Characterization of Vitronectin Effect in 3D Ewing Sarcoma Models: A Digital Microscopic Analysis of Two Cell Lines
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Monolayer Cultures (2D)
2.2. Hydrogel Cultures (3D)
2.3. Two-Dimensional and Three-Dimensional Sample Preparation for Digital Analysis
2.4. Digital Microscopic Analyses
2.5. Detection of VN in Culture Media
2.6. Statistical Analysis
3. Results
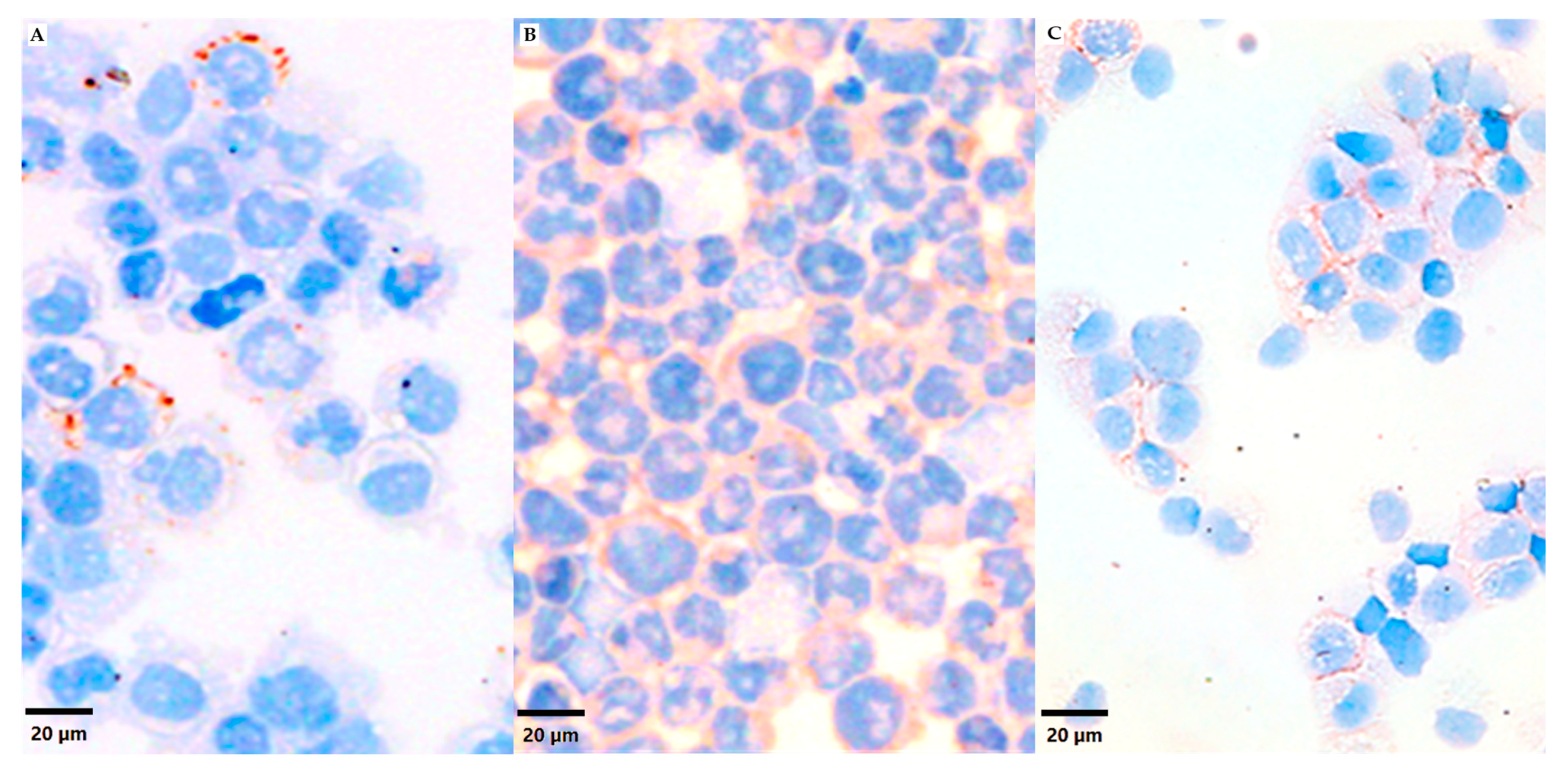
3.1. VN Cell Expression and Secretion to Culture Media in 2D Models
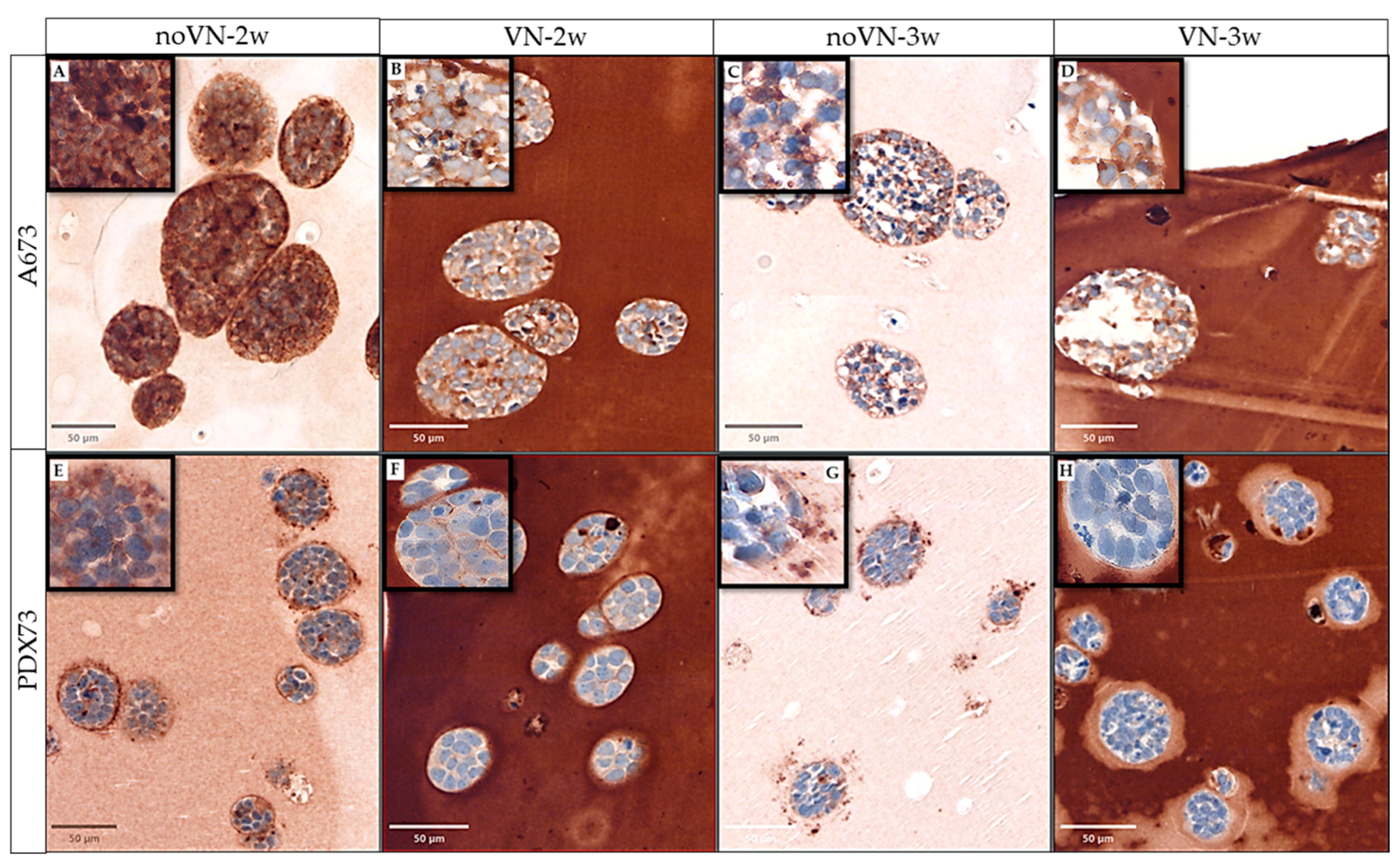
3.2. VN Cell Expression and Secretion to the ECM in 3D Models
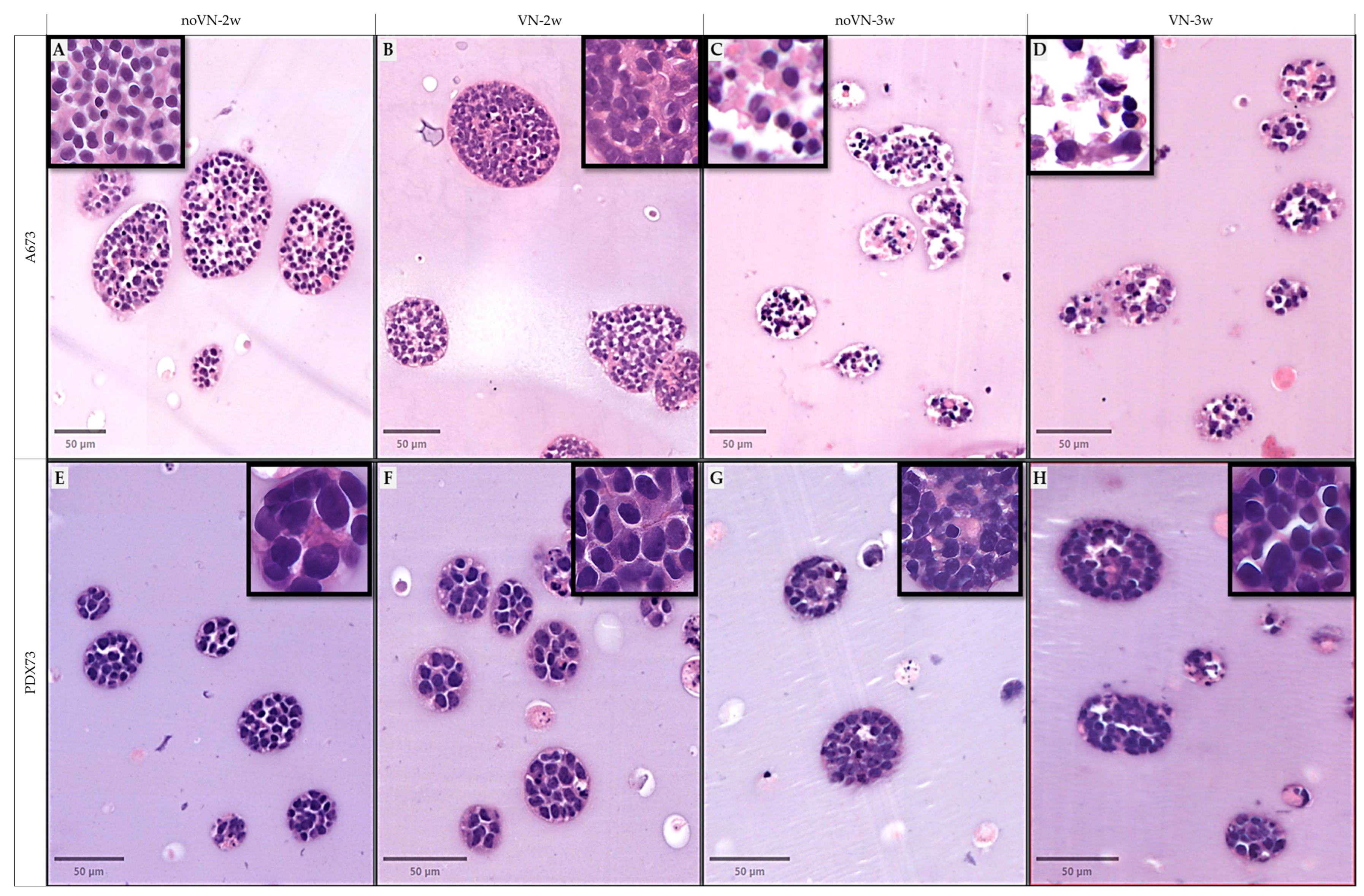
3.3. Digital Analisis and Morphometric Parameters of Clusters and Cells of 3D Models
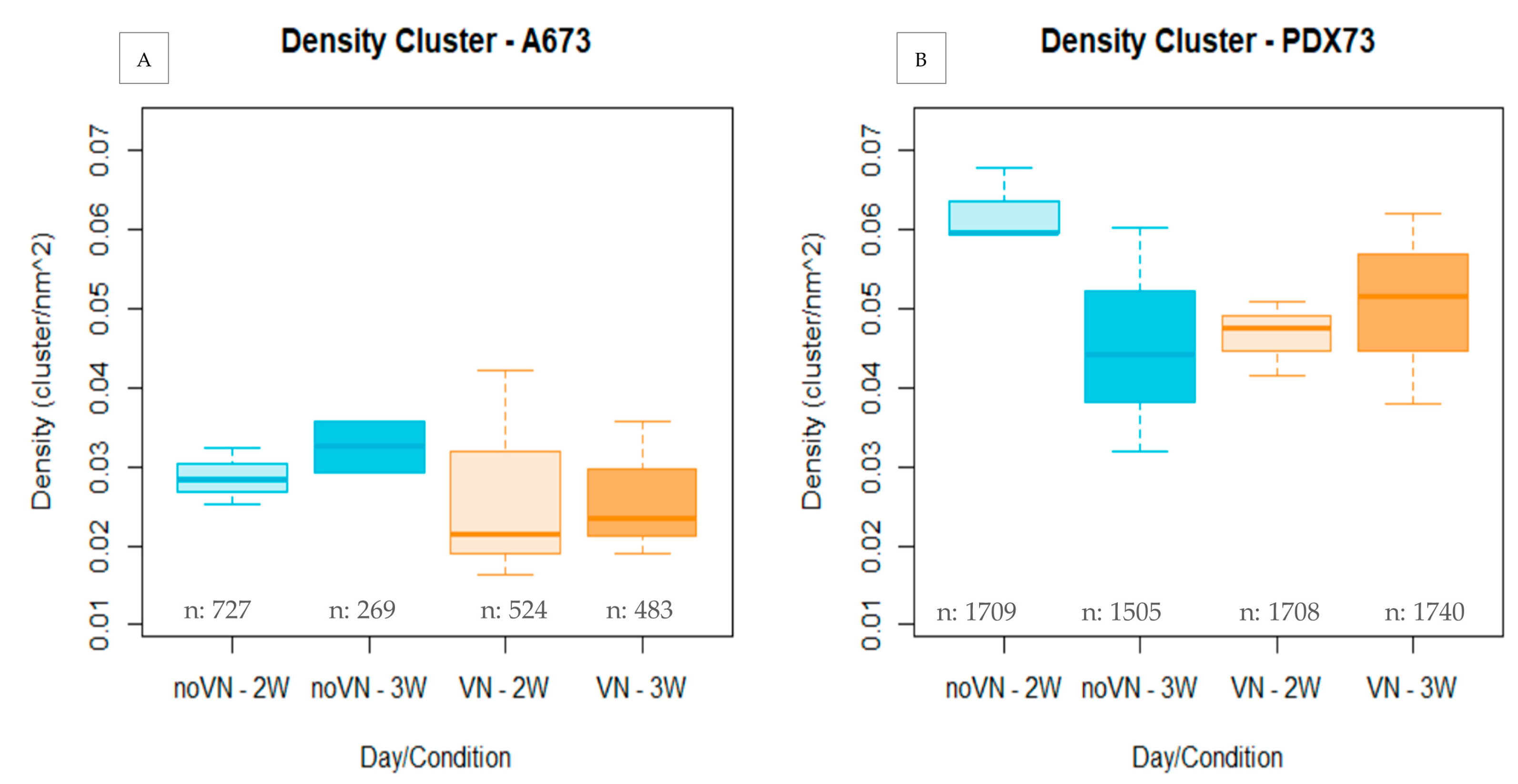
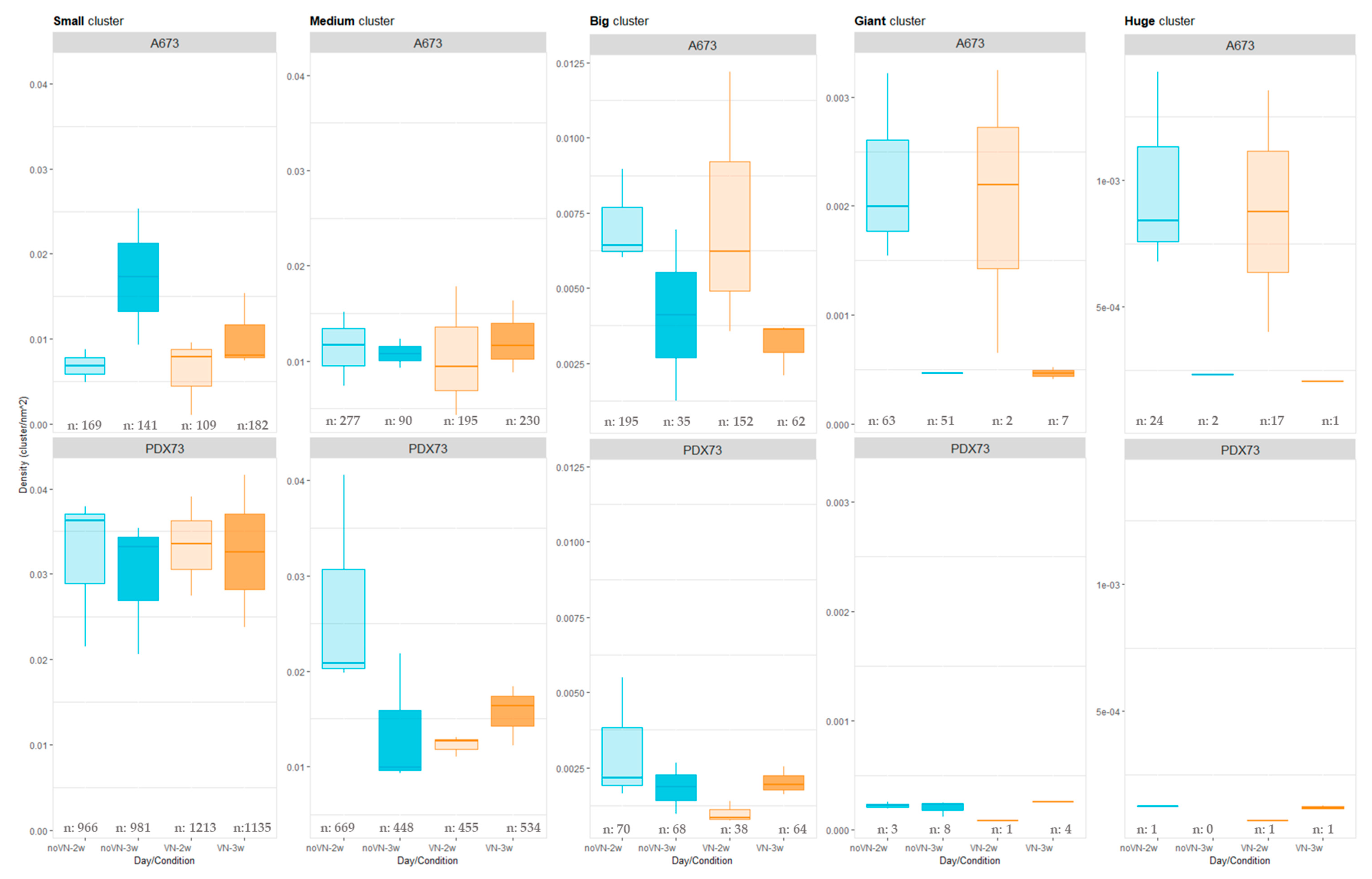
3.3.1. Cluster Density
Cluster Density Classified by Cluster Size
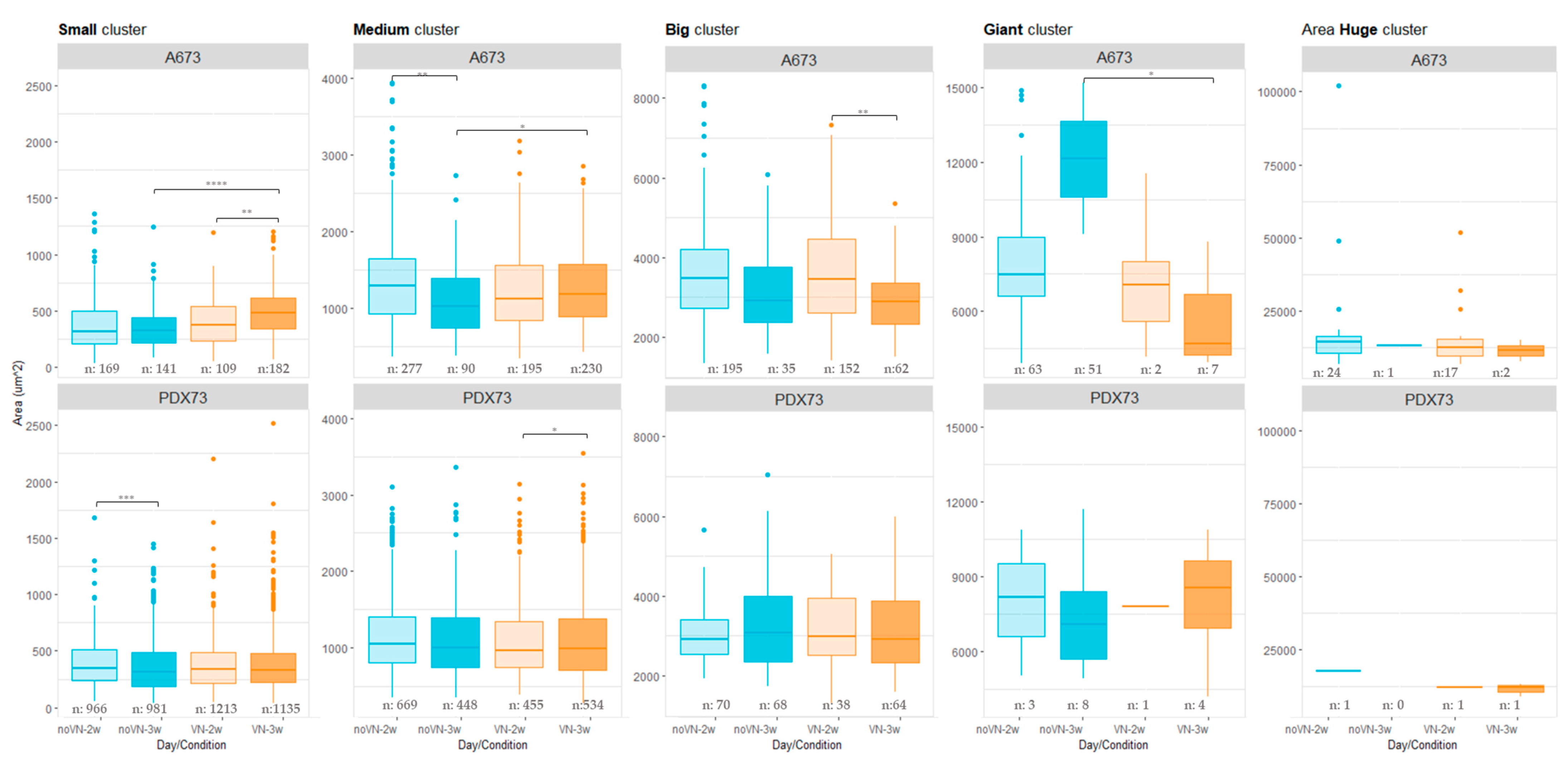
3.3.2. Cluster Areas
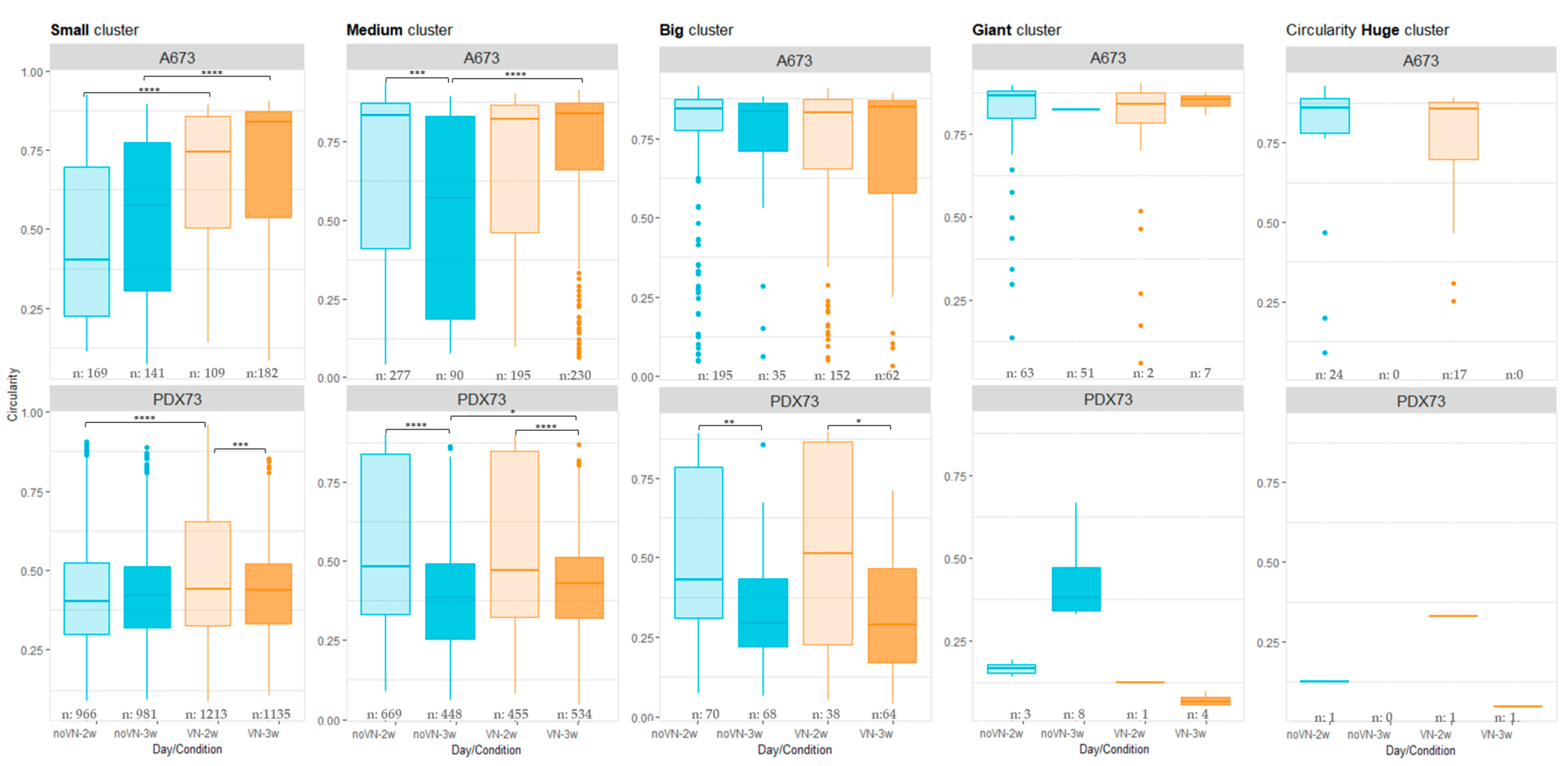
3.3.3. Circularity of Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, E.A.; Abdessalam, S.; Aldrink, J.H.; Austin, M.; Heaton, T.E.; Bruny, J.; Ehrlich, P.; Dasgupta, R.; Baertschiger, R.M.; Lautz, T.B.; et al. Update on Neuroblastoma. J. Pediatr. Surg. 2019, 54, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Grünewald, T.G.P.; Cidre-Aranaz, F.; Surdez, D.; Tomazou, E.M.; de Álava, E.; Kovar, H.; Sorensen, P.H.; Delattre, O.; Dirksen, U. Ewing Sarcoma. Nat. Rev. Dis. Primers 2018, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, P.; Yáñez, Y.; Juan, A.; Segura, V.; Balaguer, J.; Torres, B.; Oltra, S.; Castel, V.; Cañete, A. Review: Ewing Sarcoma Predisposition. Pathol. Oncol. Res. 2020, 26, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M. Small Round Cell Tumors of Bone. Arch. Pathol. Lab Med. 2007, 131, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T. Diagnosis and Treatment of Ewing Sarcoma of the Bone: A Review Article. J. Orthop. Sci. 2015, 20, 250–263. [Google Scholar] [CrossRef]
- Zöllner, S.K.; Amatruda, J.F.; Bauer, S.; Collaud, S.; de Álava, E.; DuBois, S.G.; Hardes, J.; Hartmann, W.; Kovar, H.; Metzler, M.; et al. Ewing Sarcoma—Diagnosis, Treatment, Clinical Challenges and Future Perspectives. J. Clin. Med. 2021, 10, 1685. [Google Scholar] [CrossRef]
- Eaton, B.R.; Claude, L.; Indelicato, D.J.; Vatner, R.; Yeh, B.; Schwarz, R.; Laack, N. Ewing Sarcoma. Pediatr. Blood Cancer 2021, 68, e28355. [Google Scholar] [CrossRef]
- Setty, B.A.; Gikandi, A.; DuBois, S.G. Ewing Sarcoma Drug Therapy: Current Standard of Care and Emerging Agents. Pediatr. Drugs 2023, 25, 389–397. [Google Scholar] [CrossRef]
- Sanegre, S.; Lucantoni, F.; Burgos-Panadero, R.; de La Cruz-Merino, L.; Noguera, R.; Álvaro Naranjo, T. Integrating the Tumor Microenvironment into Cancer Therapy. Cancers 2020, 12, 1677. [Google Scholar] [CrossRef]
- Burgos-Panadero, R.; Lucantoni, F.; Gamero-Sandemetrio, E.; Cruz-Merino, L.d.l.; Álvaro, T.; Noguera, R. The Tumour Microenvironment as an Integrated Framework to Understand Cancer Biology. Cancer Lett. 2019, 461, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New Horizons in Tumor Microenvironment Biology: Challenges and Opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Noguera, R.; Nieto, O.A.; Tadeo, I.; Fariñas, F.; Álvaro, T. Extracellular Matrix, Biotensegrity and Tumor Microenvironment. An Update and Overview. Histol. Histopathol. 2012, 27, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Pickup, M.W.; Mouw, J.K.; Weaver, V.M. The Extracellular Matrix Modulates the Hallmarks of Cancer. EMBO Rep. 2014, 15, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Elgundi, Z.; Papanicolaou, M.; Major, G.; Cox, T.R.; Melrose, J.; Whitelock, J.M.; Farrugia, B.L. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Front. Oncol. 2020, 9, 1482. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science (1979) 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- López-Carrasco, A.; Martín-Vañó, S.; Burgos-Panadero, R.; Monferrer, E.; Berbegall, A.P.; Fernández-Blanco, B.; Navarro, S.; Noguera, R. Impact of Extracellular Matrix Stiffness on Genomic Heterogeneity in MYCN-Amplified Neuroblastoma Cell Line. J. Exp. Clin. Cancer Res. 2020, 39, 226. [Google Scholar] [CrossRef]
- Ben-David, U.; Ha, G.; Tseng, Y.-Y.; Greenwald, N.F.; Oh, C.; Shih, J.; McFarland, J.M.; Wong, B.; Boehm, J.S.; Beroukhim, R.; et al. Patient-Derived Xenografts Undergo Mouse-Specific Tumor Evolution. Nat. Genet. 2017, 49, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular Matrix Remodeling in Tumor Progression and Immune Escape: From Mechanisms to Treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Hawkins, A.G.; Julian, C.M.; Konzen, S.; Treichel, S.; Lawlor, E.R.; Bailey, K.M. Microenvironmental Factors Drive Tenascin C and Src Cooperation to Promote Invadopodia Formation in Ewing Sarcoma. Neoplasia 2019, 21, 1063–1072. [Google Scholar] [CrossRef]
- Rodríguez-Núñez, P.; Romero-Pérez, L.; Amaral, A.T.; Puerto-Camacho, P.; Jordán, C.; Marcilla, D.; Grünewald, T.G.; Alonso, J.; de Alava, E.; Díaz-Martín, J. Hippo Pathway Effectors YAP1/TAZ Induce an EWS–FLI1 -opposing Gene Signature and Associate with Disease Progression in Ewing Sarcoma. J. Pathol. 2020, 250, 374–386. [Google Scholar] [CrossRef]
- Bierbaumer, L.; Katschnig, A.M.; Radic-Sarikas, B.; Kauer, M.O.; Petro, J.A.; Högler, S.; Gurnhofer, E.; Pedot, G.; Schäfer, B.W.; Schwentner, R.; et al. YAP/TAZ Inhibition Reduces Metastatic Potential of Ewing Sarcoma Cells. Oncogenesis 2021, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, E.; Warren, M.; Triche, T.J.; Amatruda, J.F. Dysregulated Heparan Sulfate Proteoglycan Metabolism Promotes Ewing Sarcoma Tumor Growth. eLlife 2022, 11, e69734. [Google Scholar] [CrossRef] [PubMed]
- Wullkopf, L.; West, A.-K.V.; Leijnse, N.; Cox, T.R.; Madsen, C.D.; Oddershede, L.B.; Erler, J.T. Cancer Cells’ Ability to Mechanically Adjust to Extracellular Matrix Stiffness Correlates with Their Invasive Potential. Mol. Biol. Cell 2018, 29, 2378–2385. [Google Scholar] [CrossRef] [PubMed]
- Aveic, S.; Davtalab, R.; Vogt, M.; Weber, M.; Buttler, P.; Tonini, G.P.; Fischer, H. Calcium Phosphate Scaffolds with Defined Interconnecting Channel Structure Provide a Mimetic 3D Niche for Bone Marrow Metastasized Tumor Cell Growth. Acta Biomater. 2019, 88, 527–539. [Google Scholar] [CrossRef]
- DeClerck, Y.A.; Mercurio, A.M.; Stack, M.S.; Chapman, H.A.; Zutter, M.M.; Muschel, R.J.; Raz, A.; Matrisian, L.M.; Sloane, B.F.; Noel, A.; et al. Proteases, Extracellular Matrix, and Cancer. Am. J. Pathol. 2004, 164, 1131–1139. [Google Scholar] [CrossRef]
- Shi, K.; Lan, R.-L.; Tao, X.; Wu, C.-Y.; Hong, H.-F.; Lin, J.-H. Vitronectin Significantly Influences Prognosis in Osteosarcoma. Int. J. Clin. Exp. Pathol. 2015, 8, 11364–11371. [Google Scholar]
- Tas, F.; Karabulut, S.; Bilgin, E.; Tastekin, D.; Duranyildiz, D. Clinical Significance of Serum Fibronectin and Vitronectin Levels in Melanoma Patients. Melanoma Res. 2014, 24, 475–479. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Yang, G.; Fu, C.; Jiang, G.; Hu, Q. Vitronectin Silencing Inhibits Hepatocellular Carcinoma in Vitro and in Vivo. Future Oncol. 2015, 11, 251–258. [Google Scholar] [CrossRef]
- Bera, A.; Subramanian, M.; Karaian, J.; Eklund, M.; Radhakrishnan, S.; Gana, N.; Rothwell, S.; Pollard, H.; Hu, H.; Shriver, C.D.; et al. Functional Role of Vitronectin in Breast Cancer. PLoS ONE 2020, 15, e0242141. [Google Scholar] [CrossRef]
- Ciereszko, A.; Dietrich, M.A.; Słowińska, M.; Nynca, J.; Ciborowski, M.; Kisluk, J.; Michalska-Falkowska, A.; Reszec, J.; Sierko, E.; Nikliński, J. Identification of Protein Changes in the Blood Plasma of Lung Cancer Patients Subjected to Chemotherapy Using a 2D-DIGE Approach. PLoS ONE 2019, 14, e0223840. [Google Scholar] [CrossRef]
- Schneider, G.; Suszynska, M.; Kakar, S.; Ratajczak, M.Z. Vitronectin in the Ascites of Human Ovarian Carcinoma Acts as a Potent Chemoattractant for Ovarian Carcinoma: Implication for Metastasis by Cancer Stem Cells. J. Cancer Stem Cell Res. 2016, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Panadero, R.; Noguera, I.; Cañete, A.; Navarro, S.; Noguera, R. Vitronectin as a Molecular Player of the Tumor Microenvironment in Neuroblastoma. BMC Cancer 2019, 19, 479. [Google Scholar] [CrossRef]
- Lopez-Carrasco, A.; Diaz-Martin, J.; Machado, I.; Navarro, S.; Alava, E.; Noguera, R. The Morphology of the Extracellular Matrix as a Classifier of Types and Subtypes of Ewing and Ewing-like Sarcomas: A Morpho-Molecular Study. Histol. Histopathol. 2021, 37 (Suppl. S1), 109. [Google Scholar]
- Guerrieri, A.N.; Bellotti, C.; Penzo, M.; Columbaro, M.; Pannella, M.; De Vita, A.; Gambarotti, M.; Mercatali, L.; Laranga, R.; Dozza, B.; et al. A Novel Patient-Derived Immortalised Cell Line of Myxofibrosarcoma: A Tool for Preclinical Drugs Testing and the Generation of near-Patient Models. BMC Cancer 2023, 23, 1194. [Google Scholar] [CrossRef]
- Kopper, O.; de Witte, C.J.; Lõhmussaar, K.; Valle-Inclan, J.E.; Hami, N.; Kester, L.; Balgobind, A.V.; Korving, J.; Proost, N.; Begthel, H.; et al. An Organoid Platform for Ovarian Cancer Captures Intra- and Interpatient Heterogeneity. Nat. Med. 2019, 25, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Braekeveldt, N.; Wigerup, C.; Gisselsson, D.; Mohlin, S.; Merselius, M.; Beckman, S.; Jonson, T.; Börjesson, A.; Backman, T.; Tadeo, I.; et al. Neuroblastoma Patient-derived Orthotopic Xenografts Retain Metastatic Patterns and Geno- and Phenotypes of Patient Tumours. Int. J. Cancer 2015, 136, E252–E261. [Google Scholar] [CrossRef] [PubMed]
- Rae, C.; Amato, F.; Braconi, C. Patient-Derived Organoids as a Model for Cancer Drug Discovery. Int. J. Mol. Sci. 2021, 22, 3483. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Garcia, J.; Jubelin, C.; Loussouarn, A.; Goumard, M.; Griscom, L.; Renodon-Cornière, A.; Heymann, M.-F.; Heymann, D. In Vitro Three-Dimensional Cell Cultures for Bone Sarcomas. J. Bone Oncol. 2021, 30, 100379. [Google Scholar] [CrossRef]
- Santoro, M.; Lamhamedi-Cherradi, S.-E.; Menegaz, B.A.; Ludwig, J.A.; Mikos, A.G. Flow Perfusion Effects on Three-Dimensional Culture and Drug Sensitivity of Ewing Sarcoma. Proc. Natl. Acad. Sci. USA 2015, 112, 10304–10309. [Google Scholar] [CrossRef]
- Monferrer, E.; Sanegre, S.; Martín-Vañó, S.; García-Lizarribar, A.; Burgos-Panadero, R.; López-Carrasco, A.; Navarro, S.; Samitier, J.; Noguera, R. Digital Image Analysis Applied to Tumor Cell Proliferation, Aggressiveness, and Migration-Related Protein Synthesis in Neuroblastoma 3D Models. Int. J. Mol. Sci. 2020, 21, 8676. [Google Scholar] [CrossRef]
- López-Carrasco, A.; Vieco-Martí, I.; Granados-Aparici, S.; Acevedo-León, D.; Noguera, R. Vitronectin Levels in Plasma of Neuroblastoma Patients and Culture Media of 3D Models: A Prognostic Circulating Biomarker ? Int. J. Mol. Sci. 2024, 25, 8733. [Google Scholar] [CrossRef] [PubMed]
- Sand, L.; Szuhai, K.; Hogendoorn, P. Sequencing Overview of Ewing Sarcoma: A Journey across Genomic, Epigenomic and Transcriptomic Landscapes. Int. J. Mol. Sci. 2015, 16, 16176–16215. [Google Scholar] [CrossRef]
- Hasturk, O.; Jordan, K.E.; Choi, J.; Kaplan, D.L. Enzymatically Crosslinked Silk and Silk-Gelatin Hydrogels with Tunable Gelation Kinetics, Mechanical Properties and Bioactivity for Cell Culture and Encapsulation. Biomaterials 2019, 232, 119720. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.K.; Gardner, J.M.; Lucas, D.R.; McHugh, J.B.; Patel, R.M. Ewing Sarcoma. Semin. Diagn. Pathol. 2014, 31, 39–47. [Google Scholar] [CrossRef]
- Lamhamedi-Cherradi, S.-E.; Santoro, M.; Ramammoorthy, V.; Menegaz, B.A.; Bartholomeusz, G.; Iles, L.R.; Amin, H.M.; Livingston, J.A.; Mikos, A.G.; Ludwig, J.A. 3D Tissue-Engineered Model of Ewing’s Sarcoma. Adv. Drug Deliv. Rev. 2014, 79–80, 155–171. [Google Scholar] [CrossRef]
- Hashimoto, K.; Nishimura, S.; Oka, N.; Akagi, M. Outcomes of Comprehensive Treatment for Primary Osteosarcoma. SAGE Open Med. 2020, 8, 205031212092317. [Google Scholar] [CrossRef] [PubMed]
- Cacho-Díaz, B.; García-Botello, D.R.; Wegman-Ostrosky, T.; Reyes-Soto, G.; Ortiz-Sánchez, E.; Herrera-Montalvo, L.A. Tumor Microenvironment Differences between Primary Tumor and Brain Metastases. J. Transl. Med. 2020, 18, 1. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.-J. Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Abdollahiyan, P.; Oroojalian, F.; Baradaran, B.; de la Guardia, M.; Mokhtarzadeh, A. Advanced Mechanotherapy: Biotensegrity for Governing Metastatic Tumor Cell Fate via Modulating the Extracellular Matrix. J. Control. Release 2021, 335, 596–618. [Google Scholar] [CrossRef]
- Tadeo, I.; Berbegall, A.P.; Escudero, L.M.; Álvaro, T.; Noguera, R. Biotensegrity of the Extracellular Matrix: Physiology, Dynamic Mechanical Balance, and Implications in Oncology and Mechanotherapy. Front. Oncol. 2014, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital Pathology and Artificial Intelligence in Translational Medicine and Clinical Practice. Mod. Pathol. 2022, 35, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Y.; Li, Y. Artificial Intelligence Applications in Pediatric Oncology Diagnosis. Explor. Target. Antitumor Ther. 2023, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.G.; Ardon, O.; Reuter, V.E.; Sirintrapun, S.J.; England, C.; Klimstra, D.S.; Hameed, M.R. Integrating Digital Pathology into Clinical Practice. Mod. Pathol. 2022, 35, 152–164. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered Glycosylation in Cancer: A Promising Target for Biomarkers and Therapeutics. Biochim. Et Biophys. Acta (BBA)-Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef]
- Schneider, G.; Bryndza, E.; Poniewierska-Baran, A.; Serwin, K.; Suszynska, M.; Sellers, Z.P.; Merchant, M.L.; Kaliappan, A.; Ratajczak, J.; Kucia, M.; et al. Evidence That Vitronectin Is a Potent Migration-Enhancing Factor for Cancer Cells Chaperoned by Fibrinogen: A Novel View of the Metastasis of Cancer Cells to Low-Fibrinogen Lymphatics and Body Cavities. Oncotarget 2016, 7, 69829–69843. [Google Scholar] [CrossRef][Green Version]
- Chen, M.H.; Lu, C.; Sun, J.; Chen, X.D.; Dai, J.X.; Cai, J.Y.; Chen, X.L. Diagnostic and Prognostic Value of Serum Vitronectin Levels in Human Glioma. J. Neurol. Sci. 2016, 371, 54–59. [Google Scholar] [CrossRef]
- Kadowaki, M.; Sangai, T.; Nagashima, T.; Sakakibara, M.; Yoshitomi, H.; Takano, S.; Sogawa, K.; Umemura, H.; Fushimi, K.; Nakatani, Y.; et al. Identification of Vitronectin as a Novel Serum Marker for Early Breast Cancer Detection Using a New Proteomic Approach. J. Cancer Res. Clin. Oncol. 2011, 137, 1105–1115. [Google Scholar] [CrossRef]
- Turan, T.; Torun, M.; Atalay, F.; Gönenç, A. Assessment of Vitronectin, Soluble Epithelial-Cadherin and TGF-Β1 as a Serum Biomarker with Predictive Value for Endometrial and Ovarian Cancers. Turk. J. Pharm. Sci. 2017, 14, 141–147. [Google Scholar] [CrossRef]
- Repetto, O.; Caggiari, L.; De Zorzi, M.; Elia, C.; Mussolin, L.; Buffardi, S.; Pillon, M.; Muggeo, P.; Casini, T.; Steffan, A.; et al. Quantitative Plasma Proteomics to Identify Candidate Biomarkers of Relapse in Pediatric/Adolescent Hodgkin Lymphoma. Int. J. Mol. Sci. 2022, 23, 9911. [Google Scholar] [CrossRef]
- Braoudaki, M.; Lambrou, G.I.; Vougas, K.; Karamolegou, K.; Tsangaris, G.T.; Tzortzatou-Stathopoulou, F. Protein Biomarkers Distinguish between High- and Low-Risk Pediatric Acute Lymphoblastic Leukemia in a Tissue Specific Manner. J. Hematol. Oncol. 2013, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Stepanek, O.; Brdicka, T.; Angelisova, P.; Horvath, O.; Spicka, J.; Stockbauer, P.; Man, P.; Horejsi, V. Interaction of Late Apoptotic and Necrotic Cells with Vitronectin. PLoS ONE 2011, 6, e19243. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Ta, M. A Novel Role of Vitronectin in Promoting Survival of Mesenchymal Stem Cells under Serum Deprivation Stress. Stem Cell Res. Ther. 2020, 11, 181. [Google Scholar] [CrossRef] [PubMed]
- Leavesley, D.I.; Kashyap, A.S.; Croll, T.; Sivaramakrishnan, M.; Shokoohmand, A.; Hollier, B.G.; Upton, Z. Vitronectin—Master Controller or Micromanager? IUBMB Life 2013, 65, 807–818. [Google Scholar] [CrossRef]
- Cox, T.R. The Matrix in Cancer. Nat. Rev. Cancer 2021, 21, 217–238. [Google Scholar] [CrossRef]
- Heyman, L.; Leroy-Dudal, J.; Fernandes, J.; Seyer, D.; Dutoit, S.; Carreiras, F. Mesothelial Vitronectin Stimulates Migration of Ovarian Cancer Cells. Cell Biol. Int. 2010, 34, 493–502. [Google Scholar] [CrossRef]
| Number of Cells | Classification | Color Code |
|---|---|---|
| <9 | Small | Cyan |
| >10 and 38< | Medium | Green |
| >39 and 95< | Big | Yellow |
| >96 and 169< | Giant | Magenta |
| >170 | Huge | Blue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Carrasco, A.; Parra-Haro, K.; Vieco-Martí, I.; Granados-Aparici, S.; Díaz-Martín, J.; Salguero-Aranda, C.; Acevedo-León, D.; de Álava, E.; Navarro, S.; Noguera, R. Characterization of Vitronectin Effect in 3D Ewing Sarcoma Models: A Digital Microscopic Analysis of Two Cell Lines. Cancers 2024, 16, 3347. https://doi.org/10.3390/cancers16193347
López-Carrasco A, Parra-Haro K, Vieco-Martí I, Granados-Aparici S, Díaz-Martín J, Salguero-Aranda C, Acevedo-León D, de Álava E, Navarro S, Noguera R. Characterization of Vitronectin Effect in 3D Ewing Sarcoma Models: A Digital Microscopic Analysis of Two Cell Lines. Cancers. 2024; 16(19):3347. https://doi.org/10.3390/cancers16193347
Chicago/Turabian StyleLópez-Carrasco, Amparo, Karina Parra-Haro, Isaac Vieco-Martí, Sofía Granados-Aparici, Juan Díaz-Martín, Carmen Salguero-Aranda, Delia Acevedo-León, Enrique de Álava, Samuel Navarro, and Rosa Noguera. 2024. "Characterization of Vitronectin Effect in 3D Ewing Sarcoma Models: A Digital Microscopic Analysis of Two Cell Lines" Cancers 16, no. 19: 3347. https://doi.org/10.3390/cancers16193347
APA StyleLópez-Carrasco, A., Parra-Haro, K., Vieco-Martí, I., Granados-Aparici, S., Díaz-Martín, J., Salguero-Aranda, C., Acevedo-León, D., de Álava, E., Navarro, S., & Noguera, R. (2024). Characterization of Vitronectin Effect in 3D Ewing Sarcoma Models: A Digital Microscopic Analysis of Two Cell Lines. Cancers, 16(19), 3347. https://doi.org/10.3390/cancers16193347






