Expression of CK17 and SOX2 in Vulvar Intraepithelial Neoplasia: A Comprehensive Analysis of 150 Vulvar Lesions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Categorization of Vulvar Lesions
2.2. Tissue Processing
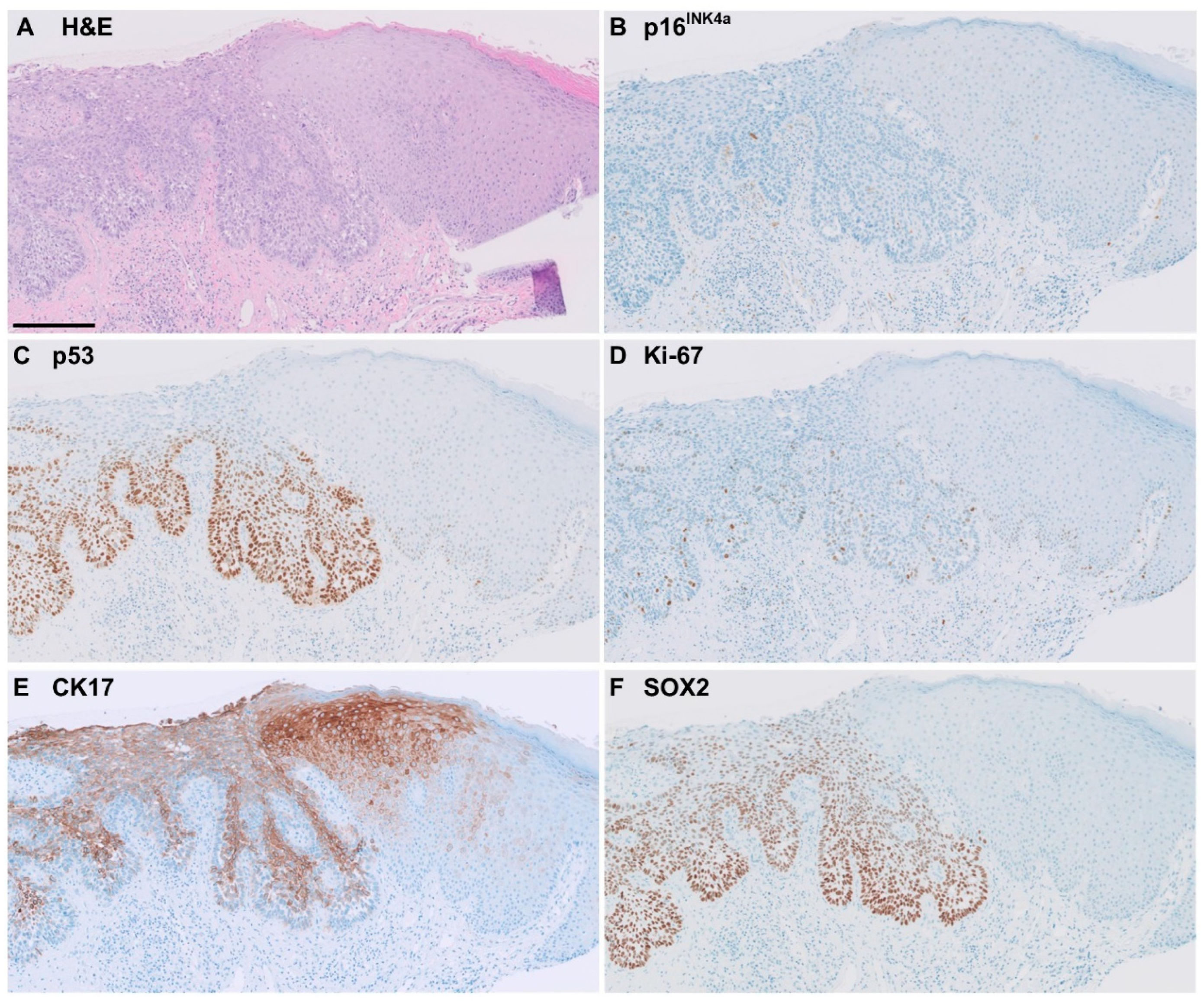
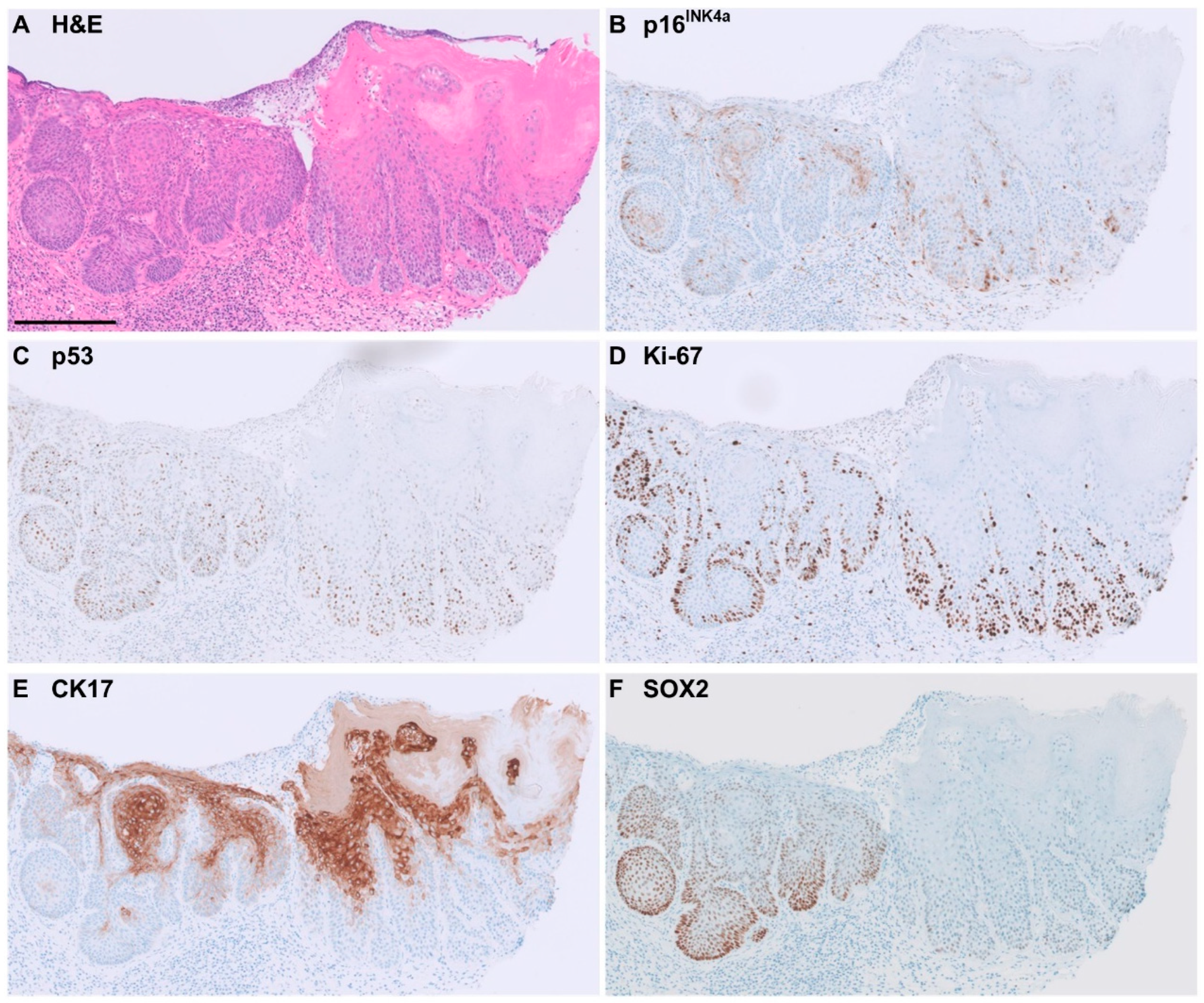
2.3. Immunohistochemical Staining Patterns for p16INK4a, p53, Ki-67, CK1, and SOX2
2.4. Statistical Analysis
3. Results
3.1. Vulvar Disease Categories
3.2. CK17 Immunohistochemistry
3.3. SOX2 Immunohistochemistry
3.4. Performance of Markers for Accurate Diagnosis of HPV-Independent VIN
3.5. Prognostic Value of CK17 and SOX2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Classification of Tumours, Female Genital Tumours, 5th ed.; IARC Press: Lyon, France, 2020.
- Thuijs, N.B.; van Beurden, M.; Duin, S.; Heideman, D.A.M.; Berkhof, J.; Steenbergen, R.D.M.; Bleeker, M.C.G. High-grade vulvar intraepithelial neoplasia: Comprehensive characterization and long-term vulvar carcinoma risk. Histopathology 2024, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Almadani, N.; Thompson, E.F.; Tessier-Cloutier, B.; Chen, J.; Ho, J.; Senz, J.; McConechy, M.K.; Chow, C.; Ta, M.; et al. Lesions Classification of Vulvar Squamous Cell Carcinoma and Precursor Lesions by p16 and p53 Immunohistochemistry: Considerations, Caveats, and an Algorithmic Approach. Mod. Pathol. 2023, 36, 100145. [Google Scholar] [CrossRef] [PubMed]
- Parra-Herran, C.; Nucci, M.R.; Singh, N.; Rakislova, N.; Howitt, B.E.; Hoang, L.; Gilks, C.B.; Bosse, T.; Watkins, J.C. HPV-independent, p53-wild-type vulvar intraepithelial neoplasia: A review of nomenclature and the journey to characterize verruciform and acanthotic precursor lesions of the vulva. Mod. Pathol. 2022, 35, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Heller, D.S.; Day, T.; Allbritton, J.I.; Scurry, J.; Radici, G.; Welch, K.; Preti, M.; Committee, I.D.P.D. Diagnostic Criteria for Differentiated Vulvar Intraepithelial Neoplasia and Vulvar Aberrant Maturation. J. Low. Genit. Tract. Dis. 2021, 25, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.G.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E.; et al. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) Consensus Statements on Pre-invasive Vulvar Lesions. J. Low. Genit. Tract. Dis. 2022, 26, 229–244. [Google Scholar] [CrossRef]
- Roy, S.F.; Wong, J.; Le Page, C.; Tran-Thanh, D.; Barkati, M.; Pina, A.; Trinh, V.Q.; Rahimi, K. DEVIL, VAAD and vLSC constitute a spectrum of HPV-independent, p53-independent intra-epithelial neoplasia of the vulva. Histopathology 2021, 79, 975–988. [Google Scholar] [CrossRef]
- Neville, G.; Chapel, D.B.; Crum, C.P.; Song, S.J.; Yoon, J.Y.; Lee, K.R.; Kolin, D.L.; Hirsch, M.S.; Nucci, M.R.; Parra-Herran, C. Interobserver reproducibility of the diagnosis of differentiated exophytic vulvar intraepithelial lesion (DEVIL) and the distinction from its mimics. Histopathology 2021, 79, 957–965. [Google Scholar] [CrossRef]
- Dasgupta, S.; Ewing-Graham, P.C.; van Kemenade, F.J.; van Doorn, H.C.; Noordhoek Hegt, V.; Koljenovic, S. Differentiated vulvar intraepithelial neoplasia (dVIN): The most helpful histological features and the utility of cytokeratins 13 and 17. Virchows Arch. 2018, 473, 739–747. [Google Scholar] [CrossRef]
- Dasgupta, S.; Koljenovic, S.; van den Bosch, T.P.P.; Swagemakers, S.M.A.; van der Hoeven, N.M.A.; van Marion, R.; van der Spek, P.J.; van Doorn, H.C.; van Kemenade, F.J.; Ewing-Graham, P.C. Evaluation of Immunohistochemical Markers, CK17 and SOX2, as Adjuncts to p53 for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia (dVIN). Pharmarceutical 2021, 14, 324. [Google Scholar] [CrossRef]
- Cook, E.; Van de Vijver, K.; Parra-Herran, C. Diagnosis of verruciform acanthotic vulvar intra-epithelial neoplasia (vaVIN) using CK17, SOX2 and GATA3 immunohistochemistry. Histopathology 2024, 84, 1212–1223. [Google Scholar] [CrossRef]
- Hartsough, E.M.; Watkins, J.; Nazarian, R.M. D2-40 and CK17 Immunohistochemistry as a Diagnostic Adjunct for HPV-Independent Squamous Lesions in the Vulva and Their Role in Defining Atypical Lichen Sclerosus. Am. J. Surg. Pathol. 2024. [Google Scholar] [CrossRef] [PubMed]
- McMullen-Tabry, E.R.; Schechter, S.A.; Wang, G.Y.; Sciallis, A.P.; Hrycaj, S.M.; Chan, M.P.; Skala, S.L. p53/CK17 Dual Stain Improves Accuracy of Distinction Between Differentiated Vulvar Intraepithelial Neoplasia and Its Mimics. Int. J. Gynecol. Pathol. 2022, 41, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Podoll, M.B.; Singh, N.; Gilks, C.B.; Moghadamfalahi, M.; Sanders, M.A. Assessment of CK17 as a Marker for the Diagnosis of Differentiated Vulvar Intraepithelial Neoplasia. Int. J. Gynecol. Pathol. 2017, 36, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Troyanovsky, S.M.; Guelstein, V.I.; Tchipysheva, T.A.; Krutovskikh, V.A.; Bannikov, G.A. Patterns of expression of keratin 17 in human epithelia: Dependency on cell position. J. Cell. Sci. 1989, 93, 419–426. [Google Scholar] [CrossRef]
- Wilson, C.L.; Dean, D.; Lane, E.B.; Dawber, R.P.; Leigh, I.M. Keratinocyte differentiation in psoriatic scalp: Morphology and expression of epithelial keratins. Br. J. Dermatol. 1994, 131, 191–200. [Google Scholar] [CrossRef]
- Sanguansin, S.; Kosanwat, T.; Juengsomjit, R.; Poomsawat, S. Diagnostic Value of Cytokeratin 17 during Oral Carcinogenesis: An Immunohistochemical Study. Int. J. Dent. 2021, 2021, 089549. [Google Scholar] [CrossRef]
- Watanabe, H.; Ma, Q.; Peng, S.; Adelmant, G.; Swain, D.; Song, W.; Fox, C.; Francis, J.M.; Pedamallu, C.S.; DeLuca, D.S.; et al. SOX2 and p63 colocalize at genetic loci in squamous cell carcinomas. J. Clin. Invest. 2014, 124, 1636–1645. [Google Scholar] [CrossRef]
- Bass, A.J.; Watanabe, H.; Mermel, C.H.; Yu, S.; Perner, S.; Verhaak, R.G.; Kim, S.Y.; Wardwell, L.; Tamayo, P.; Gat-Viks, I.; et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat. Genet. 2009, 41, 1238–1242. [Google Scholar] [CrossRef]
- Maier, S.; Wilbertz, T.; Braun, M.; Scheble, V.; Reischl, M.; Mikut, R.; Menon, R.; Nikolov, P.; Petersen, K.; Beschorner, C.; et al. SOX2 amplification is a common event in squamous cell carcinomas of different organ sites. Hum. Pathol. 2011, 42, 1078–1088. [Google Scholar] [CrossRef]
- Voss, F.O.; Thuijs, N.B.; Duin, S.; Ozer, M.; van Beurden, M.; Berkhof, J.; Steenbergen, R.D.M.; Bleeker, M.C.G. Clinical validation of methylation biomarkers for optimal detection of high-grade vulvar intraepithelial neoplasia. Int. J. Cancer 2023, 153, 783–791. [Google Scholar] [CrossRef]
- Casparie, M.; Tiebosch, A.T.; Burger, G.; Blauwgeers, H.; van de Pol, A.; van Krieken, J.H.; Meijer, G.A. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell. Oncol. 2007, 29, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Te Grootenhuis, N.C.; Pouwer, A.W.; de Bock, G.H.; Hollema, H.; Bulten, J.; van der Zee, A.G.J.; de Hullu, J.A.; Oonk, M.H.M. Margin status revisited in vulvar squamous cell carcinoma. Gynecol. Oncol. 2019, 154, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.F.; Wong, R.W.C.; Trevisan, G.; Tessier-Cloutier, B.; Almadani, N.; Chen, J.; Cheng, A.; Karnezis, A.; McConechy, M.K.; Lum, A.; et al. p53-Abnormal “Fields of Dysplasia” in Human Papillomavirus-Independent Vulvar Squamous Cell Carcinoma Impacts Margins and Recurrence Risk. Mod. Pathol. 2023, 36, 100010. [Google Scholar] [CrossRef]
- Kitamura, R.; Toyoshima, T.; Tanaka, H.; Kawano, S.; Kiyosue, T.; Matsubara, R.; Goto, Y.; Hirano, M.; Oobu, K.; Nakamura, S. Association of cytokeratin 17 expression with differentiation in oral squamous cell carcinoma. J. Cancer Res. Clin. Oncol. 2012, 138, 1299–1310. [Google Scholar] [CrossRef]
- Nazarian, R.M.; Primiani, A.; Doyle, L.A.; Linskey, K.R.; Duncan, L.M.; Odze, R.D.; Zukerberg, L.R. Cytokeratin 17: An adjunctive marker of invasion in squamous neoplastic lesions of the anus. Am. J. Surg. Pathol. 2014, 38, 78–85. [Google Scholar] [CrossRef]
- Nobusawa, A.; Sano, T.; Negishi, A.; Yokoo, S.; Oyama, T. Immunohistochemical staining patterns of cytokeratins 13, 14, and 17 in oral epithelial dysplasia including orthokeratotic dysplasia. Pathol. Int. 2014, 64, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Ohkura, S.; Kondoh, N.; Hada, A.; Arai, M.; Yamazaki, Y.; Sindoh, M.; Takahashi, M.; Matsumoto, I.; Yamamoto, M. Differential expression of the keratin-4, -13, -14, -17 and transglutaminase 3 genes during the development of oral squamous cell carcinoma from leukoplakia. Oral Oncol. 2005, 41, 607–613. [Google Scholar] [CrossRef]
- Brustmann, H.; Brunner, A. Immunohistochemical expression of SOX2 in vulvar intraepithelial neoplasia and squamous cell carcinoma. Int. J. Gynecol. Pathol. 2013, 32, 323–328. [Google Scholar] [CrossRef]

| Imunohistochemical Marker | Expression Pattern | HPV-Independent VIN | HPV-Associated SIL | Non-Dysplastic | Inconclusive | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p53 Mutant | p53 Wild-Type | HSIL | LSIL | ||||||||||
| n = 30 | (%) | n = 16 | (%) | n = 58 | (%) | n = 4 | (%) | n = 37 | (%) | n = 5 | (%) | ||
| P53 | Wild-type | 0 | (0) | 16 | (100) | 58 | (100) | 4 | (100) | 37 | (100) | 4 | (80) |
| Mutant | 30 | (100) | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (20) | |
| CK17 | Negative | 8 | (27) | 0 | (0) | 50 | (86) | 4 | (100) | 27 | (73) | 2 | (40) |
| No expression | 0 | (0) | 0 | (0) | 16 | (28) | 2 | (50) | 17 | (46) | 1 | (20) | |
| Patchy | 8 | (27) | 0 | (0) | 34 | (59) | 2 | (50) | 10 | (27) | 1 | (20) | |
| Positive | 22 | (73) | 16 | (100) | 8 | (14) | 0 | (0) | 9 | (24) | 3 | (60) | |
| Full-epithelial | 8 | (27) | 6 | (38) | 1 | (2) | 0 | (0) | 3 | (8) | 1 | (20) | |
| Partial thickness | 14 | (47) | 10 | (63) | 7 | (12) | 0 | (0) | 6 | (16) | 2 | (40) | |
| Not judgeable | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | |
| SOX2 | Negative | 17 | 14 | 57 | 4 | (100) | 35 | (95) | 5 | (100) | |||
| No expression | 11 | (37) | 9 | (56) | 53 | (91) | 3 | (75) | 24 | (65) | 2 | (40) | |
| Patchy | 6 | (20) | 5 | (31) | 4 | (7) | 1 | (25) | 11 | (30) | 3 | (60) | |
| Positive | 13 | (43) | 2 | (13) | 1 | (2) | 0 | (0) | 1 | (3) | 0 | (0) | |
| Full-epithelial | 8 | (27) | 0 | (0) | 1 | (2) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Partial thickness | 5 | (17) | 2 | (13) | 0 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | |
| Not judgeable | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | |
| P16INK4a | Negative | 29 | (97) | 16 | (100) | 0 | (0) | 3 | (75) | 36 | (97) | 5 | (100) |
| Block-positive | 1 | (3) | 0 | (0) | 58 | (100) | 1 | (25) | 0 | (0) | 0 | (0) | |
| Not judgeable | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1 | (3) | 0 | (0) | |
| Ki-67 | Not increased | 4 | (13) | 2 | (13) | 0 | (0) | 0 | (0) | 20 | (54) | 1 | (20) |
| Increased | 26 | (87) | 14 | (88) | 58 | (100) | 4 | (100) | 17 | (46) | 4 | (80) | |
| HPV-Independent VIN | Immunohistochemical Marker | Sensitivity | Specificity | Accuracy | |||
|---|---|---|---|---|---|---|---|
| % (95% CI) | % (95% CI) | % (95% CI) | |||||
| All | P53 | 65 | (50–79) | 100 | (90–100) | 80 | (71–89) |
| n = 46 | CK17 | 83 | (69–92) | 75 | (58–88) | 79 | (69–87) |
| SOX2 | 33 | (20–48) | 97 | (85–99.9) | 61 | (70–88) | |
| P53/CK17 | 100 | (92–100) | 76 | (59–88) | 89 | (80–95) | |
| P53/SOX2 | 70 | (54–82) | 95 | (82–99) | 81 | (71–89) | |
| P53/CK17/SOX2 | 100 | (92–100) | 70 | (53–84) | 87 | (78–93) | |
| P53 mutant | CK17 | 73 | (54–88) | 75 | (58–88) | 74 | (62–84) |
| n = 30 | SOX2 | 43 | (26–63) | 97 | (85–99.9) | 73 | (60–83) |
| P53 wild-type | CK17 | 100 | (79–100) | 75 | (58–88) | 83 | (70–92) |
| n = 16 | SOX2 | 13 | (2–38) | 97 | (85–99.9) | 71 | (57–83) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thuijs, N.B.; Voss, F.O.; Ewing-Graham, P.C.; Dasgupta, S.; Berkhof, J.; Bulten, J.; van de Vijver, K.; Bleeker, M.C.G. Expression of CK17 and SOX2 in Vulvar Intraepithelial Neoplasia: A Comprehensive Analysis of 150 Vulvar Lesions. Cancers 2024, 16, 3966. https://doi.org/10.3390/cancers16233966
Thuijs NB, Voss FO, Ewing-Graham PC, Dasgupta S, Berkhof J, Bulten J, van de Vijver K, Bleeker MCG. Expression of CK17 and SOX2 in Vulvar Intraepithelial Neoplasia: A Comprehensive Analysis of 150 Vulvar Lesions. Cancers. 2024; 16(23):3966. https://doi.org/10.3390/cancers16233966
Chicago/Turabian StyleThuijs, Nikki B., Féline O. Voss, Patricia C. Ewing-Graham, Shatavisha Dasgupta, Johannes Berkhof, Johan Bulten, Koen van de Vijver, and Maaike C. G. Bleeker. 2024. "Expression of CK17 and SOX2 in Vulvar Intraepithelial Neoplasia: A Comprehensive Analysis of 150 Vulvar Lesions" Cancers 16, no. 23: 3966. https://doi.org/10.3390/cancers16233966
APA StyleThuijs, N. B., Voss, F. O., Ewing-Graham, P. C., Dasgupta, S., Berkhof, J., Bulten, J., van de Vijver, K., & Bleeker, M. C. G. (2024). Expression of CK17 and SOX2 in Vulvar Intraepithelial Neoplasia: A Comprehensive Analysis of 150 Vulvar Lesions. Cancers, 16(23), 3966. https://doi.org/10.3390/cancers16233966






