 10.1 |
| Children in single-parent families, % | 4.6 | 4.8 | 4.9 | 5.1 | 5.0 | 5.3 | 5.0 | 4.9 | 4.9 |
| Unemployed job seekers, % | 3.4 | 3.5 | 3.7 | 3.8 | 3.7 | 3.9 | 3.9 | 3.7 | 3.6 |
| Average net taxable income per inhabitant, € | 21,080.0 | 21,050.0 | 20,670.0 | 20,560.0 | 20,660.0 | 20,510.0 | 20,570.0 | 21,170.0 | 21,190.0 |
| Home ownership, % | 84.6 | 83.3 | 81.0 | 79.2 | 80.1 | 78.0 | 78.5 | 81.3 | 83.0 |
| Social housing, per 100 private households | 0.0 | 0.0 | 0.2 | 0.4 | 0.2 | 0.6 | 0.3 | 0.0 | 0.0 |
| Cars, per 100 households | 134.8 | 132.3 | 129.8 | 127.8 | 129.8 | 127.2 | 127.8 | 130.8 | 133.9 |
| Variables/Outcome | Q10 | Q20 | Q30 | Q40 | Q50 | Q60 | Q70 | Q80 | Q90 |
|---|---|---|---|---|---|---|---|---|---|
| Median screening interval difference, days | −6.0 | 1.0 | 3.5 | 6.0 | 7.5 | 9.5 | 12.0 | 15.0 | 20.0 |
| Interval difference range, days | ≤−1.5 | (−1.5, 2.0) | (2.0, 4.5) | (4.5, 6.5) | (6.5, 8.5) | (8.5, 10.5) | (10.5, 13.0) | (13.0, 17.0) | (17.0, 24.0) |
| Male percentage, % | 50.8 | 51.1 | 50.4 | 50.4 | 50.6 | 50.5 | 51.1 | 51.1 | 51.2 |
| ≥60 years old percentage, % | 49.2 | 48.9 | 50.0 | 50.5 | 49.3 | 48.8 | 48.7 | 48.0 | 47.3 |
| Participation history rate, % | 52.9 | 53.9 | 56.0 | 54.5 | 55.0 | 56.4 | 54.6 | 54.3 | 53.9 |
| Population density, /km2 | 480.0 | 1250.0 | 1450.0 | 1620.0 | 1490.0 | 1420.0 | 1330.0 | 1160.0 | 940.0 |
| Same address as last year, % | 93.0 | 92.6 | 92.6 | 92.3 | 92.4 | 92.2 | 92.4 | 92.7 | 93.1 |
| Belgian origin nationality, % | 93.0 | 91.5 | 91.1 | 90.9 | 90.7 | 91.8 | 91.6 | 91.9 | 92.6 |
| Living alone, % | 9.9 | 10.4 | 10.6 | 11.1 | 10.7 | 10.6 | 10.6 | 10.1 | 10.0 |
| Children in single-parent families, % | 4.7 | 4.8 | 5.0 | 5.0 | 5.1 | 4.8 | 5.0 | 4.8 | 4.8 |
| Unemployed job seekers, % | 3.6 | 3.7 | 3.9 | 3.9 | 3.8 | 3.6 | 3.7 | 3.5 | 3.5 |
| Average net taxable income per inhabitant, € | 20,900.0 | 20,790.0 | 20,620.0 | 20,660.0 | 20,450.0 | 20,810.0 | 20,920.0 | 21,130.0 | 21,280.0 |
| Home ownership, % | 83.9 | 81.9 | 81.3 | 79.7 | 79.0 | 79.7 | 79.8 | 82.5 | 84.1 |
| Social housing, per 100 private households | 0.0 | 0.0 | 0.3 | 0.4 | 0.4 | 0.3 | 0.3 | 0.0 | 0.0 |
| Cars, per 100 households | 134.6 | 130.9 | 130.0 | 127.8 | 128.6 | 129.5 | 129.7 | 132.0 | 133.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, S.; Ding, L.; Greuter, M.J.W.; Tran, T.N.; Sidorenkov, G.; Hoeck, S.; Goossens, M.; Van Hal, G.; de Bock, G.H. The Association of the COVID-19 Pandemic with the Uptake of Colorectal Cancer Screening Varies by Socioeconomic Status in Flanders, Belgium. Cancers 2024, 16, 3983. https://doi.org/10.3390/cancers16233983
Zheng S, Ding L, Greuter MJW, Tran TN, Sidorenkov G, Hoeck S, Goossens M, Van Hal G, de Bock GH. The Association of the COVID-19 Pandemic with the Uptake of Colorectal Cancer Screening Varies by Socioeconomic Status in Flanders, Belgium. Cancers. 2024; 16(23):3983. https://doi.org/10.3390/cancers16233983
Chicago/Turabian StyleZheng, Senshuang, Lilu Ding, Marcel J. W. Greuter, Thuy Ngan Tran, Grigory Sidorenkov, Sarah Hoeck, Mathieu Goossens, Guido Van Hal, and Geertruida H. de Bock. 2024. "The Association of the COVID-19 Pandemic with the Uptake of Colorectal Cancer Screening Varies by Socioeconomic Status in Flanders, Belgium" Cancers 16, no. 23: 3983. https://doi.org/10.3390/cancers16233983
APA StyleZheng, S., Ding, L., Greuter, M. J. W., Tran, T. N., Sidorenkov, G., Hoeck, S., Goossens, M., Van Hal, G., & de Bock, G. H. (2024). The Association of the COVID-19 Pandemic with the Uptake of Colorectal Cancer Screening Varies by Socioeconomic Status in Flanders, Belgium. Cancers, 16(23), 3983. https://doi.org/10.3390/cancers16233983







