Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Cohort
2.2. Stem Cell Mobilization, Apheresis and TreoMel HDCT Treatment Schedule
2.3. Assessment of Treosulfan Pharmacokinetics
2.4. Response Assessment and Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Treatment before HDCT/ASCT
3.3. HDCT/ASCT Therapy
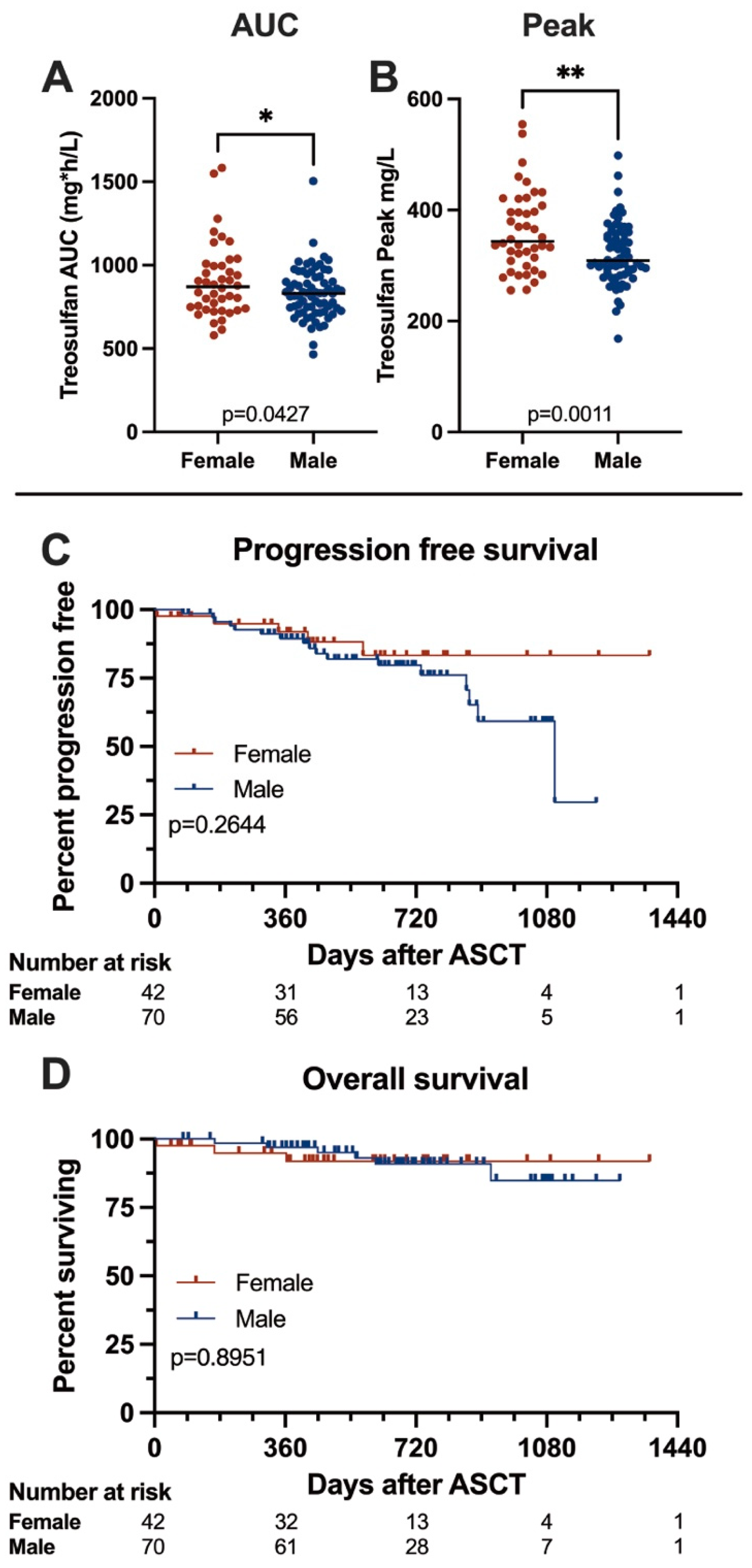
3.4. Treosulfan Pharmacodynamics
3.5. Outcome
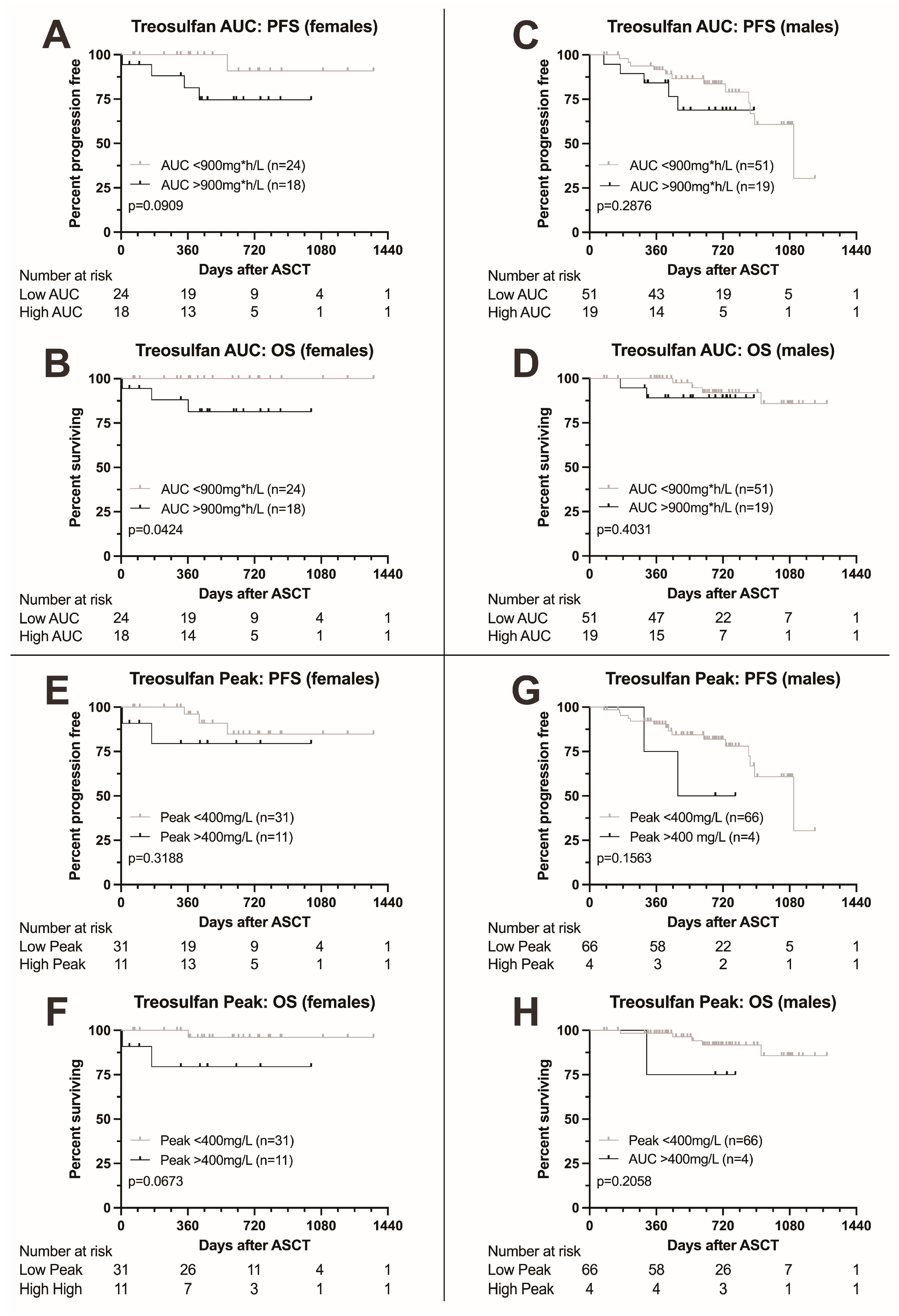
3.6. Outcome According to Treosulfan Exposure
3.7. Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özdemir, B.C.; Oertelt-Prigione, S.; Adjei, A.A.; Borchmann, S.; Haanen, J.B.; Letsch, A.; Mir, O.; Quaas, A.; Verhoeven, R.H.A.; Wagner, A.D. Investigation of sex and gender differences in oncology gains momentum: ESMO announces the launch of a Gender Medicine Task Force. Ann. Oncol. 2022, 33, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.D. Chapter 1 Gender Differences in Pharmacological Response. Int. Rev. Neurobiol. 2008, 83, 1–10. [Google Scholar] [PubMed]
- Hunt, C.M.; Westerkam, W.R.; Stave, G.M. Effect of age and gender on the activity of human hepatic CYP3A. Biochem. Pharmacol. 1992, 44, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mueller, F.; Büchel, B.; Köberle, D.; Schürch, S.; Pfister, B.; Krähenbühl, S.; Froehlich, T.K.; Largiader, C.R.; Joerger, M. Gender-specific elimination of continuous-infusional 5-fluorouracil in patients with gastrointestinal malignancies: Results from a prospective population pharmacokinetic study. Cancer Chemother. Pharmacol. 2013, 71, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Grothey, A.; Andre, T.; Dixon, J.G.; Wolmark, N.; Haller, D.G.; Allegra, C.J.; De Gramont, A.; Vancutsem, E.; Alberts, S.R.; et al. Sex and Adverse Events of Adjuvant Chemotherapy in Colon Cancer: An Analysis of 34 640 Patients in the ACCENT Database. J. Natl. Cancer Inst. 2021, 113, 400–407. [Google Scholar] [CrossRef]
- Dobbs, N.A.; Twelves, C.J.; Gillies, H.; James, C.A.; Harper, P.G.; Rubens, R.D. Gender affects doxorubicin pharmacokinetics in patients with normal liver biochemistry. Cancer Chemother. Pharmacol. 1995, 36, 473–476. [Google Scholar] [CrossRef]
- Ngo, L.; Hee, S.W.; Lim, L.C.; Tao, M.; Quek, R.; Yap, S.P.; Loong, E.L.; Sng, I.; Hwan-Cheong, T.L.; Ang, M.K.; et al. Prognostic factors in patients with diffuse large B cell lymphoma: Before and after the introduction of rituximab. Leuk. Lymphoma 2008, 49, 462–469. [Google Scholar] [CrossRef]
- Gotta, V.; Bouchet, S.; Widmer, N.; Schuld, P.; Decosterd, L.A.; Buclin, T.; Mahon, F.X.; Csajka, C.; Molimard, M. Large-scale imatinib dose-concentration-effect study in CML patients under routine care conditions. Leuk. Res. 2014, 38, 764–772. [Google Scholar] [CrossRef]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; Leblanc, M.; Minasian, L.M.; Gotay, C.C.; Henry, N.L.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 1474–1486. [Google Scholar] [CrossRef]
- Pfreundschuh, M.; Murawski, N.; Zeynalova, S.; Ziepert, M.; Loeffler, M.; Hänel, M.; Dierlamm, J.; Keller, U.; Dreyling, M.; Truemper, L.; et al. Optimization of rituximab for the treatment of DLBCL: Increasing the dose for elderly male patients. Br. J. Haematol. 2017, 179, 410–420. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Trümper, L.; Iyer, S.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. The ECHELON-2 Trial: 5-year results of a randomized, phase III study of brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma. Ann. Oncol. 2022, 33, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.; Cairns, D.; Menzies, T.; Boyd, K.; Davies, F.; Cook, G.; Drayson, M.; Gregory, W.; Jenner, M.; Jones, J.; et al. Sex Differences in Multiple Myeloma Biology but not Clinical Outcomes: Results from 3894 Patients in the Myeloma XI Trial. Clin. Lymphoma Myeloma Leuk. 2021, 21, 667–675. [Google Scholar] [CrossRef]
- Boyd, K.D.; Ross, F.M.; Chiecchio, L.; Dagrada, G.P.; Konn, Z.J.; Tapper, W.J.; Walker, B.A.; Wardell, C.P.; Gregory, W.M.; Szubert, A.J.; et al. Gender disparities in the tumor genetics and clinical outcome of multiple myeloma. Cancer Epidemiol. Biomarkers Prev. 2011, 20, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Posch, D.; Rabitsch, W.; Wohlfarth, P.; Leiner, M.; Porpaczy, E.; Drach, J.; Raderer, M.; Lamm, W. Gender-Specific Aspects in Patients with Multiple Myeloma Undergoing Autologous Stem Cell Transplantation: A Single-Center Experience. Oncology 2017, 93, 295–301. [Google Scholar] [CrossRef]
- Derman, B.A.; Langerman, S.S.; Maric, M.; Jakubowiak, A.; Zhang, W.; Chiu, B.C.H. Sex differences in outcomes in multiple myeloma. Br. J. Haematol. 2021, 192, e66–e69. [Google Scholar] [CrossRef]
- Ali, M.O.; Al Hadidi, S. High dose (conditioning) regimens used prior to autologous stem cell transplantation in multiple myeloma. Transplant. Cell Ther. 2022, 28, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Jacobus, S.J.; Weller, E.A.; Hassoun, H.; Lonial, S.; Raje, N.S.; Medvedova, E.; McCarthy, P.L.; Libby, E.N.; Voorhees, P.M.; et al. Triplet Therapy, Transplantation, and Maintenance until Progression in Myeloma. N. Engl. J. Med. 2022, 387, 132–147. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Bashir, Q.; Thall, P.F.; Milton, D.R.; Fox, P.S.; Kawedia, J.D.; Kebriaei, P.; Shah, N.; Patel, K.; Andersson, B.S.; Nieto, Y.L.; et al. Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: An open-label, randomised, phase 3 trial. Lancet Haematol. 2019, 6, e266–e275. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Gillich, C.; Akhoundova, D.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Seipel, K.; Daskalakis, M.; Bacher, U.; Pabst, T. Efficacy and Safety of High-Dose Chemotherapy with Treosulfan and Melphalan in Multiple Myeloma. Cancers 2023, 15, 2699. [Google Scholar] [CrossRef] [PubMed]
- Gurevich, E.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Taleghani, B.M.; Bacher, U.; Pabst, T. Comparison of Melphalan Combined with Treosulfan or Busulfan as High-Dose Chemotherapy before Autologous Stem Cell Transplantation in AML. Cancers 2022, 14, 1024. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised international staging system for multiple myeloma: A report from international myeloma working group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Romański, M.; Wachowiak, J.; Główka, F.K. Treosulfan Pharmacokinetics and its Variability in Pediatric and Adult Patients Undergoing Conditioning Prior to Hematopoietic Stem Cell Transplantation: Current State of the Art, In-Depth Analysis, and Perspectives. Clin. Pharmacokinet. 2018, 57, 1255–1265. [Google Scholar] [CrossRef]
- Beelen, D.W.; Trenschel, R.; Casper, J.; Freund, M.; Hilger, R.A.; Scheulen, M.E.; Basara, N.; Fauser, A.A.; Hertenstein, B.; Mylius, H.A.; et al. Dose-escalated treosulphan in combination with cyclophosphamide as a new preparative regimen for allogeneic haematopoietic stem cell transplantation in patients with an increased risk for regimen-related complications. Bone Marrow Transplant. 2005, 35, 233–241. [Google Scholar] [CrossRef]
- Pai, A.A.; Mohanan, E.; Panetta, J.C.; Kulkarni, U.P.; Illangeswaran, R.S.S.; Balakrishnan, B.; Jayaraman, A.; Edison, E.S.; Lakshmi, K.M.; Devasia, A.J.; et al. Treosulfan Exposure Predicts Thalassemia-Free Survival in Patients with Beta Thalassemia Major Undergoing Allogeneic Hematopoietic Cell Transplantation. Clin. Pharmacol. Ther. 2024, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- van der Stoep, M.Y.E.C.; Bertaina, A.; ten Brink, M.H.; Bredius, R.G.; Smiers, F.J.; Wanders, D.C.M.; Moes, D.J.A.R.; Locatelli, F.; Guchelaar, H.J.; Zwaveling, J.; et al. High interpatient variability of treosulfan exposure is associated with early toxicity in paediatric HSCT: A prospective multicentre study. Br. J. Haematol. 2017, 179, 772–780. [Google Scholar] [CrossRef]
- van der Stoep, M.Y.E.C.; Bertaina, A.; Moes, D.J.A.R.; Algeri, M.; Bredius, R.G.M.; Smiers, F.J.W.; Berghuis, D.; Buddingh, E.P.; Mohseny, A.B.; Guchelaar, H.J.; et al. Impact of Treosulfan Exposure on Early and Long-Term Clinical Outcomes in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients: A Prospective Multicenter Study. Transplant. Cell Ther. 2022, 28, 99.e1–99.e7. [Google Scholar] [CrossRef]
- Ayçiçek, S.G.; Akhoundova, D.; Bacher, U.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Pabst, T. Determinants of Interpatient Variability in Treosulfan Pharmacokinetics in AML Patients Undergoing Autologous Stem Cell Transplantation. Int. J. Mol. Sci. 2024, 25, 8215. [Google Scholar] [CrossRef]
- Moreau, P.; Karamanesht, I.I.; Domnikova, N.; Kyselyova, M.Y.; Vilchevska, K.V.; Doronin, V.A.; Schmidt, A.; Hulin, C.; Leleu, X.; Esseltine, D.L.; et al. Pharmacokinetic, pharmacodynamic and covariate analysis of subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma. Clin. Pharmacokinet. 2012, 51, 823–829. [Google Scholar] [CrossRef]

| Females | %/Range | Males | %/Range | p-Value | s | All Patients | %/Range | |
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Female, n (%) | 42 | 37.5 | 70 | 62.5 | — | 112 | 100 | |
| Median age at diagnosis, years (range) | 64.3 | (36.1–73.8) | 60.8 | (37.9–73.4) | 0.0413 | * | 61.8 | (36.1–73.8) |
| Median age at HDCT/ASCT, years, (range) | 64.8 | (36.5–74.3) | 61.8 | (38.2–73.8) | 0.0390 | * | 62.3 | (36.5–74.3) |
| Median body weight at HDCT/ASCT, kg (range) | 65.5 | (47–109) | 80.0 | (60–130.5) | 0.0001 | **** | 76.0 | (47–130) |
| Median eGFR, mL/min/1.73 m2 (range) | 94.5 | (48–124) | 97.1 | (34–125) | 0.4371 | n.s. | 96 | (34–125) |
| R-ISS Stage, n (%) | ||||||||
| I | 7 | 16.7 | 19 | 27.1 | 0.2515 | n.s. | 26 | 23.2 |
| II | 23 | 54.8 | 29 | 41.4 | 0.1785 | n.s. | 52 | 46.4 |
| III | 10 | 23.8 | 17 | 24.3 | >0.9999 | n.s. | 27 | 24.1 |
| No data | 2 | 4.8 | 5 | 7.1 | 0.7095 | n.s. | 7 | 6.3 |
| Cytogenetics, n (%) | ||||||||
| High risk | 13 | 31.0 | 11 | 15.7 | 0.0944 | n.s. | 24 | 21.4 |
| Standard risk | 21 | 50.0 | 43 | 61.4 | 0.2453 | n.s. | 64 | 57.1 |
| No data | 8 | 19.0 | 16 | 22.9 | 0.8125 | n.s. | 24 | 21.4 |
| Paraprotein Subtype, n (%) | ||||||||
| IgA | 7 | 16.7 | 9 | 12.9 | 0.5879 | n.s. | 16 | 14.3 |
| IgG | 28 | 66.7 | 49 | 70 | 0.8336 | n.s. | 77 | 68.8 |
| IgM | 0 | 0 | 1 | 1.4 | 0.4365 | n.s. | 1 | 0.9 |
| Light chain only | 7 | 16.7 | 11 | 15.7 | >0.9999 | n.s. | 18 | 16.1 |
| Kappa light chain | 25 | 59.5 | 48 | 68.6 | 0.4131 | n.s. | 73 | 65.2 |
| Lambda light chain | 17 | 40.5 | 22 | 31.4 | 0.4131 | n.s. | 39 | 34.8 |
| Females | %/Range | Males | %/Range | p-Value | s | All Patients | % | |
|---|---|---|---|---|---|---|---|---|
| Induction regimens, n (%) | ||||||||
| Bortezomib/lenalidomide/dexamethasone | 29 | 69 | 63 | 90.0 | 0.0095 | ** | 92 | 82.1 |
| Daratumumab/bortezomib/lenalidomide/dexamethasone | 9 | 21.4 | 4 | 5.7 | 0.0161 | * | 13 | 11.6 |
| Other | 4 | 9.5 | 3 | 4.3 | 0.4218 | n.s. | 7 | 6.3 |
| Dartumumab in induction | 10 | 23.8 | 4 | 5.7 | 0.0075 | ** | 14 | 12.5 |
| Cycles of induction therapy, median (range) | 4 | (2–4) | 4 | (2–6) | 0.4126 | n.s. | 4 | (2–6) |
| HDCT/ASCT | ||||||||
| Time to HDCT from first diagnosis, months, median (range) | 5.3 | (3.3–60.9) | 4.6 | (2.9–75.3) | 0.0056 | ** | 4.8 | (2.9–75.3) |
| HD regimen: treosulfan/melphalan, n (%) | 42 | 100 | 70 | 100 | >0.9999 | n.s. | 112 | 100 |
| CD34+ cells transfused, median (range) | 256.7 | (98.5–462) | 239.8 | (90–1369) | 0.3814 | n.s. | 249.9 | (90–1369) |
| Treosulfan AUC (mg*h/L), median (range) | 869.9 | (578–1583) | 830.5 | (465–1504) | 0.0427 | * | 833.0 | (465–1583) |
| Treosulfan peak (mg/L), median (range) | 343.8 | (256–554) | 309.0 | (168–498) | 0.0011 | ** | 330.0 | (168–554) |
| Maintenance therapy, n (%) | ||||||||
| Lenalidomide | 39 | 92.9 | 65 | 92.9 | >0.9999 | n.s. | 104 | 92.9 |
| Daratumumab | 0 | 0 | 1 | 1.4 | >0.9999 | n.s. | 1 | 0.9 |
| Other | 0 | 0 | 3 | 4.3 | 0.2905 | n.s. | 3 | 2.7 |
| No maintenance | 3 | 7.1 | 1 | 1.4 | 0.1146 | n.s. | 4 | 3.6 |
| Females | %/Range | Males | %/Range | p-Value | s | All Patients | %/Range | |
|---|---|---|---|---|---|---|---|---|
| Response before HDCT/ASCT, n (%) | ||||||||
| CR | 7 | 16.7 | 11 | 15.7 | >0.9999 | n.s. | 18 | 16.1 |
| VGPR | 23 | 54.8 | 26 | 37.1 | 0.0793 | n.s. | 49 | 43.8 |
| PR | 5 | 11.9 | 24 | 34.3 | 0.0133 | * | 29 | 25.9 |
| SD | 0 | 0 | 2 | 2.9 | 0.5270 | n.s. | 2 | 1.8 |
| No Info | 7 | 16.7 | 7 | 10 | 0.3788 | n.s. | 14 | 12.5 |
| VGPR or better | 30 | 71.4 | 37 | 52.9 | 0.0729 | n.s. | 67 | 59.8 |
| Survival | ||||||||
| Progression-free survival, months, median (range) | n.r. | (0.25–44.81) | 36.7 | (2.56–40.53) | 0.2644 | n.s. | n.r. | (0.25–44.81) |
| Overall survival, months, median (range) | n.r. | (0.25–44.81) | n.r. | (2.63–42.70) | 0.8951 | n.s. | n.r. | (0.25–44.81) |
| Follow up, months, median (range) | — | — | — | — | — | 30.95 | — | |
| Best response after HDCT/ASCT, n (%) | ||||||||
| sCR | 27 | 64.3 | 43 | 61.4 | >0.9999 | n.s. | 70 | 62.5 |
| CR | 12 | 28.6 | 12 | 17.1 | 0.1629 | n.s. | 24 | 21.4 |
| VGPR | 2 | 4.8 | 7 | 10 | 0.4795 | n.s. | 9 | 8 |
| PR | 0 | 0 | 4 | 5.7 | 0.2950 | n.s. | 4 | 3.6 |
| SD | 0 | 0 | 1 | 1.4 | >0.9999 | n.s. | 1 | 0.9 |
| No Info | 1 | 2.4 | 3 | 4.3 | >0.9999 | n.s. | 4 | 3.6 |
| Females | %/Range | Males | %/Range | p-Value | s | All Patients | %/Range | |
|---|---|---|---|---|---|---|---|---|
| Hematologic recovery | ||||||||
| ANC recovery to >0.5 × 109/L, days, median (range) | 11 | (2–16) | 12 | (10–26) | 0.0119 | * | 11 | (2–26) |
| Platelet recovery to >100 × 109/L, days, median (range) | 12 | (7–38) | 13 | (9–52) | 0.2132 | n.s. | 12 | (7–52) |
| Platelet transfusions, units, median (range) | 3 | (0–30) | 2 | (0–16) | 0.2348 | n.s. | 3 | (0–30) |
| RBC transfusions, units, median (range) | 1 | (0–15) | 0 | (0–19) | 0.0173 | * | 1 | (0–19) |
| Engraftment syndrome, n (%) | 6 | 14.3 | 5 | 7.1 | 0.3253 | n.s. | 11 | 9.8 |
| Hospitalization | ||||||||
| Inpatient stay, days, median (range) | 24 | (12–89) | 21 | (16–38) | 0.0007 | *** | 22 | (12–89) |
| ICU admission, n (%) | 5 | 11.9 | 2 | 2.9 | 0.1007 | n.s. | 7 | 6.3 |
| Infectious complications | ||||||||
| Neutropenic fever, n (%) | 41 | 97.6 | 68 | 97.1 | >0.9999 | n.s. | 109 | 97.3 |
| Neutropenic colitis, n (%) | 28 | 66.7 | 56 | 80 | 0.1223 | n.s. | 84 | 75 |
| Bacteriemia, n (%) | 17 | 40.5 | 23 | 32.9 | 0.4240 | n.s. | 40 | 35.7 |
| Central line associated blood stream infection, n (%) | 3 | 7.1 | 18 | 25.7 | 0.0732 | n.s. | 21 | 18.8 |
| SARS-CoV2 infection, n (%) | 4 | 9.5 | 2 | 2.9 | 0.1949 | n.s. | 6 | 5.4 |
| Metabolism | ||||||||
| Parenteral nutrition, n (%) | 40 | 95.2 | 60 | 85.7 | 0.2051 | n.s. | 100 | 89.3 |
| Duration of PN, days, median (range) | 10 | (2–25) | 8 | (2–20) | 0.0025 | ** | ||
| Refeeding syndrome in patients requiring PN, n (%) | 13 | 32.5 | 33 | 55 | 0.0401 | * | 46 | 46 |
| Mucositis, n (%) | 19 | 47.5 | 37 | 61.7 | 0.5585 | n.s. | 56 | 50 |
| Acute kidney injury, n (%) | 4 | 9.5 | 3 | 4.3 | 0.4218 | n.s. | 7 | 6.3 |
| Hepatic injury, n (%) | 2 | 4.8 | 3 | 4.3 | >0.9999 | n.s. | 5 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heini, A.D.; Kammermann, K.; Bacher, U.; Jeker, B.; Hayoz, M.; Aebi, Y.; Largiadèr, C.R.; Nilius, H.; Pabst, T. Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma. Cancers 2024, 16, 3364. https://doi.org/10.3390/cancers16193364
Heini AD, Kammermann K, Bacher U, Jeker B, Hayoz M, Aebi Y, Largiadèr CR, Nilius H, Pabst T. Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma. Cancers. 2024; 16(19):3364. https://doi.org/10.3390/cancers16193364
Chicago/Turabian StyleHeini, Alexander D., Karin Kammermann, Ulrike Bacher, Barbara Jeker, Michael Hayoz, Yolanda Aebi, Carlo R. Largiadèr, Henning Nilius, and Thomas Pabst. 2024. "Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma" Cancers 16, no. 19: 3364. https://doi.org/10.3390/cancers16193364
APA StyleHeini, A. D., Kammermann, K., Bacher, U., Jeker, B., Hayoz, M., Aebi, Y., Largiadèr, C. R., Nilius, H., & Pabst, T. (2024). Gender-Specific Prognostic Impact of Treosulfan Levels in High-Dose Chemotherapy for Multiple Myeloma. Cancers, 16(19), 3364. https://doi.org/10.3390/cancers16193364









