Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Controls
2.2. Enzyme-Linked Immunosorbent Assay
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants
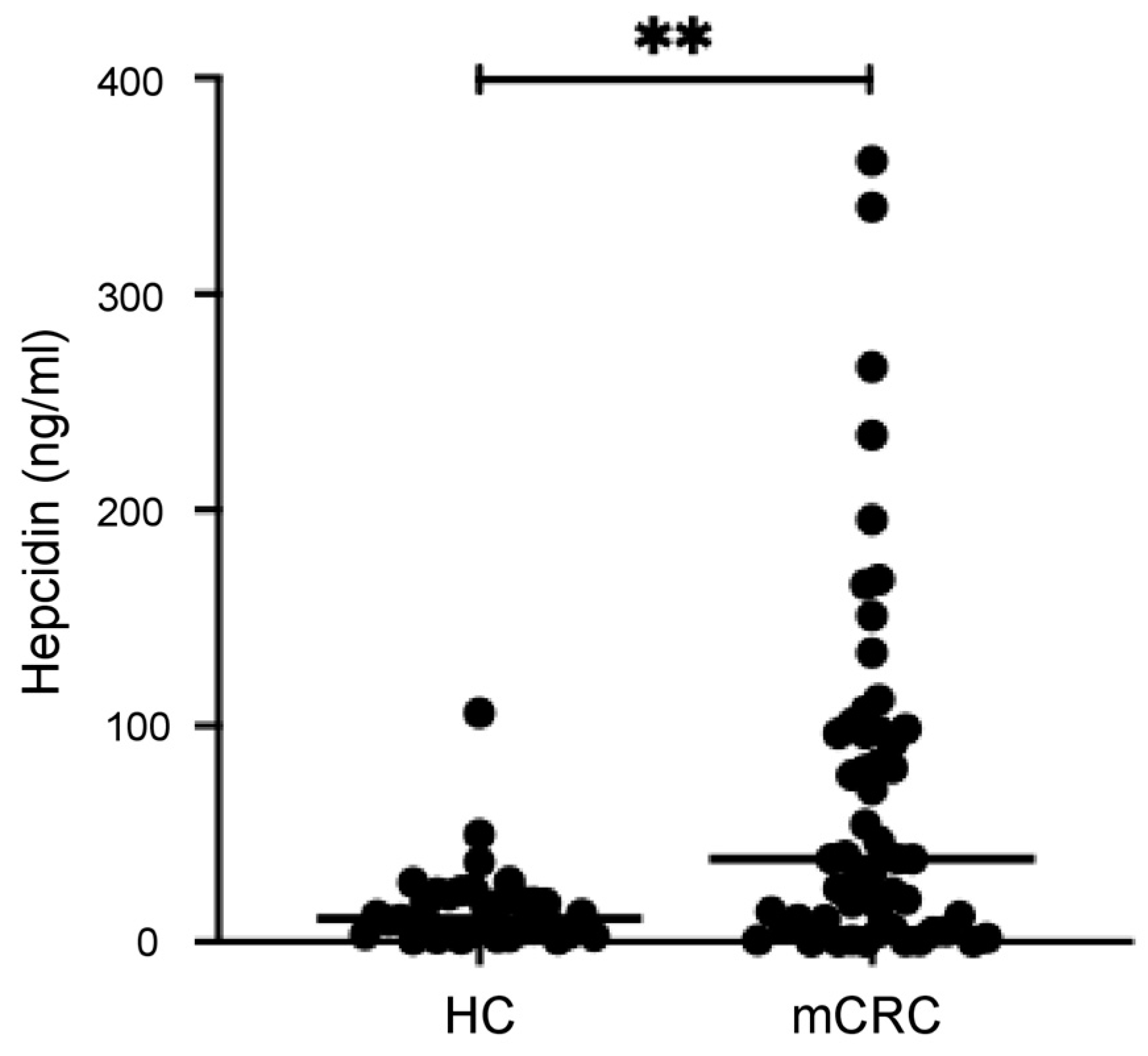
3.2. Serum Hepcidin Levels Are Higher in mCRC Patients than in the Controls
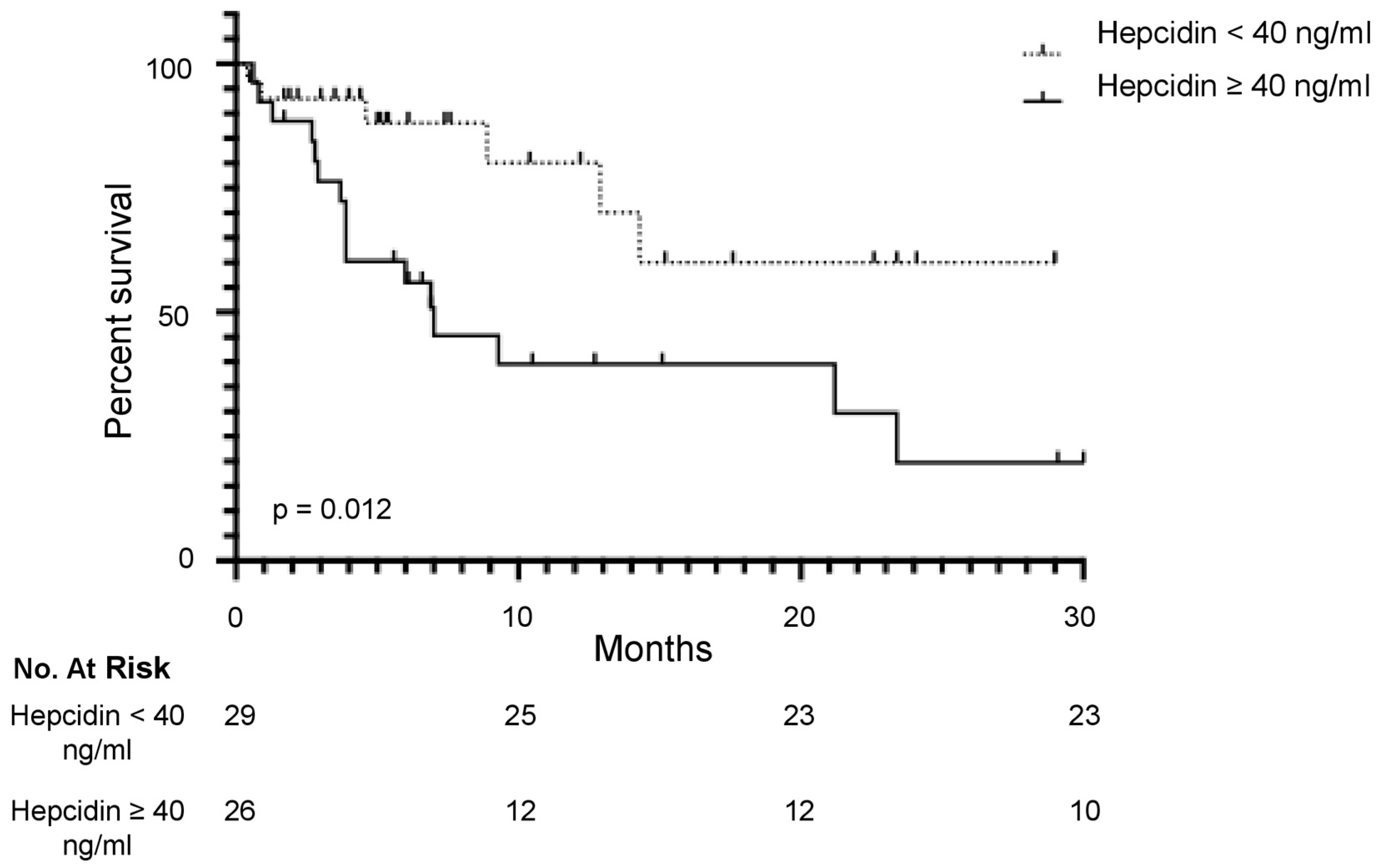
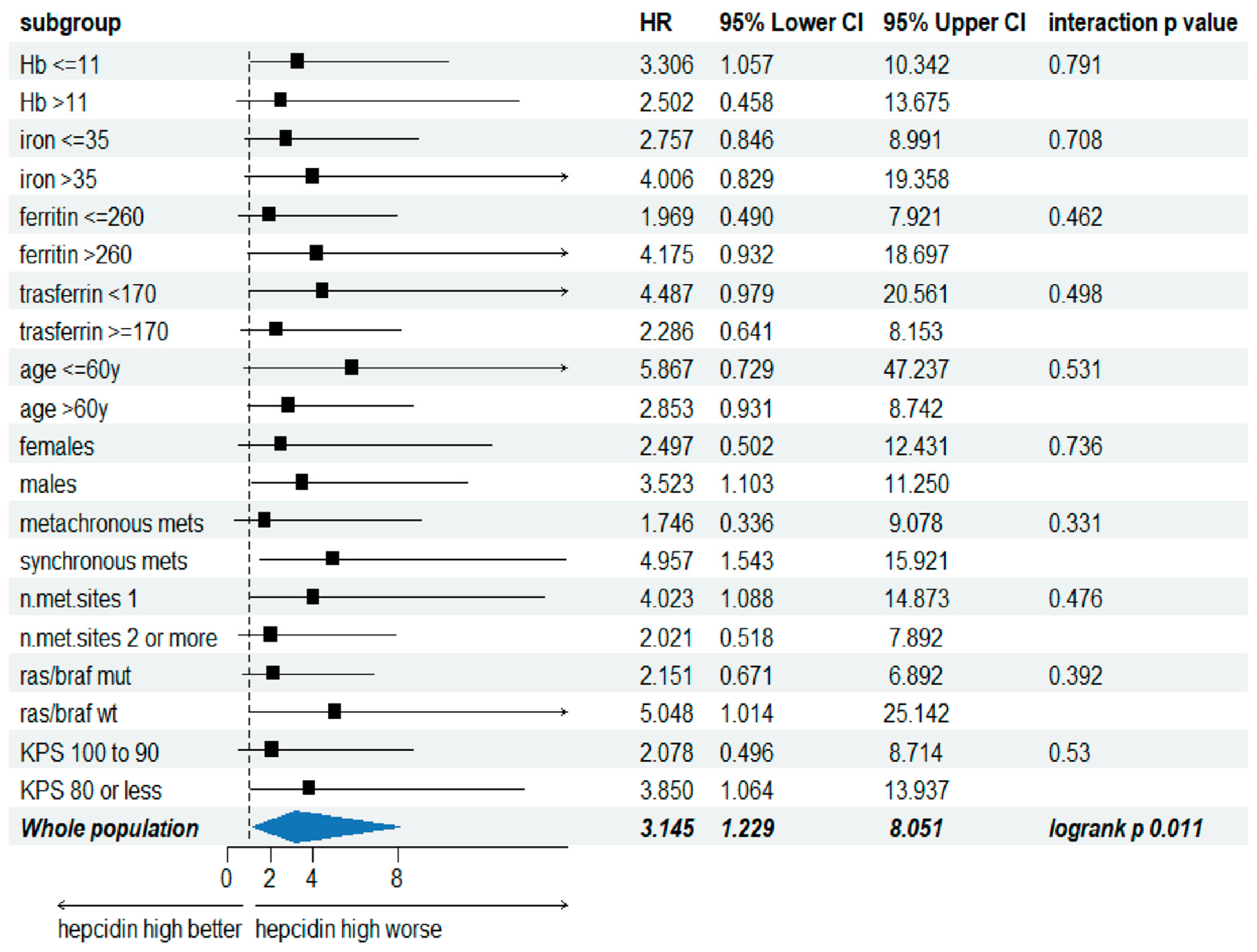
3.3. Serum Hepcidin Level Is a Predictive Factor of the Overall Survival in mCRC
3.4. Correlation Between Serum Hepcidin and Iron Metabolism-Related Factors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic Colorectal Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Andre, T.; Amonkar, M.; Norquist, J.M.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.J.A.; Smith, D.; Garcia-Carbonero, R.; et al. Health-Related Quality of Life in Patients with Microsatellite Instability-High or Mismatch Repair Deficient Metastatic Colorectal Cancer Treated with First-Line Pembrolizumab versus Chemotherapy (KEYNOTE-177): An Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Koopman, M.; Kortman, G.A.M.; Mekenkamp, L.; Ligtenberg, M.J.L.; Hoogerbrugge, N.; Antonini, N.F.; Punt, C.J.A.; van Krieken, J.H.J.M. Deficient Mismatch Repair System in Patients with Sporadic Advanced Colorectal Cancer. Br. J. Cancer 2009, 100, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Koessler, T.; Alsina, M.; Arnold, D.; Ben-Aharon, I.; Collienne, M.; Lutz, M.P.; Neuzillet, C.; Obermannova, R.; Peeters, M.; Sclafani, F.; et al. ESMO Congress 2021: Highlights from the EORTC Gastrointestinal Tract Cancer Group’s Perspective. ESMO Open 2022, 7, 100392. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal Cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the Iron Homeostatic Hormone Hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef]
- Di Grazia, A.; Di Fusco, D.; Franzè, E.; Colella, M.; Strimpakos, G.; Salvatori, S.; Formica, V.; Laudisi, F.; Maresca, C.; Colantoni, A.; et al. Hepcidin Upregulation in Colorectal Cancer Associates with Accumulation of Regulatory Macrophages and Epithelial–Mesenchymal Transition and Correlates with Progression of the Disease. Cancers 2022, 14, 5294. [Google Scholar] [CrossRef]
- Sornjai, W.; Nguyen Van Long, F.; Pion, N.; Pasquer, A.; Saurin, J.-C.; Marcel, V.; Diaz, J.J.; Mertani, H.C.; Smith, D.R. Iron and Hepcidin Mediate Human Colorectal Cancer Cell Growth. Chem. Biol. Interact. 2020, 319, 109021. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Goyert, J.W.; Solanki, S.; Kerk, S.A.; Chen, B.; Castillo, C.; Hsu, P.P.; Do, B.T.; Singhal, R.; Dame, M.K.; et al. Hepcidin Sequesters Iron to Sustain Nucleotide Metabolism and Mitochondrial Function in Colorectal Cancer Epithelial Cells. Nat. Metab. 2021, 3, 969–982. [Google Scholar] [CrossRef]
- Di Grazia, A.; Franzè, E.; Frascatani, R.; Laudisi, F.; Pacifico, T.; Tomassini, L.; Di Fusco, D.; Formica, V.; Sica, G.; Stolfi, C.; et al. Targeting Hepcidin in Colorectal Cancer Triggers a TNF-Dependent-Gasdermin E-Driven Immunogenic Cell Death Response. Exp. Hematol. Oncol. 2024, 13, 95. [Google Scholar] [CrossRef] [PubMed]
- Tomosugi, N.; Kawabata, H.; Wakatabe, R.; Higuchi, M.; Yamaya, H.; Umehara, H.; Ishikawa, I. Detection of Serum Hepcidin in Renal Failure and Inflammation by Using ProteinChip System. Blood 2006, 108, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A New Mouse Liver-Specific Gene, Encoding a Protein Homologous to Human Antimicrobial Peptide Hepcidin, Is Overexpressed during Iron Overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Valore, E.V.; Territo, M.; Schiller, G.; Lichtenstein, A.; Ganz, T. Hepcidin, a Putative Mediator of Anemia of Inflammation, Is a Type II Acute-Phase Protein. Blood 2003, 101, 2461–2463. [Google Scholar] [CrossRef] [PubMed]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; Van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; Den Heijer, M.; et al. Serum Hepcidin: Reference Ranges and Biochemical Correlates in the General Population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef]
- Demir, H.; Beypinar, I.; Urvay, S.; Davarcı, S.E.; Baykara, M. Prognostic Role of Pre-Operative Serum Ferritin Level in Stage 2 Colon Cancer. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6473–6479. [Google Scholar] [CrossRef]
- Giessen-Jung, C.; Nagel, D.; Glas, M.; Spelsberg, F.; Lau-Werner, U.; Modest, D.P.; Schulz, C.; Heinemann, V.; Di Gioia, D.; Stieber, P. Preoperative Serum Markers for Individual Patient Prognosis in Stage I–III Colon Cancer. Tumor Biol. 2015, 36, 7897–7906. [Google Scholar] [CrossRef]
- Słomka, A.; Łęcka, M.; Styczyński, J. Hepcidin in Children and Adults with Acute Leukemia or Undergoing Hematopoietic Cell Transplantation: A Systematic Review. Cancers 2022, 14, 4936. [Google Scholar] [CrossRef]
- El-Mahdy, R.I.; Zakhary, M.M.; Maximous, D.W.; Mokhtar, A.A.; El Dosoky, M.I. Circulating Osteocyte-Related Biomarkers (Vitamin D, Sclerostin, Dickkopf-1), Hepcidin, and Oxidative Stress Markers in Early Breast Cancer: Their Impact in Disease Progression and Outcome. J. Steroid Biochem. Mol. Biol. 2020, 204, 105773. [Google Scholar] [CrossRef]
- Liu, J.; Chen, S.; Ye, X. The Effect of Red Blood Cell Transfusion on Plasma Hepcidin and Growth Differentiation Factor 15 in Gastric Cancer Patients: A Prospective Study. Ann. Transl. Med. 2019, 7, 466. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, L.; Ma, Y.; Wu, X.; Jin, L.; Yu, F. Increased Hepcidin Expression in Non-Small Cell Lung Cancer Tissue and Serum Is Associated with Clinical Stage. Thorac. Cancer 2014, 5, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Victor, M.; Evgeniy, H.; Gergana, T.; Julia, P.; Vasil, V.; Borislav, M.; Ivo, B.; Zlatina, G.; Kamen, T. Serum Hepcidin Levels in Multiple Myeloma. Clin. Lab. 2017, 63, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Traeger, L.; Ellermann, I.; Wiethoff, H.; Ihbe, J.; Gallitz, I.; Eveslage, M.; Moritz, R.; Herrmann, E.; Schrader, A.J.; Steinbicker, A.U. Serum Hepcidin and GDF-15 Levels as Prognostic Markers in Urothelial Carcinoma of the Upper Urinary Tract and Renal Cell Carcinoma. BMC Cancer 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Ando, M.; Tsuchiya, K.; Nitta, K. Serum Hepcidin-25 Level Linked with High Mortality in Patients with Non-Hodgkin Lymphoma. Ann. Hematol. 2015, 94, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Xiao, X.; Liang, N.; Dong, L.; Wei, S.; Mo, L.; Zhao, W.; Cai, Y. Clinical Significance of Serum Iron Metabolism-Related Markers in Patients with Nasopharyngeal Carcinoma. ORL J. Otorhinolaryngol. Relat. Spec. 2023, 85, 223–230. [Google Scholar] [CrossRef]
- Joachim, J.H.; Mehta, K.J. Hepcidin in Hepatocellular Carcinoma. Br. J. Cancer 2022, 127, 185–192. [Google Scholar] [CrossRef]
- Formica, V.; Riondino, S.; Morelli, C.; Guerriero, S.; D’Amore, F.; Di Grazia, A.; Del Vecchio Blanco, G.; Sica, G.; Arkenau, H.-T.; Monteleone, G.; et al. HIF2α, Hepcidin and Their Crosstalk as Tumour-Promoting Signalling. Br. J. Cancer 2023, 129, 222–236. [Google Scholar] [CrossRef]
| Characteristics | Hepcidin ≥ 40 ng/mL (N = 26) | Hepcidin < 40 ng/mL (N = 29) | p-Value |
|---|---|---|---|
| Age (years), median [range] | 63 (44–91) | 70 (41–85) | 0.1085 |
| Female gender, n (%) | 10 (38.5) | 7 (24) | 0.4000 |
| Disease localization, n (%): | |||
| Rectal | 5 (19.2) | 2 (7) | 0.0057 |
| Colon | 21 (80.8) | 27 (93) | |
| Metastasis, n (%): | |||
| 1 | 16 (61.5) | 21 (72.4) | 0.7289 |
| >1 | 10 (38.5) | 8 (27.6) | |
| Sync, n (%): | 16 (61.5) | 18 (62) | |
| Meta, n (%): | 10 (38.5) | 11 (38) | 0.5883 |
| RAS/BRAF, n (%): | |||
| WT | 9 (34.6) | 13 (44.8) | 0.3982 |
| MUT | 17 (65.4) | 16 (55.2) | |
| BMI, n (%): | |||
| >21.7 | 14 (54) | 25 (86) | 0.2118 |
| ≤21.7 | 12(46) | 4 (14) | |
| KPS, n (%): | |||
| <80 | 13 (50) | 10 (34.5) | 0.3213 |
| 90–100 | 13 (50) | 19 (65.5) | |
| CEA, n (%): | |||
| >1.82 | 20 (77) | 15 (52) | 0.1694 |
| ≤1.82 | 6 (23) | 14 (48) | |
| Hb, n (%): | |||
| >11.5 | 10 (38.5) | 15 (52) | 0.8969 |
| ≤11.5 | 16 (61.5) | 14 (48) |
| Variable | p | Hazard Ratio | 95% CI |
|---|---|---|---|
| CEA | 0.1370 | 1.0009 | 0.9997–1.0020 |
| Hepcidin > 40 ng/mL | 0.0460 | 2.6796 | 1.0175–7.0569 |
| kPS ≤ 80 | 0.0291 | 2.9528 | 1.1168–7.8073 |
| Number of metastatic sites ≥2 | 0.7149 | 1.1984 | 0.4538–3.1648 |
| RAS/BRAF wild-type | 0.8760 | 1.0786 | 0.4171–2.7895 |
| Ferritin Concentration (ng/mL) | Iron Concentration (μg/dL) | Transferrin Concentration (mg/dL) | Hemoglobin Concentration (g/dL) | ||
|---|---|---|---|---|---|
| Iron | Correlation | 0.146 | |||
| Concentration | Coefficient | ||||
| (μg/dL) | |||||
| p | 0.2888 | ||||
| n | 55 | ||||
| Transferrin | Correlation | −0.408 | 0.129 | ||
| Concentration | Coefficient | ||||
| (mg/dL) | |||||
| p | 0.0020 | 0.3469 | |||
| n | 55 | 55 | |||
| Hemoglobin | Correlation | 0.022 | 0.264 | −0.174 | |
| Concentration | Coefficient | ||||
| (g/dL) | |||||
| p | 0.8736 | 0.0513 | 0.2033 | ||
| n | 55 | 55 | 55 | ||
| Hepcidin | Correlation | 0.399 | 0.159 | −0.207 | −0.126 |
| Level (ng/mL) | Coefficient | ||||
| p | 0.0025 | 0.2469 | 0.1287 | 0.3577 | |
| n | 55 | 55 | 55 | 55 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Formica, V.; Di Grazia, A.; Bonomo, M.V.; Frascatani, R.; Mancone, R.; Monteleone, G. Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment. Cancers 2024, 16, 3977. https://doi.org/10.3390/cancers16233977
Formica V, Di Grazia A, Bonomo MV, Frascatani R, Mancone R, Monteleone G. Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment. Cancers. 2024; 16(23):3977. https://doi.org/10.3390/cancers16233977
Chicago/Turabian StyleFormica, Vincenzo, Antonio Di Grazia, Maria Vittoria Bonomo, Rachele Frascatani, Roberto Mancone, and Giovanni Monteleone. 2024. "Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment" Cancers 16, no. 23: 3977. https://doi.org/10.3390/cancers16233977
APA StyleFormica, V., Di Grazia, A., Bonomo, M. V., Frascatani, R., Mancone, R., & Monteleone, G. (2024). Circulating Hepcidin Levels Are an Independent Predictor of Survival in Microsatellite Stable Metastatic Colorectal Cancer Patient Candidates for Standard First-Line Treatment. Cancers, 16(23), 3977. https://doi.org/10.3390/cancers16233977






