Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sarcoma Centers
2.2. Identifications for Study Cohorts and Study Endpoints
- 5-year risk of local recurrence.
- Local recurrence rate for the entire observation period.
- Proportion of patients that received postoperative RT.
- Five-year overall survival.
- Overall survival for the entire observation period.
2.3. Patients
- Upper extremity tumor: from or distal to the shoulder.
- Lower extremity tumor: from the pelvic area to the toes (excluding genital and peritoneal tumors).
- Trunk wall tumors: from the clavicle to the top of the pelvis (excluding tumors located in the mamma, retroperitoneal, intraabdominal, head, and neck).
- 4.
- Patients who did not undergo surgical treatment of their STS.
- 5.
- Grade 1 or borderline tumors (Trojani grading system [13])
- 6.
- Tumors that were not considered deep-seated. We defined deep-seated tumor location as having a tumor located under or through the facia.
- 7.
- Patients operated on in another hospital than SC1 or SC2.
- 8.
- Patients younger than 18 years at the time of operation.
- 9.
- Patients with a tumor removed with an intralesional margin (defined by the pathologist).
- 10.
- Patients that had received pre- or postoperative chemotherapy within three months of the primary operation.
- 11.
- Patients that had received preoperative radiation.
- 12.
- Metastases within three months of surgery.
- 13.
- Patients who had an amputation as primary surgery.
- 14.
- Patients operated on for a local recurrence.
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. Patient and Tumor Characteristics
3.2. Non-RT Patient Characteristics
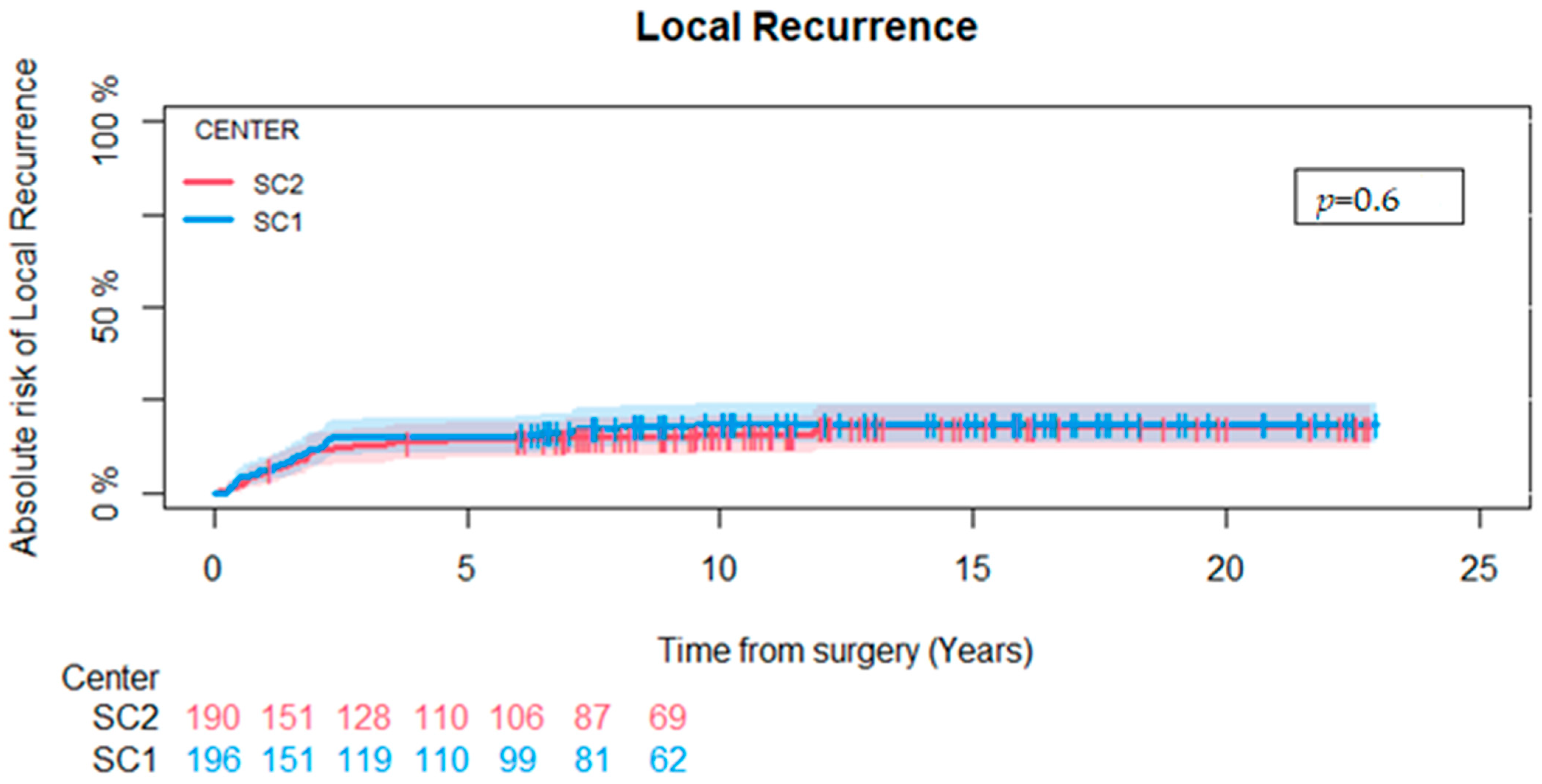
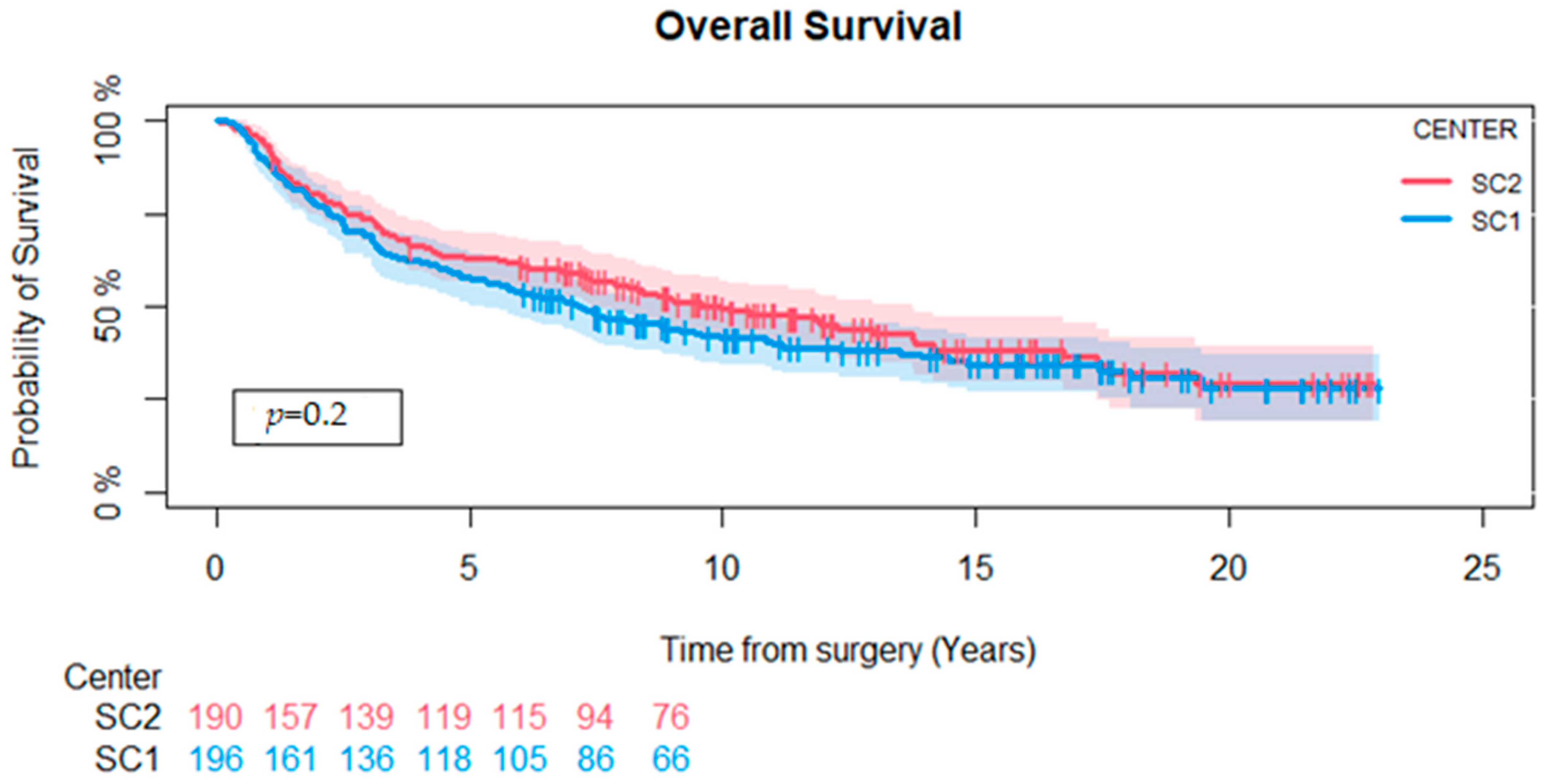
3.3. Local Recurrence, Amputation, and Overall Survival
3.4. Cause-Specific Cox Regression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lahat, G.; Lazar, A.; Lev, D. Sarcoma epidemiology and etiology: Potential environmental and genetic factors. Surg. Clin. N. Am. 2008, 88, 451–481. [Google Scholar] [CrossRef] [PubMed]
- Grimer, R.; Judson, I.; Peake, D.; Seddon, B. Guidelines for the management of soft tissue sarcomas. Sarcoma 2010, 2010, 506182. [Google Scholar] [CrossRef] [PubMed]
- Hoefkens, F.; Dehandschutter, C.; Somville, J.; Meijnders, P.; Van Gestel, D. Soft tissue sarcoma of the extremities: Pending questions on surgery and radiotherapy. Radiat. Oncol. 2016, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.H.; Raut, C. Radiation therapy for extremity soft tissue sarcoma: In the absence of a clear survival benefit, why do we give it? Ann. Surg. Oncol. 2014, 21, 2463–2465. [Google Scholar] [CrossRef] [PubMed]
- Simões, R.; Augustin, Y.; Gulliford, S.; Dehbi, H.-M.; Hoskin, P.; Miles, E.; Harrington, K.; Miah, A.B. Toxicity, normal tissue and dose-volume planning parameters for radiotherapy in soft tissue sarcoma of the extremities: A systematic review of the literature. Radiother. Oncol. 2023, 186, 109739. [Google Scholar] [CrossRef] [PubMed]
- Safwat, A. Radiotherapy of Localized Soft Tissue Sarcoma: Center for Clinical Practice Guidelines. 2018. Available online: https://www.dmcg.dk/Kliniske-retningslinjer/kliniske-retningslinjer-opdelt-paa-dmcg/sarkomer/radiotherapy-of-localized-soft-tissue-sarcoma/ (accessed on 4 March 2022).
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. Relat. Res. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Trovik, C.S.; Skjeldal, S.; Bauer, H.; Rydholm, A.; Jebsen, N. Reliability of Margin Assessment after Surgery for Extremity Soft Tissue Sarcoma: The SSG Experience. Sarcoma 2012, 2012, 290698. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA A Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Jørgensen, P.H.; Lausten, G.S.; Pedersen, A.B. The Danish Sarcoma Database. Clin. Epidemiol. 2016, 8, 685–690. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Keller, J.; Safwat, A.; Baerentzen, S.; Pedersen, A.B. Population-based Aarhus Sarcoma Registry: Validity, completeness of registration, and incidence of bone and soft tissue sarcomas in western Denmark. Clin. Epidemiol. 2013, 5, 45–56. [Google Scholar] [CrossRef]
- Schmidt, M.; Pedersen, L.; Sørensen, H.T. The Danish Civil Registration System as a tool in epidemiology. Eur. J. Epidemiol. 2014, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Trojani, M.; Contesso, G.; Coindre, J.M.; Rouesse, J.; Bui, N.B.; De Mascarel, A.; Goussot, J.F.; David, M.; Bonichon, F.; Lagarde, C. Soft-tissue sarcomas of adults; study of pathological prognostic variables and definition of a histopathological grading system. Int. J. Cancer 1984, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- van Praag, V.M.; Rueten-Budde, A.J.; Jeys, L.M.; Laitinen, M.K.; Pollock, R.; Aston, W.; van der Hage, J.A.; Dijkstra, P.D.S.; Ferguson, P.C.; Griffin, A.M.; et al. A prediction model for treatment decisions in high-grade extremity soft-tissue sarcomas: Personalised sarcoma care (PERSARC). Eur. J. Cancer (1990) 2017, 83, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Grimer, R.; Gaston, C.; Francis, M.; Charman, J.; Graunt, P.; Uchida, A.; Sudo, A.; Jeys, L. The value of C-reactive protein and comorbidity in predicting survival of patients with high grade soft tissue sarcoma. Eur. J. Cancer (1990) 2013, 49, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Willeumier, J.; Fiocco, M.; Nout, R.; Dijkstra, S.; Aston, W.; Pollock, R.; Hartgrink, H.; Bovée, J.; van de Sande, M. High-grade soft tissue sarcomas of the extremities: Surgical margins influence only local recurrence not overall survival. Int. Orthop. 2015, 39, 935–941. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, X.; Feng, Y.; Yang, Z.; Chen, X.; Wand, J.; Ma, S.; Zhang, Z.; Guo, X. Local recurrence is correlated with decreased overall survival in patients with intermediate high-grade localized primary soft tissue sarcoma of extremity and abdominothoracic wall. Asia-Pac. J. Clin. Oncol. 2018, 14, e109–e115. [Google Scholar] [CrossRef]
- Lee, D.W.; Kim, H.S.; Han, I. Actual long-term survival after resection of stage III soft tissue sarcoma. BMC Cancer 2021, 21, 21. [Google Scholar] [CrossRef]
- Kungwengwe, G.; Clancy, R.; Vass, J.; Slade, R.; Sandhar, S.; Dobbs, T.D.; Bragg, T.W.H. Preoperative versus Post-operative Radiotherapy for Extremity Soft tissue Sarcoma: A Systematic Review and Meta-analysis of Long-term Survival. J. Plast. Reconstr. Aesthetic Surg. 2021, 74, 2443–2457. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef]
- Lebas, A.; Le Fevre, C.; Waissi, W.; Chambrelant, I.; Brinkert, D.; Noel, G. Factors Influencing Long-Term Local Recurrence, Distant Metastasis, and Survival in Patients with Soft Tissue Sarcoma of the Extremities Treated with Radiotherapy. Cancers 2024, 16, 1789. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Safwat, A.; Jørgensen, P.H.; Hansen, B.H.; Baerentzen, S.; Pedersen, A.B.; Keller, J. Prognostic factors for local recurrence and mortality in adult soft tissue sarcoma of the extremities and trunk wall: A cohort study of 922 consecutive patients. Acta Orthop. 2014, 85, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Sabolch, A.; Feng, M.; Griffith, K.; Rzasa, C.; Gadzala, L.; Feng, F.; Biermann, J.S.; Chugh, R.; Ray, M.; Ben-Josef, E. Risk factors for local recurrence and metastasis in soft tissue sarcomas of the extremity. Am. J. Clin. Oncol. 2012, 35, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Alektiar, K.M.; Velasco, J.; Zelefsky, M.J.; Woodruff, J.M.; Lewis, J.J.; Brennan, M.F. Adjuvant radiotherapy for margin-positive high-grade soft tissue sarcoma of the extremity. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Novais, E.N.; Demiralp, B.; Alderete, J.; Larson, M.C.; Rose, P.S.; Sim, F.H. Do Surgical Margin and Local Recurrence Influence Survival in Soft Tissue Sarcomas? Clin. Orthop. Relat. Res. 2010, 468, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Daigeler, A.; Zmarsly, I.; Hirsch, T.; Goertz, O.; Steinau, H.U.; Lehnhardt, M.; Harati, K. Long-term outcome after local recurrence of soft tissue sarcoma: A retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br. J. Cancer 2014, 110, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.A.; Beltrami, G.; Scoccianti, G.; Frenos, F.; Capanna, R. Combining limb-sparing surgery with radiation therapy in high-grade soft tissue sarcoma of extremities—Is it effective? Eur. J. Surg. Oncol. 2016, 42, 1057–1063. [Google Scholar] [CrossRef]
- Jebsen, N.L.; Trovik, C.S.; Bauer, H.C.; Rydholm, A.; Monge, O.R.; Hall, K.S.; Alvegård, T.; Bruland, O.S. Radiotherapy to improve local control regardless of surgical margin and malignancy grade in extremity and trunk wall soft tissue sarcoma: A Scandinavian sarcoma group study. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 1196–1203. [Google Scholar] [CrossRef]
- Fletcher, C.D.M.; Unni, K.K.; Mertens, F.; Fletcher, C.D.M. Pathology and Genetics of Tumours of Soft Tissue and Bone; IARC Press: Lyon, France, 2002. [Google Scholar]
- Daugaard, S. Current soft-tissue sarcoma classifications. Eur. J. Cancer (1990) 2004, 40, 543–548. [Google Scholar] [CrossRef]
- Gamboa, A.C.; Gronchi, A.; Cardona, K. Soft-tissue sarcoma in adults: An update on the current state of histiotype-specific management in an era of personalized medicine. CA A Cancer J. Clin. 2020, 70, 200–229. [Google Scholar] [CrossRef]
- Maretty-Nielsen, K.; Aggerholm-Pedersen, N.; Safwat, A.; Baerentzen, S.; Pedersen, A.B.; Keller, J. Prevalence and prognostic impact of comorbidity in soft tissue sarcoma: A population-based cohort study. Acta Oncol. 2014, 53, 1188–1196. [Google Scholar] [CrossRef]
- Raedkjaer, M.; Maretty-Kongstad, K.; Bead-Hansen, T.; Jorgensen, P.H.; Safwat, A.; Vedsted, P.; Petersen, M.M.; Hovgaard, T.; Nymark, T.; Keller, J. The impact of comorbidity on mortality in Danish sarcoma patients from 2000–2013: A nationwide population-based multicentre study. PLoS ONE 2018, 13, e0198933. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, C.; Springfield, D.S.; Marcus, K.J.; Perez-Atayde, A.R.; Gebhardt, M.C. Factors Predicting Local Recurrence, Metastasis, and Survival in Pediatric Soft Tissue Sarcoma in Extremities. Clin. Orthop. Relat. Res. 2010, 468, 3019–3027. [Google Scholar] [CrossRef] [PubMed]
- Saxby, N.E.; An, Q.; Miller, B.J. Local Recurrence of Soft Tissue Sarcoma Revisited: Is there a Role for “Selective” Radiation? Iowa Orthop. J. 2022, 42, 239–248. [Google Scholar] [PubMed]
- Nandra, R.; Hwang, N.; Matharu, G.S.; Reddy, K.; Grimer, R. One-year mortality in patients with bone and soft tissue sarcomas as an indicator of delay in presentation. Ann. R. Coll. Surg. Engl. 2015, 97, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, F.; Sayyed, R.; Jones, R.L.; Hayes, A.J. Risk factors for the development of local recurrence in extremity soft-tissue sarcoma. Expert. Rev. Anticancer Ther. 2022, 22, 83–95. [Google Scholar] [CrossRef]
- Müller, J.A.; Delank, K.S.; Laudner, K.; Wittenberg, I.; Zeh, A.; Vordermark, D.; Medenwald, D. Clinical characteristics of sarcoma patients: A population-based data analysis from a German clinical cancer registry. J. Cancer Res. Clin. Oncol. 2023, 149, 17051–17069. [Google Scholar] [CrossRef]
- Fiore, M.; Ford, S.; Callegaro, D.; Sangalli, C.; Colombo, C.; Radaelli, S.; Frezza, A.M.; Renne, S.L.; Casali, P.G.; Gronchi, A. Adequate Local Control in High-Risk Soft Tissue Sarcoma of the Extremity Treated with Surgery Alone at a Reference Centre: Should Radiotherapy Still be a Standard? Ann. Surg. Oncol. 2018, 25, 1536–1543. [Google Scholar] [CrossRef]
- Karakousis, C.P.; Zografos, G.C. Radiation therapy for high grade soft tissue sarcomas of the extremities treated with limb-preserving surgery. Eur. J. Surg. Oncol. 2002, 28, 431–436. [Google Scholar] [CrossRef]
- Lemma, J.; Jäämaa, S.; Repo, J.P.; Santti, K.; Salo, J.; Blomqvist, C.P.; Sampo, M.M. Local relapse of soft tissue sarcoma of the extremities or trunk wall operated on with wide margins without radiation therapy. BJS Open 2023, 7, zrac172. [Google Scholar] [CrossRef]
- Pisters, P.W.; Pollock, R.E.; Lewis, V.O.; Yasko, A.W.; Cormier, J.N.; Respondek, P.M.; Feig, B.W.; Hunt, K.K.; Lin, P.P.; Zagars, G.; et al. Long-term results of prospective trial of surgery alone with selective use of radiation for patients with T1 extremity and trunk soft tissue sarcomas. Ann. Surg. 2007, 246, 675–681, discussion 681–672. [Google Scholar] [CrossRef]

| Radiation | Yes | No | |||||
|---|---|---|---|---|---|---|---|
| Treatment Center | Overall | SC1 | SC2 | p- Value 2 | SC1 | SC2 | p- Value 2 |
| Overall | (n = 386) | (n = 163) | (n = 121) | (n = 33) | (n = 69) | ||
| Sex | 0.6 | 0.8 | |||||
| Female | 176 (45%) | 81 (50%) | 56 (46%) | 12 (36%) | 27 (40%) | ||
| Male | 210 (55%) | 82 (50%) | 65 (54%) | 21 (64%) | 41 (60%) | ||
| Age (years) 1 | 61 (18–95) | 60 (19–86) | 60 (18–85) | 0.7 | 69 (27–92) | 60 (18–95) | 0.034 |
| Histological grade | <0.001 | 0.044 | |||||
| Grade 2 | 121 (32%) | 32 (20%) | 50 (41%) | 8 (24%) | 31 (45%) | ||
| Grade 3 | 265 (68%) | 131 (80%) | 71 (59%) | 25 (76%) | 38 (55%) | ||
| Location | 0.046 | 0.7 | |||||
| Lower Extremity | 273 (71%) | 105 (64%) | 93 (77%) | 24 (74%) | 51 (74%) | ||
| Truncal | 42 (11%) | 16 (10%) | 11 (9%) | 4 (12%) | 11 (16%) | ||
| Upper Extremity | 71 (18%) | 42 (26%) | 17 (14%) | 5 (15%) | 7 (10%) | ||
| Tumor size | 0.054 | 0.087 | |||||
| <5 cm | 55 (14%) | 26 (16%) | 10 (8%) | 3 (9%) | 16 (23%) | ||
| ≥5 cm | 331 (86%) | 137 (84%) | 111 (92%) | 30 (91%) | 53 (77%) | ||
| Surgical margin | <0.001 | 0.4 | |||||
| Marginal | 204 (53%) | 74 (45%) | 96 (79%) | 13 (39%) | 21 (30%) | ||
| Wide | 182 (47%) | 89 (55%) | 25 (21%) | 20 (61%) | 48 (70%) | ||
| Treatment Center | Overall | SC1 | SC2 |
|---|---|---|---|
| Overall | (n = 386) | (n = 196) | (n = 190) |
| Sarcoma NOS | 90 (23%) | 33 (17%) | 57 (30%) |
| Malignant fibrous histiocytoma | 37 (10%) | 23 (12%) | 14 (7.5%) |
| Leiomyosarcoma | 36 (9%) | 24 (12%) | 12 (6%) |
| Myxoid liposarcoma | 34 (9%) | 13 (6.5%) | 21 (11%) |
| Undifferentiated pleomorphic sarcoma | 29 (8%) | 15 (7.5%) | 14 (7.5%) |
| Other | 160 (41%) | 88 (45%) | 72 (38%) |
| Treatment Center | Overall n = 102 | SC1 n = 33 | SC2 n = 69 |
|---|---|---|---|
| Surgical margin considered sufficient to skip radiation therapy | 47 (46%) [7] | 2 (6%) [0] | 45 (65%) [7] |
| Clinical factors (wound complications, co-morbidity) | 27 (26%) [6] | 16 (48%) [2] | 11 (16%) [5] |
| Patient factors (patient refusal or psychosocial comorbidity) | 12 (12%) [5] | 4 (12%) [2] | 8 (12%) [3] |
| Death before radiation | 4 (4%) [0] | 2 (6%) [0] | 2 (3%) [0] |
| No explanation | 12(12%) [3] | 9 (27%) [1] | 3 (4%) [2] |
| Hazard Ratio; (95% CI) | p Value | |
|---|---|---|
| Crude | ||
| Center (reference = SC1) | 0.85; (0.52–1.37) | 0.5 |
| Adjusted | ||
| Center (reference = SC1) | 0.83; (0.50–1.37) | 0.48 |
| Age at diagnosis | 1.02; (1.00–1.03) | 0.06 |
| Tumor location (Truncus vs. lower extremity) | 0.34; (0.18–0.64) | <0.001 |
| Tumor location (Truncus vs. upper extremity) | 0.40; (0.18–0.87) | 0.02 |
| Higher histological grade (grade 2 vs. grade 3) | 1.07; (0.63–1.83) | 0.76 |
| Sex (male) | 1.32; (0.80–2.16) | 0.28 |
| Size (>5 cm) | 1.42; (0.64–3.15) | 0.38 |
| Surgical margin (wide vs. marginal) | 0.96; (0.58–1.59) | 0.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorn, A.; Iljazi, A.; Engelmann, B.E.; Aggerholm-Pedersen, N.; Baad-Hansen, T.; Petersen, M.M. Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall. Cancers 2024, 16, 3423. https://doi.org/10.3390/cancers16193423
Thorn A, Iljazi A, Engelmann BE, Aggerholm-Pedersen N, Baad-Hansen T, Petersen MM. Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall. Cancers. 2024; 16(19):3423. https://doi.org/10.3390/cancers16193423
Chicago/Turabian StyleThorn, Andrea, Afrim Iljazi, Bodil Elisabeth Engelmann, Ninna Aggerholm-Pedersen, Thomas Baad-Hansen, and Michael Mørk Petersen. 2024. "Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall" Cancers 16, no. 19: 3423. https://doi.org/10.3390/cancers16193423
APA StyleThorn, A., Iljazi, A., Engelmann, B. E., Aggerholm-Pedersen, N., Baad-Hansen, T., & Petersen, M. M. (2024). Evaluation of Two Different Approaches for Selecting Patients for Postoperative Radiotherapy in Deep-Seated High-Grade Soft Tissue Sarcomas in the Extremities and Trunk Wall. Cancers, 16(19), 3423. https://doi.org/10.3390/cancers16193423







