A Clinico-Genetic Score Incorporating Disease-Free Intervals and Chromosome 8q Copy Numbers: A Novel Prognostic Marker for Recurrence and Survival Following Liver Resection in Patients with Liver Metastases of Uveal Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
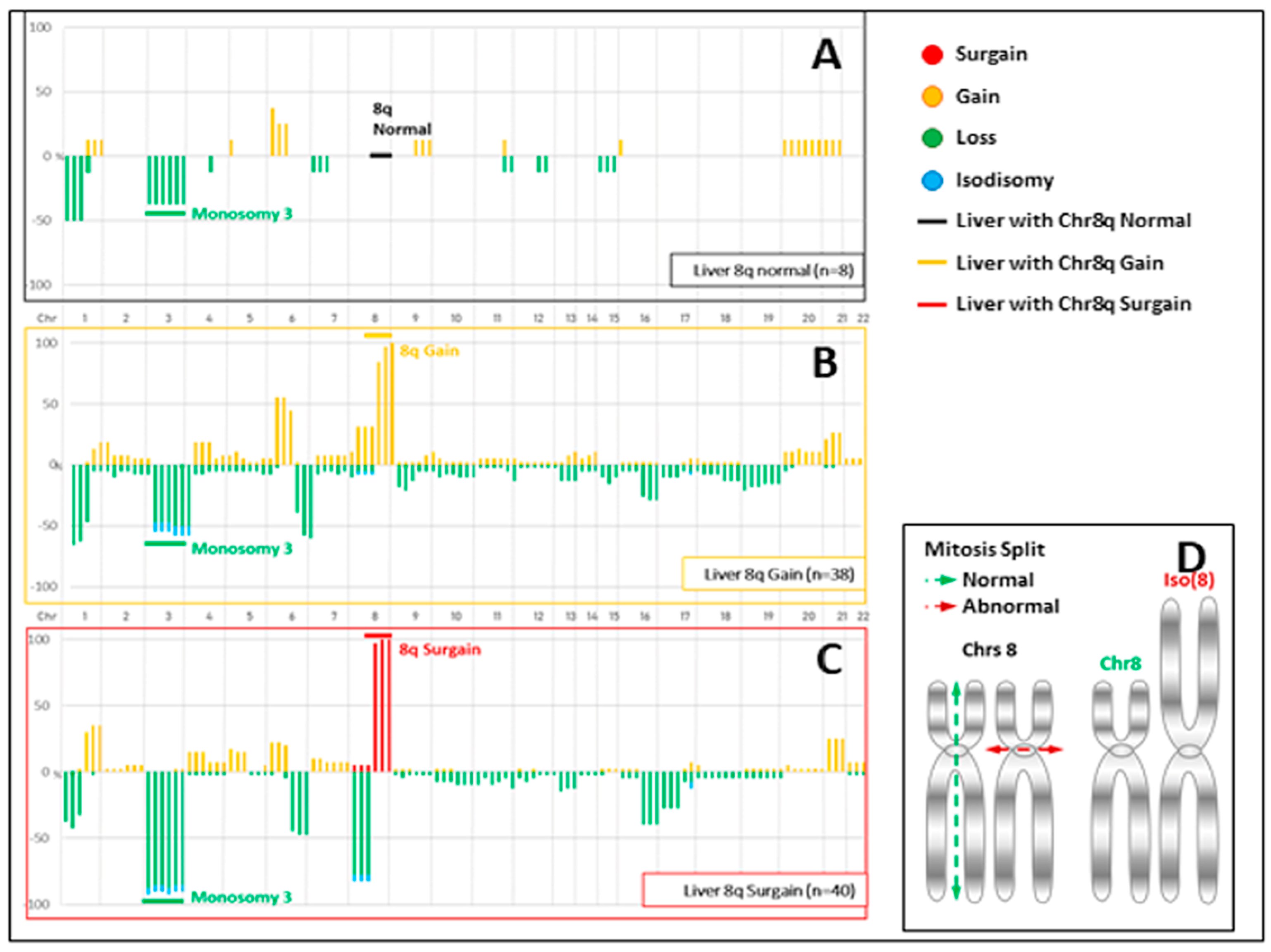
3. Results
3.1. Independent Prognostic Factors Associated with RFS and OS
3.2. Preoperative Clinico-Genetic Risk Score
3.3. Comparison of CNVs and Mutations between UM and Resected Liver Metastases
4. Discussion
4.1. DFI
4.2. Chromosome 8q Surgain
4.3. Scientific Rationale for Our Clinico-Genetic Score
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carvajal, R.D.; Sacco, J.J.; Jager, M.J.; Eschelman, D.J.; Olofsson Bagge, R.; Harbour, J.W.; Chieng, N.D.; Patel, S.P.; Joshua, A.M.; Piperno-Neumann, S. Advances in the clinical management of uveal melanoma. Nat. Rev. Clin. Oncol. 2023, 20, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilhol, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019, 30, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Rantala, E.S.; Hernberg, M.; Kivelä, T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019, 29, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N. Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCN2024). NCCN Clinical Practice Guidelines in Oncology for Melanoma: Uveal. Version 1.2024-23 May 2024. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1488 (accessed on 23 May 2024).
- Bethlehem, M.S.; Katsarelias, D.; Olofsson Bagge, R. Meta-Analysis of Isolated Hepatic Perfusion and Percutaneous Hepatic Perfusion as a Treatment for Uveal Melanoma Liver Metastases. Cancers 2021, 13, 4726. [Google Scholar] [CrossRef] [PubMed]
- Olofsson Bagge, R.; Nelson, A.; Shafazand, A.; All-Eriksson, C.; Cahlin, C.; Elander, N.; Helgadottir, H.; Kiilgaard, J.F.; Kinhult, S.; Ljuslinder, I.; et al. Isolated Hepatic Perfusion with Melphalan for Patients With Isolated Uveal Melanoma Liver Metastases: A Multicenter, Randomized, Open-Label, Phase III Trial (the SCANDIUM Trial). J. Clin. Oncol. 2023, 41, 3042–3050. [Google Scholar] [CrossRef]
- Zager, J.S.; Orloff, M.; Ferrucci, P.F.; Choi, J.; Eschelman, D.J.; Glazer, E.S.; Ejaz, A.; Howard, J.H.; Richtig, E.; Ochsenreither, S.; et al. Efficacy and Safety of the Melphalan/Hepatic Delivery System in Patients with Unresectable Metastatic Uveal Melanoma: Results from an Open-Label, Single-Arm, Multicenter Phase 3 Study. Ann. Surg. Oncol. 2024, 31, 5340–5351. [Google Scholar] [CrossRef]
- Gomez, D.; Wetherill, C.; Cheong, J.; Jones, L.; Marshall, E.; Damato, B.; Coupland, S.E.; Ghaneh, P.; Poston, G.J.; Malik, H.Z.; et al. The Liverpool uveal melanoma liver metastases pathway: Outcome following liver resection. J. Surg. Oncol. 2014, 109, 542–547. [Google Scholar] [CrossRef]
- Trivedi, D.B.; Aldulaimi, N.; Karydis, I.; Wheater, M.; Modi, S.; Stedman, B.; Karavias, D.; Primrose, J.; Pearce, N.; Takhar, A.S. Liver resection for metastatic uveal melanoma: Experience from a supra-regional centre and review of literature. Melanoma Res. 2023, 33, 71–79. [Google Scholar] [CrossRef]
- Mariani, P.; Piperno-Neumann, S.; Servois, V.; Berry, M.G.; Dorval, T.; Plancher, C.; Couturier, J.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Desjardins, L.; et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur. J. Surg. Oncol. 2009, 35, 1192–1197. [Google Scholar] [CrossRef]
- Mariani, P.; Dureau, S.; Savignoni, A.; Lumbroso-Le Rouic, L.; Levy-Gabriel, C.; Piperno-Neumann, S.; Rodrigues, M.J.; Desjardins, L.; Cassoux, N.; Servois, V. Development of a prognostic nomogram for liver metastasis of uveal melanoma patients selected by liver MRI. Cancers 2019, 11, 863. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.C.; Bas, Z.; Malkani, K.; Ganguly, A.; Shields, C.L.; Jager, M.J. Adding the Cancer Genome Atlas Chromosome Classes to American Joint Committee on Cancer System Offers More Precise Prognostication in Uveal Melanoma. Ophthalmology 2022, 129, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Rantala, E.S.; Hernberg, M.M.; Piperno-Neumann, S.; Grossniklaus, H.E.; Kivelä, T.T. Metastatic uveal melanoma: The final frontier. Prog. Retin. Eye Res. 2022, 90, 101041. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.H.; Cebulla, C.M.; Verma, V.; Christopher, B.N.; Carson, W.E., 3rd; Olencki, T.; Davidorf, F.H. Monosomy 3 status of uveal melanoma metastases is associated with rapidly progressive tumors and short survival. Exp. Eye Res. 2012, 100, 26–31. [Google Scholar] [CrossRef]
- Nathan, P.; Cohen, V.; Coupland, S.; Curtis, K.; Damato, B.; Evans, J.; Fenwick, S.; Kirkpatrick, L.; Li, O.; Marshall, E.; et al. Uveal melanoma UK national guidelines. Eur. J. Cancer 2015, 51, 2404–2412. [Google Scholar] [CrossRef]
- Cassoux, N.; Rodrigues, M.J.; Plancher, C.; Asselain, B.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Piperno-Neumann, S.; Dendale, R.; Sastre, X.; Desjardins, L.; et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 2014, 98, 769–774. [Google Scholar] [CrossRef]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017, 32, 204–220.e15. [Google Scholar] [CrossRef] [PubMed]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M.; Statistics Subcommittee of the NCI-EORTC Working Group on Cancer Diagnostics. REporting recommendations for tumor MARKer prognostic studies (REMARK). Nat. Rev. Clin. Oncol. 2005, 2, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Sellam, A.; Desjardins, L.; Barnhill, R.; Plancher, C.; Asselain, B.; Savignoni, A.; Pierron, G.; Cassoux, N. Fine needle aspiration biopsy in uveal melanoma: Technique, complications and outcomes. Am. J. Ophthalmol. 2016, 162, 28–34. [Google Scholar] [CrossRef]
- Matet, A.; Aït Raïs, K.; Malaise, D.; Angi, M.; Dendale, R.; Tick, S.; Lumbroso-Le Rouic, L.; Lévy-Gabriel, C.; Rodrigues, M.; Pierron, G.; et al. Comparative Cytogenetic Abnormalities in Paired Choroidal Melanoma Samples Obtained Before and After Proton Beam Irradiation by Transscleral Fine-Needle Aspiration Biopsy and Endoresection. Cancers 2019, 11, 1173. [Google Scholar] [CrossRef]
- Vacher, S.; Suybeng, V.; Girard, E.; Masliah Planchon, J.; Thomson, G.; Le Goux, C.; Garinet, S.; Schnitzler, A.; Chemlali, W.; Firlej, V.; et al. Genomic Instability Signature of Palindromic Non-Coding Somatic Mutations in Bladder. Cancer Cancers 2020, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Valpione, S.; Moser, J.C.; Parrozzani, R.; Bazzi, M.; Mansfield, A.S.; Mocellin, S.; Pigozzo, J.; Midena, E.; Markovic, S.N.; Aliberti, C.; et al. Development and external validation of a prognostic nomogram for metastatic uveal melanoma. PLoS ONE 2015, 10, e0120181. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Versluis, M.; de Lange, M.J.; van Pelt, S.I.; Ruivenkamp, C.A.; Kroes, W.G.; Cao, J.; Jager, M.J.; Luyten, G.P.; van der Velden, P.A. Digital PCR validates 8q dosage as prognostic tool in uveal melanoma. PLoS ONE 2015, 10, e0116371. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Alsafadi, S.; Baulande, S.; Mariani, O.; Marande, B.; Ait Rais, K.; Van der Kooij, M.K.; Kapiteijn, E.; et al. Evolutionary Routes in Metastatic Uveal Melanomas Depend on MBD4 Alterations. Clin. Cancer Res. 2019, 25, 5513–5524. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Bagger, M.M.; Yu, R.; Chang, D.; Liu, S.; Vemula, S.; Weier, J.F.; Wadt, K.; Heegaard, S.; Bastian, B.C.; et al. The genetic evolution of metastatic uveal melanoma. Nat. Genet. 2019, 51, 1123–1130. [Google Scholar] [CrossRef]
- Terai, M.; Shimada, A.; Chervoneva, I.; Hulse, L.; Danielson, M.; Swensen, J.; Orloff, M.; Wedegaertner, P.B.; Benovic, J.L.; Aplin, A.E.; et al. Prognostic Values of G-Protein Mutations in Metastatic Uveal Melanoma. Cancers 2021, 13, 5749. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Larkin, J.; Carvajal, R.D.; Luke, J.J.; Schwartz, G.K.; Hodi, F.S.; Sablin, M.P.; Shoushtari, A.N.; Szpakowski, S.; Chowdhury, N.R.; et al. Genomic Profiling of Metastatic Uveal Melanoma and Clinical Results of a Phase I Study of the Protein Kinase C Inhibitor AEB071. Mol. Cancer Ther. 2020, 19, 1031–1039. [Google Scholar] [CrossRef]
- Ehlers, J.P.; Worley, L.; Onken, M.D.; Harbour, J.W. DDEF1 is located in an amplified region of chromosome 8q and is overexpressed in uveal melanoma. Clin. Cancer Res. 2005, 11, 3609–3613. [Google Scholar] [CrossRef]
- Eskelin, S.; Pyrhönen, S.; Summanen, P.; Hahka-Kemppinen, M.; Kivelä, T. Tumor doubling times in metastatic malignant melanoma of the uvea: Tumor progression before and after treatment. Ophthalmology 2000, 107, 1443–1449. [Google Scholar] [CrossRef]
- Grossniklaus, H.E. Understanding Uveal Melanoma Metastasis to the Liver: The Zimmerman Effect and the Zimmerman Hypothesis. Ophthalmology 2019, 126, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Okamura, Y.; Ohshima, K.; Uesaka, K.; Sugiura, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Nagashima, T.; Yamaguchi, K. Molecular characterization-based multi-omics analyses in primary liver cancer using the Japanese version of the genome atlas. J. Hepatobiliary Pancreat. Sci. 2023, 30, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Alshalalfa, M.; Nguyen, T.T.; Stopsack, K.H.; Khan, A.; Franco, I.; Seldon, C.; Swami, N.; Jin, W.; Meiyappan, K.; Ton, M.; et al. Chromosome 8q arm overexpression is associated with worse prostate cancer prognosis. Urol. Oncol. 2023, 41, 106.e17–106.e23. [Google Scholar] [CrossRef] [PubMed]
- Smit, K.N.; Jager, M.J.; de Klein, A.; Kiliҫ, E. Uveal melanoma: Towards a molecular understanding. Prog. Retin. Eye Res. 2020, 75, 100800. [Google Scholar] [CrossRef]
- Hassel, J.C.; Piperno-Neumann, S.; Rutkowski, P.; Baurain, J.F.; Schlaak, M.; Butler, M.O.; Sullivan, R.J.; Dummer, R.; Kirkwood, J.M.; Orloff, M.; et al. Three-Year Overall Survival with Tebentafusp in Metastatic Uveal Melanoma. N. Engl. J. Med. 2023, 389, 2256–2266. [Google Scholar] [CrossRef]


| Variables | All (n = 86) | Chromosome 8q Surgain | p-Value | ||
|---|---|---|---|---|---|
| Absent (n = 46) | Present (n = 40) | ||||
| Age (years) | ≤50 | 36 (42%) | 22 (48%) | 14 (35%) | 0.23 |
| >50 | 50 (58%) | 24 (52%) | 26 (65%) | ||
| Gender | Female | 46 (53%) | 24 (52%) | 22 (55%) | 0.79 |
| Male | 40 (47%) | 22 (48%) | 18 (45%) | ||
| Ocular UM thickness (mm) | ≤20 | 81 (94%) | 43 (93%) | 38 (95%) | 0.76 |
| >20 | 5 (6%) | 3 (7%) | 2 (5%) | ||
| Ocular UM largest basal diameter (mm) | <10 | 56 (65%) | 27 (59%) | 29 (73%) | 0.18 |
| >10 | 30 (35%) | 19 (41%) | 11 (28%) | ||
| AJCC tumor category | T1/2/3 | 52 (60%) | 29 (63%) | 23 (58%) | 0.60 |
| T4 | 34 (40%) | 17 (37%) | 17 (43%) | ||
| Ciliary body extension | Absent | 55 (68%) | 32 (74%) | 23 (61%) | 0.18 |
| Present | 26 (32%) | 11 (26%) | 15 (39%) | ||
| Treatment of ocular UM | I-Disk or Proton beam irradiation | 50 (58%) | 29 (63%) | 21 (53%) | 0.32 |
| Enucleation | 36 (42%) | 17 (37%) | 19 (48%) | ||
| Performance status | 0 | 84 (98%) | 46 (100%) | 38 (95%) | 0.12 |
| 1 | 2 (2%) | 0 (0%) | 2 (5%) | ||
| Disease-free interval (months) | ≤24 | 32 (37%) | 9 (20%) | 23 (58%) | <0.001 |
| >24 | 54 (63%) | 37 (80%) | 17 (43%) | ||
| LDH level | ≤ULN | 34 (92%) | 20 (100%) | 14 (82%) | 0.05 |
| >ULN | 3 (8%) | 0 (0%) | 3 (18%) | ||
| Number of liver lesions in MRI | ≤2 | 56 (65%) | 30 (65%) | 26 (65%) | 0.98 |
| >2 | 30 (35%) | 16 (35%) | 14 (35%) | ||
| Largest lesion size in MRI (mm) | ≤20 | 60 (70%) | 32 (70%) | 28 (70%) | 0.97 |
| >20 | 26 (30%) | 14 (30%) | 12 (30%) | ||
| Largest lesion area in MRI (mm2) | ≤250 | 46 (53%) | 24 (52%) | 22 (55%) | 0.79 |
| >250 | 40 (47%) | 22 (48%) | 18 (45%) | ||
| Type of liver surgery | Coelioscopy | 2 (2%) | 1 (2%) | 1 (3%) | 0.92 |
| Laparotomy | 84 (98%) | 45 (98%) | 39 (98%) | ||
| Capsular miliary disease | Absent | 40 (47%) | 26 (57%) | 14 (35%) | 0.05 |
| Present | 46 (53%) | 20 (43%) | 26 (65%) | ||
| Parenchymal miliary disease | Absent | 70 (81%) | 40 (87%) | 30 (75%) | 0.16 |
| Present | 16 (19%) | 6 (13%) | 10 (25%) | ||
| Major hepatectomy | Absent | 48 (56%) | 26 (57%) | 22 (55%) | 0.89 |
| Present | 38 (44%) | 20 (43%) | 18 (45%) | ||
| Macroscopically resection | Complete (R0/R1) | 80 (93%) | 44 (96%) | 36 (90%) | 0.31 |
| Incomplete (R2) | 6 (7%) | 2 (4%) | 4 (10%) | ||
| Bleeding loss (mL) | >100 | 46 (53%) | 21 (46%) | 25 (63%) | 0.12 |
| ≤100 | 40 (47%) | 25 (54%) | 15 (38%) | ||
| Variables | All (n = 86) | Chromosome 8q Surgain | p-Value | ||
|---|---|---|---|---|---|
| Absent (n = 46) | Present (n = 40) | ||||
| Liver histopathology | Fusiform | 21 (24%) | 18 (39%) | 3 (8%) | <0.001 |
| Epithelioid/mixed | 65 (76%) | 28 (61%) | 37 (93%) | ||
| GNAQ mutation | Present | 43 (50%) | 19 (41%) | 24 (60%) | 0.08 |
| Absent | 43 (50%) | 27 (59%) | 16 (40%) | ||
| GNA11 mutation | Present | 39 (45%) | 23 (50%) | 16 (40%) | 0.35 |
| Absent | 47 (55%) | 23 (50%) | 24 (60%) | ||
| CYSLTR2 mutation | Present | 1 (1%) | 1 (3%) | 0 (0%) | 0.33 |
| Absent | 71 (99%) | 36 (97%) | 35 (100%) | ||
| SF3B1 mutation | Present | 23 (27%) | 20 (43%) | 3 (8%) | <0.001 |
| Absent | 63 (73%) | 26 (57%) | 37 (93%) | ||
| BAP1 mutation | Present | 51 (59%) | 20 (43%) | 31 (78%) | 0.001 |
| Absent | 35 (41%) | 26 (57%) | 9 (23%) | ||
| EIF1AX mutation | Present | 7 (8%) | 6 (13%) | 1 (3%) | 0.07 |
| Absent | 79 (92%) | 40 (87%) | 39 (98%) | ||
| Cassoux classification | Low risk | 5 (6%) | 5 (11%) | 0 (0%) | <0.001 |
| Intermediate risk | 23 (27%) | 20 (43%) | 3 (8%) | ||
| High risk | 58 (67%) | 21 (46%) | 37 (93%) | ||
| TCGA classification | Low risk | 5 (6%) | 5 (11%) | 0 (0%) | <0.001 |
| Intermediate risk | 23 (27%) | 20 (43%) | 3 (8%) | ||
| High risk | 21 (24%) | 21 (46%) | 0 (0%) | ||
| Very high risk | 37 (43%) | 0 (0%) | 37 (93%) | ||
| Variable | Multivariable Analysis | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Age (years), >50 vs. ≤50 | - | - |
| Ciliary body extension, present vs. absent | - | - |
| Disease-free interval (months), ≤24 vs. >24 | 2.2 (1.2–3.8) | 0.007 |
| Capsular miliary disease, present vs. absent | - | - |
| BAP1 mutation, present vs. absent | - | - |
| Cassoux classification, high vs. low | - | - |
| intermediate vs. low | - | - |
| TCGA classification, very high vs. high | - | - |
| intermediate vs. high | - | - |
| low vs. high | - | - |
| Chromosome 8q surgain, present vs. absent | 2.2 (1.3–3.7) | 0.005 |
| Variable | Multivariable Analysis | |
|---|---|---|
| HR (95% CI) | p-Value | |
| Age (years), >50 vs. ≤50 | - | - |
| Ciliary body extension, present vs. absent | - | - |
| Disease-free interval (months), ≤24 vs. >24 | 2.7 (1.5–4.7) | <0.001 |
| Capsular miliary disease, present vs. absent | - | - |
| BAP1 mutation, present vs. absent | - | - |
| Cassoux classification, high vs. low | - | - |
| intermediate vs. low | - | - |
| TCGA classification, very high vs. high | - | - |
| intermediate vs. high | - | - |
| low vs. high | - | - |
| Chromosome 8q surgain, present vs. absent | 2.9 (1.6–5.2) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariani, P.; Pierron, G.; Ait Rais, K.; Bouhadiba, T.; Rodrigues, M.; Malaise, D.; Lumbroso-Le Rouic, L.; Barnhill, R.; Stern, M.-H.; Servois, V.; et al. A Clinico-Genetic Score Incorporating Disease-Free Intervals and Chromosome 8q Copy Numbers: A Novel Prognostic Marker for Recurrence and Survival Following Liver Resection in Patients with Liver Metastases of Uveal Melanoma. Cancers 2024, 16, 3407. https://doi.org/10.3390/cancers16193407
Mariani P, Pierron G, Ait Rais K, Bouhadiba T, Rodrigues M, Malaise D, Lumbroso-Le Rouic L, Barnhill R, Stern M-H, Servois V, et al. A Clinico-Genetic Score Incorporating Disease-Free Intervals and Chromosome 8q Copy Numbers: A Novel Prognostic Marker for Recurrence and Survival Following Liver Resection in Patients with Liver Metastases of Uveal Melanoma. Cancers. 2024; 16(19):3407. https://doi.org/10.3390/cancers16193407
Chicago/Turabian StyleMariani, Pascale, Gaëlle Pierron, Khadija Ait Rais, Toufik Bouhadiba, Manuel Rodrigues, Denis Malaise, Livia Lumbroso-Le Rouic, Raymond Barnhill, Marc-Henri Stern, Vincent Servois, and et al. 2024. "A Clinico-Genetic Score Incorporating Disease-Free Intervals and Chromosome 8q Copy Numbers: A Novel Prognostic Marker for Recurrence and Survival Following Liver Resection in Patients with Liver Metastases of Uveal Melanoma" Cancers 16, no. 19: 3407. https://doi.org/10.3390/cancers16193407
APA StyleMariani, P., Pierron, G., Ait Rais, K., Bouhadiba, T., Rodrigues, M., Malaise, D., Lumbroso-Le Rouic, L., Barnhill, R., Stern, M.-H., Servois, V., & Ramtohul, T. (2024). A Clinico-Genetic Score Incorporating Disease-Free Intervals and Chromosome 8q Copy Numbers: A Novel Prognostic Marker for Recurrence and Survival Following Liver Resection in Patients with Liver Metastases of Uveal Melanoma. Cancers, 16(19), 3407. https://doi.org/10.3390/cancers16193407








