Simple Summary
Lifestyle dietary changes implemented by people who are overweight or obese to improve health generally focus on caloric restriction, changes in macronutrient intake, or both. Findings from reports of the impact of these changes on cancer are mixed, with many subjected to secondary analysis bias. An impact on cancer endpoints requires long-term (≥1 year) intervention. There is a clear need for more reports evaluating the role of diet in cancer risk reduction in overweight/obese individuals who are at increased risk of obesity-related cancers, especially reports focusing on cancers other than breast. Pharmacologic and metabolic/bariatric surgery-MBS strategies have demonstrated promise to deliver both greater initial weight loss and better weight loss maintenance). The impact of weight loss on reducing cancer risk may depend on the (1) amount and (2) maintenance of weight loss.
Abstract
Background: Most randomized controlled trials (RCTs) assessing the impact of diet on cancer have been short term (<1 year), mostly evaluating breast cancer survivors. Given the many-year interval that is generally required for an intervention to have an impact on cancer risk or prognosis, as well as the fact that lifestyle strategies such as diet modification frequently fail due to lack of adherence over the long term, we focused this systematic review only on longer-term (≥1 year) intervention reports. Diet intervention reports focused on reducing cancer risk in overweight and obese individuals target caloric restriction (every day, some days, or most hours of each day). Methods: This study is a systematic review of RCTs lasting at least 1 year, testing dietary interventions with a primary or secondary endpoint of cancer or a biomarker linked to cancer. Results: Fifty-one reports met our review criteria. Twenty of fifty-one (39%) reports are RCTs where the primary endpoint was cancer or a cancer-related biomarker, while the other reports evaluated reports where cancer or a cancer-related biomarker was a secondary endpoint. Thirteen of twenty (65%) primary reports evaluated isocaloric, and the remaining eight evaluated low-calorie diets. All but one of the primary and two secondary isocaloric diet reports evaluated the benefit of a low-fat diet (LFD), with the other three evaluating a Mediterranean diet (MedD). More LCD vs. isocaloric diet primary reports (71% vs. 38%) demonstrated cancer or cancer-related biomarker benefit; the difference in chance of benefit with secondary reports was 85% for LCD vs. 73% for isocaloric diets. Three of three MedD reports demonstrated benefit. Sixty-nine percent (20/29) of the secondary reports came from two large reports: the WHI diet modification trial (15 secondary reports) and the polyp prevention trial (5 secondary reports). Nineteen of twenty-two (86%) primary reports enrolled only women, and three enrolled both men and women. No study that met our criteria enrolled only men, comprising 1447 men in total vs. 62,054 women. Fifteen of twenty (75%) primary reports focus on healthy women or women with breast cancer. Adherence findings are discussed when provided. Conclusions: More long-term RCTs evaluating cancer and cancer-related biomarker endpoints are needed, especially for cancers at sites other than the breast.
1. Introduction
According to the American Cancer Society (ACS), no less than 18% of all cancers and about 16% of U.S. cancer deaths are linked to overweight, physical inactivity, alcohol consumption, and/or poor nutrition [1]. Diet recommendations to mitigate cancer risk have been published by multiple societies, including the ACS and the American Institute for Cancer Research (AICR). The ACS recommendations include maintaining a healthy weight and avoiding weight gain, being physically active, and choosing a diet that focuses on healthy foods (including a variety of vegetables, legumes, fruits, and whole grains) for cancer prevention and control [1]. AICR recommendations mostly mirror those of the ACS, though the AICR recommendations specifically address the breast cancer-reducing benefits of breastfeeding https://www.aicr.org/cancer-prevention/ (accessed on 5 April 2024). Thus, public health recommendations encourage the adoption of healthy dietary patterns and maintaining a healthy weight for cancer risk reduction. High vs. low adherence to diet and cancer risk reduction guidelines have consistently been associated with significant reductions in overall as well as endometrial, breast, and colorectal cancer risk and survival [2].
Many diets have been proposed for caloric restriction (CR) and/or a healthy lifestyle by altering macronutrient intake, though there is no consensus regarding whether one diet is better than another regarding weight loss or cancer risk reduction and control. “There have been no rigorous, long-term reports comparing contenders for best diet laurels using methodology that precludes bias and confounding, and for many reasons such reports are unlikely” [3]. Moreover, dietary adherence assessment is not consistently measured or consistently reported in published studies, hindering the ability to compare adherence between dietary approaches. Commonly used diets target weight loss and/or metabolic dysfunction since obesity and metabolic dysfunction are known to impact multiple common diseases such as cancer, diabetes mellitus, and cardiovascular disease (CVD) [4]. We conducted a systematic review of the literature to examine the effect of long-term (≥1 year) dietary intervention reports on cancer and cancer-related biomarkers.
2. Weight Loss and Altered Macronutrient Diets
Below we briefly address low-calorie and isocaloric diets, followed by the published studies that we identified in our systematic review. In our review of each study, we were disappointed to find the reporting of diet adherence was inconsistent from study to study, making comparisons between dietary approaches difficult. As it turns out, this lack of consistency is a recognized problem worldwide, and there has been a call by the Food and Agriculture Organization of the United Nations and the World Health Organization to provide more uniform dietary metrics [5].
2.1. Low Calorie Diet (LCD)
LCDs focus on weight loss. Caloric restriction is an important strategy to reduce overweight and obesity-associated metabolic and inflammatory perturbations associated with cancer risk [6]. LCDs focus on the restriction of calories each day, for some days per week or month (intermittent fasting [IF]), or for most hours each day (time-restricted eating, a form of IF). Diets that alter macronutrients for health benefits may also include caloric restriction, whether or not intentional. For those who have overweight or obesity, the goal is often to lose weight, resulting in a metabolically beneficial effect.
2.2. Low Fat Isocaloric Diet
Low-fat diets (LFDs) have been promoted for a healthy heart and metabolic improvement, which impacts diabetes and cancer risk. One of the largest of these reports focusing on cancer was the Women’s Health Initiative diet modification trial, which enrolled and randomized healthy postmenopausal women with a fat intake at baseline of at least 32% of their daily calories to a usual diet (control) or intervention (fat intake 20% of energy, with increased vegetables, fruits, and grains). Although the study was not designed to achieve weight loss, the investigators found a mean 3% (2.2 kg) lower body weight after one year in the intervention group (p < 0.001) [7]. After 8.5 years of study, breast cancer incidence and deaths were nonsignificantly lower in the intervention group. After 19.6 years of median follow-up, the reduction in deaths from breast cancer in the intervention group was significant (p = 0.02) [7].
2.3. Mediterranean (MedD) Isocaloric Diet
Started in 1947, the Seven Countries Study (SCS) found that a diet including olive oil, whole grains, fruits, vegetables, seafood, beans, and nuts that were widely consumed in Greece, southern Italy and Crete (MedD) resulted in the lowest rates of CVD and longest life expectancy [8]. Since the SCS, multiple reports have correlated increased adherence to a MedD with lower rates of CVD [8] and cancer [9], including colorectal (HR = 0.82), breast (HR = 0.92), gastric (HR = 0.72), and liver cancer (HR = 0.58).
The PREDIMED study was conducted to identify participant characteristics and study features that predict short and long-term adherence to a MedD [10] (Table 1). After 12 months of study, improved adherence to a MedD was greatest among men who were nondiabetic and among those with worse baseline dietary habits, whereas among women, improved compliance was greatest among those who were married and those with worse baseline dietary habits [11].

Table 1.
51 studies that met our search criteria.
3. What Predicts Long-Term Dietary Adherence?
This report focuses on reports lasting at least 1 year since dietary interventions take years to impact cancer risk. There are multiple impediments to dietary adherence, many of which are psychological, including depression, emotional eating, and poor exercise attitude and adherence [62]. Short-term success in diet adherence has been associated with openness to change, setting ambitious targets, and rewarding weight loss, but often, there is weight regain [63].
Many of the challenges with long-term adherence to lifestyle changes are similar to those related to short-term adherence, including challenges associated with adherence to lifestyle behaviors recommended for weight loss and healthy weight management (i.e., reductions in dietary intake and increases in physical activity). Given the limited number of reports evaluating long-term diet adherence, a question arises regarding whether short-term adherence provides longer-term benefits. Although the primary outcome was not a cancer endpoint, the findings of the POUNDS LOST study provide insight into the question of short- vs. long-term adherence. The study correlated diet self-monitoring and adherence during the first 6 months with changes in adiposity and CV risk factors at 24 months [64]. Early adherence was associated with changes in weight loss and waist circumference at 6 and 24 months but not with adiposity or with CV risk factors, suggesting that short-term adherence provides some, but perhaps not an optimal, long-term benefit. The Look AHEAD trial found that individuals with the greatest weight loss during the first 2 months of the intervention were more likely to achieve ≥5% weight loss through year 8, and weight loss < 3% at 2 months was associated with poor adherence to intervention meetings, fewer meal replacements, and less physical activity than those with higher initial weight loss [65]. Thus, early adherence was associated with longer-term weight loss maintenance in this study.
4. Systematic Review Methodology
The aim of this systematic review is to investigate how long-term diet reports are related to cancer risk and control. The review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 and the protocol registered in PROSPERO CRD42023438966 (PRISMA checklist available in Supplementary File S1). We selected manuscripts for review based on the criteria outlined in Supplementary Table S1, with results listed in Table 1. Briefly, we included only randomized clinical trials (RCTs) that enrolled adults in which the study evaluated the impact of a long-term dietary intervention (>1 year) on cancer or cancer-related biomarkers.
The search strategy was developed by two authors (ERS and TAC) in collaboration with a professional research librarian with expertise in systematic reviews (GB) using an iterative process and further peer reviewed. A combination of indexed terms (e.g., MeSH) and keywords were used to create a PubMed search strategy that was translated into other databases using appropriate syntax and controlled vocabulary. The following biomedical databases were searched: MEDLINE via PubMed, EMBASE (Elsevier), and Web of Science (Clarivate). The search was limited to English and human reports with no date restrictions. In addition, we performed a citation search of the references of included articles. The complete database search strategies can be found in Supplementary File S1.
For the study selection process, duplicates were removed using EndNote 21, and records were then imported into Covidence screening software (Web based version of Covidence 2024, Melbourne, Victoria, Australia: https://www.covidence.org) for the screening of records. Two authors (ERS and TAC) independently screened titles and abstracts as well as full-text records according to eligibility criteria. Any disagreements were resolved through consensus among the authors. The screening process for included articles is available in the PRISMA flow diagram (Figure 1). Data extraction from the included articles was performed by two authors (ERS and TAC) and extracted to a predefined spreadsheet.
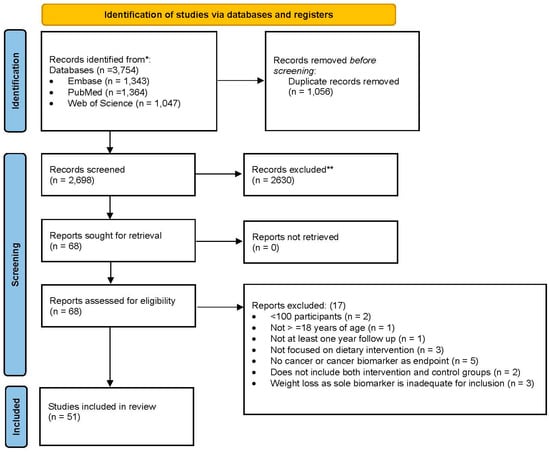
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. From [66].
The risk of bias (RoB) assessment was carried out using the NHLBI Study Quality Assessment Tools to assess the quality of RCTs) [9]. The RoB assessment was used to assess the overall quality of the evidence of the included reports and investigate whether potential heterogeneity could be explained by a difference in study risk of bias. Two reviewers (ERS and TAC) independently performed RoB and met to compare and resolve disagreements through discussion (available in Supplementary File S1).
5. Results
Given the time gap between diet intervention initiation and a measurable impact on cancer risk, many short-term reports comparing the benefits of various diets focused on non-cancer endpoints and were not included in the systematic review. The focus of our systematic review was on RCTs (i.e., reports with individuals randomized to at least two arms) lasting at least 1 year. We identified a limited number of RCTs; a total of 51 articles [7,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] were included that evaluated the impact of long-term lifestyle dietary changes on cancer or cancer-related biomarkers (Table 1). We also included RCTs with a non-cancer primary endpoint where participants were followed over time to assess cancer or cancer-related biomarkers as secondary endpoints to gain a better understanding of how studies involving lifestyle dietary changes impacted cancer or cancer-related biomarkers. Statistical analysis attempted to address bias linked to prior knowledge of the data, which is an important approach to minimize results bias [67].
Thirteen of twenty (65%) primary reports evaluated isocaloric and the remaining seven LCDs (Table 2).

Table 2.
Benefits of Isocaloric and Low-Calorie Diets on Cancer and Cancer Biomarkers.
All but one primary and two secondary isocaloric diet reports evaluated the benefit of an LFD, with the other three evaluating an MedD. Whereas more LCD primary reports vs. isocaloric diets (71% vs. 38%) demonstrated a cancer or cancer-related biomarker benefit, less difference in benefit was observed in secondary reports between LCD and isocaloric diets (86% vs. 73%, respectively). All three MedD reports demonstrated benefit. Sixty-nine percent (20/29) of the secondary reports came from two large reports: the WHI diet modification trial (15 secondary reports) and the polyp prevention trial (5 secondary reports). Sixteen of twenty (80%) studies focused on either healthy women or women with breast cancer (Table 3).

Table 3.
Population Focus of Primary Reports.
Of the primary reports, 80% enrolled only women and 20% both men and women, while no study that met our criteria enrolled only men. Primary reports in our review enrolled a total of 1447 men vs. 62,054 women.
6. Discussion
The findings from our systematic review highlight a number of points. First, most long-term RCTs, both primary and secondary reports, that met our criteria focused on healthy women or women with breast cancer. Because the reports overwhelmingly focus on women at risk for or with breast cancer, we have little to no RCT long-term trial information among men on the role of lifestyle changes on cancer, either risk or prognosis. This is especially important because men are more likely than women to develop cancer. Specifically, in the U.S., the rate of new cancer cases per 100,000 persons was 478.7 for men vs. 416.7 for women for the years 2017–2021 [68]. Moreover, the death rate from cancer is higher among U.S. men than women (173.2 vs. 126.4 per 100,000) [69]. Comparing sexes based on race/ethnicity, mortality from cancer was highest among non-Hispanic black men (208.3 per 100,000) and lowest among Asian/Pacific Islander women (82.6 per 100,000) [69]. Despite this, none of the primary reports that met our criteria focused on men, and the total number of men enrolled in primary reports in our review was only 2% vs. 98% for women. This disparity has been noted by many in the scientific community. Investigators are now addressing possible mechanism(s) for sex differences in cancer [70], risk of severe adverse events after treatment for cancer [71], outcomes in oncology clinical trials [72], and the difference in cancer incidence after receipt of an organ transplant [73]. This disparity has been noted by the National Institutes of Health (NIH). The NIH Sex and Gender Differences in Cancer Workshop Series was conducted in February through June 2024. There is also a trans-National Cancer Institute extramural awareness group that hosts webinars on sex differences in cancer.
For primary reports, it appeared that LCDs were more likely to have a beneficial impact on cancer risk vs. cancer biomarkers, whereas the difference was far less pronounced for reports where cancer was not the primary outcome of the study. This is not surprising, given their large size and long-term follow-up. Secondary data analyses, while providing the potential to answer important questions, have a risk of bias that likely exceeds the analysis of the primary outcome stated in an RCT [67]. This includes researcher bias, including analyzing data likely to demonstrate significant results, the tendency to focus on evidence that is consistent with one’s beliefs (confirmation bias), selective reporting only when the findings are significant. As a result, secondary data analysis results are often not able to be replicated [67]. Given this, it is our view that the secondary findings comparing the efficacy of LCD vs. isocaloric diets should be viewed with a level of caution, likely requiring confirmation before being viewed as true.
The identification of cancer risk reduction from weight loss is generally related to both the maximum amount of weight loss and the ability to maintain that weight loss. Dietary strategies have strived to obtain and sustain 7% to 10% total body weight loss. Lifestyle reports suggest that at least 5% sustained weight loss is required to detect a reduction in the risk of cancer or cancer-related biomarkers [74]. Our systemic analysis indicates that diet intervention reports (>1 year) may achieve a 5% weight loss, but rarely 10% or more. This raises the question as to the type of strategies needed to increase long-term weight loss and potentially greater cancer risk reduction.
There is convincing evidence that metabolic/bariatric surgery (MBS), which generally results in greater and more sustained weight loss than nonsurgical weight loss strategies, leads to a significant reduction in obesity-related cancers [75]. Ten years after surgery, diabetes remission was associated with a 60% reduction in cancer risk [76]. The prolonged weight reduction after MBS among the severely obese has also been shown to reduce death from cancer [76]. The reduction in death from cancer after a median follow-up of 20 years from surgery was 23% [77].
There are now pharmacologic weight loss strategies centered on GLP-1 receptor agonists, which can lead to a mean of 10% weight loss for at least 4 years [78] as long as the individual continues on the agent. Among the reasons to stop these medications are (1) cost and (2) unknown side effects with long-term use. Stopping the agent generally leads to weight regain. It remains unclear if these strategies will be as effective as surgery for cancer risk reduction. Reports in the future will require long-term follow-up.
A 2006 viewpoint from Pagano and Appelhans called for an end to the diet debates [79] to identify the ideal diet for disease prevention and weight loss. They justify their stance by reporting that “Numerous randomized trials comparing diets differing in macronutrient compositions … demonstrated differences in weight loss and metabolic risk factors that are small and inconsistent.… The only consistent finding among the trials is that adherence was most strongly associated with weight loss and improvement in disease-related outcomes”. Notably, the metabolic factors evaluated were generally weight loss and laboratory findings such as lipid profiles rather than cancer risk reduction endpoints. A counter view was proposed in 2013 that diets are not equivalent regarding metabolic outcomes, citing a meta-analysis comparing low-carbohydrate and low-fat diets, which demonstrated different changes in lipid profiles based on diet [80] and therefore cautioned against healthcare providers advising patients to choose whichever diet they are most likely to adhere to was premature until more is known about the safety and efficacy of different diets [81]. Reports comparing the benefits of various diets regarding cancer risk reduction endpoints are sorely needed. The reports should compare their results to what is currently the gold standard for adherence and most associated with cancer risk reduction: diet adherence after MBS.
Limitations of the study include the lack of RCTs with cancer or cancer as a primary endpoint, the lack of diversity in the RCTs that we identified, and the lack of studies that address the effects of the same dietary strategy among different ethnic age groups or health conditions. Below, we discuss possible strategies to mitigate these limitations.
7. Conclusions and Future Directions
Predictors of diet adherence include several factors, including female sex, older age, diabetic, depression, body weight, physical activity, nonsmoker, white ethnicity, higher socioeconomic status, and being married [10]. Sustained weight loss has been difficult to maintain for most, if not all, weight loss diets. Recent reports of GLP-1 agonists among obese individuals with or without T2DM lasting over 1 year demonstrate encouraging adherence rates, though the individuals have extensive support while on study and may not accurately reflect real-world findings. Social support programs appear to increase adherence to diets but may be impractical in the very long term due to cost. Few reports have evaluated long-term (>1 year) diet adherence. Among the reports that report adherence, biomarker changes may be useful in the assessment of adherence. For reports where the primary goal is a reduction in weight, weight loss may be a useful surrogate assessment for dietary adherence. On the other hand, for diets where the primary goal is metabolic improvement rather than weight loss, weight loss may not be an optimal surrogate adherence marker. Overweight and obese individuals who meet the criteria (BMI ≥ 27 with one or more weight-related comorbidities) may be directed toward pharmacologic therapy. Severely obese (a group likely to fail a long-term dietary strategy) individuals who meet the criteria (current criteria ≥ 35 BMI and considered for people with a BMI 30–34.9 with metabolic disease and fit for surgery; https://asmbs.org/news_releases/after-30-years-new-guidelines-for-weight-loss-surgery (accessed on 9 May 2024) could be encouraged to consider MBS.
Currently, findings from diet intervention reports have generally not assessed cancer risk reduction as their primary outcome because the effects of obesity on cancer risk take longer to observe than the effect on T2DM or CV risk. Few reports investigated whether there is a long-term benefit from short-term weight loss as it relates to cancer risk reduction if there is subsequent weight regain to or above baseline. Long-term adherence to changes in lifestyle, including changes in what an individual eats, has been difficult for many obese and overweight individuals, with most regaining all or most of the weight lost 12–24 months after starting the lifestyle changes. Time-restricted eating, which does not require a reduction in what or how much one eats, but rather only when, demonstrates early evidence of good diet adherence [82], though long-term studies are needed to validate this. However, reports lasting ≥1 year are needed to confirm this and assess the effects on weight loss. In conclusion, sustained dietary adherence is the most important factor in long-term improvements in health. Additional reports that address which strategies lead to long-term (≥1 year) diet adherence on weight loss, with or without the use of medication to sustain adherence, are needed. Precision nutrition approaches that assess the optimal diet for a given individual are needed. Diet intervention reports that include medications are needed to address adherence after medication is stopped, medication side effects, and impact on cancer risk. Moreover, there is a need for more RCTs that include men, studies with substantive representation of individuals from diverse ethnic and socioeconomic backgrounds, and studies that address the effects of the same dietary strategy among different ethnic groups, age groups, or health conditions. Finally, the RCTs that we reviewed rarely address hard cancer endpoints or mechanisms driving the associations. Future research should be designed to better determine if the relationships found with cancer risk are mere associations or causally related.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16193296/s1, File S1. Database Search Strategies. Table S1. Risk of Bias Table.
Author Contributions
All authors were involved in the design and writing of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors’ Disclaimer
This material should not be interpreted as representing the viewpoint of the U.S. Department of Health and Human Services, the National Institutes of Health, or the National Cancer Institute.
References
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K.; et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Garcia, D.O.; Harris, R.B.; Oren, E.; Roe, D.J.; Jacobs, E.T. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.L.; Meller, S. Can we say what diet is best for health? Annu. Rev. Public Health 2014, 35, 83–103. [Google Scholar] [CrossRef] [PubMed]
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- Machado, P.; McNaughton, S.A.; Livingstone, K.M.; Hadjikakou, M.; Russell, C.; Wingrove, K.; Sievert, K.; Dickie, S.; Woods, J.; Baker, P.; et al. Measuring Adherence to Sustainable Healthy Diets: A Scoping Review of Dietary Metrics. Adv. Nutr. 2023, 14, 147–160. [Google Scholar] [CrossRef]
- Ravussin, E.; Redman, L.M.; Rochon, J.; Das, S.K.; Fontana, L.; Kraus, W.E.; Romashkan, S.; Williamson, D.A.; Meydani, S.N.; Villareal, D.T.; et al. A 2-Year Randomized Controlled Trial of Human Caloric Restriction: Feasibility and Effects on Predictors of Health Span and Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Pan, K.; Neuhouser, M.L.; Manson, J.E.; Thomson, C.A.; Mossavar-Rahmani, Y.; Lane, D.S.; Johnson, K.C.; et al. Dietary Modification and Breast Cancer Mortality: Long-Term Follow-Up of the Women’s Health Initiative Randomized Trial. J. Clin. Oncol. 2020, 38, 1419–1428. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E. The role of the Mediterranean diet on weight loss and obesity-related diseases. Rev. Endocr. Metab. Disord. 2020, 21, 315–327. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Galbete, C.; Hoffmann, G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients 2017, 9, 1063. [Google Scholar] [CrossRef]
- Downer, M.K.; Gea, A.; Stampfer, M.; Sanchez-Tainta, A.; Corella, D.; Salas-Salvado, J.; Ros, E.; Estruch, R.; Fito, M.; Gomez-Gracia, E.; et al. Predictors of short- and long-term adherence with a Mediterranean-type diet intervention: The PREDIMED randomized trial. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 67. [Google Scholar] [CrossRef]
- Zazpe, I.; Estruch, R.; Toledo, E.; Sanchez-Tainta, A.; Corella, D.; Bullo, M.; Fiol, M.; Iglesias, P.; Gomez-Gracia, E.; Aros, F.; et al. Predictors of adherence to a Mediterranean-type diet in the PREDIMED trial. Eur. J. Nutr. 2010, 49, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Beresford, S.A.; Johnson, K.C.; Ritenbaugh, C.; Lasser, N.L.; Snetselaar, L.G.; Black, H.R.; Anderson, G.L.; Assaf, A.R.; Bassford, T.; Bowen, D.; et al. Low-fat dietary pattern and risk of colorectal cancer: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Black, H.S.; Thornby, J.I.; Wolf, J.E., Jr.; Goldberg, L.H.; Herd, J.A.; Rosen, T.; Bruce, S.; Tschen, J.A.; Scott, L.W.; Jaax, S.; et al. Evidence that a low-fat diet reduces the occurrence of non-melanoma skin cancer. Int. J. Cancer 1995, 62, 165–169. [Google Scholar] [CrossRef]
- Black, H.S. Influence of dietary factors on actinically-induced skin cancer. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1998, 422, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; de Lange, T.; Tonstad, S.; Berstad, P. Exploring the effect of a lifestyle intervention on cancer risk: 43-year follow-up of the randomized Oslo diet and antismoking study. J. Intern. Med. 2018, 284, 282–291. [Google Scholar] [CrossRef]
- Boyd, N.F.; Cousins, M.; Beaton, M.; Fishell, E.; Wright, B.; Fish, E.; Kriukov, V.; Lockwood, G.; Tritchler, D.; Hanna, W.; et al. Clinical trial of low-fat, high-carbohydrate diet in subjects with mammographic dysplasia: Report of early outcomes. JNCI J. Natl. Cancer Inst. 1988, 80, 1244–1248. [Google Scholar] [CrossRef]
- Boyd, N.F.; Lockwood, G.A.; Greenberg, C.V.; Martin, L.J.; Tritchler, D.L. Effects of a low-fat high-carbohydrate diet on plasma sex hormones in premenopausal women: Results from a randomized controlled trial. Canadian Diet and Breast Cancer Prevention Study Group. Br. J. Cancer 1997, 76, 127–135. [Google Scholar] [CrossRef]
- Brown, J.C.; Sturgeon, K.; Sarwer, D.B.; Troxel, A.B.; DeMichele, A.M.; Denlinger, C.S.; Schmitz, K.H. The effects of exercise and diet on sex steroids in breast cancer survivors. Endocr. Relat. Cancer 2022, 29, 485–493. [Google Scholar] [CrossRef]
- Bruno, E.; Krogh, V.; Gargano, G.; Grioni, S.; Bellegotti, M.; Venturelli, E.; Panico, S.; Santucci de Magistris, M.; Bonanni, B.; Zagallo, E.; et al. Adherence to Dietary Recommendations after One Year of Intervention in Breast Cancer Women: The DIANA-5 Trial. Nutrients 2021, 13, 2990. [Google Scholar] [CrossRef]
- Byrd, D.A.; Gomez, M.; Hogue, S.; Murphy, G.; Sampson, J.N.; Vogtmann, E.; Albert, P.; Freedman, N.D.; Sinha, R.; Loftfield, E. Circulating Bile Acids and Adenoma Recurrence in the Context of Adherence to a High-Fiber, High-Fruit and Vegetable, and Low-Fat Dietary Intervention. Clin. Transl. Gastroenterol. 2022, 13, e00533. [Google Scholar] [CrossRef]
- Caan, B.J.; Aragaki, A.; Thomson, C.A.; Stefanick, M.L.; Chlebowski, R.; Hubbell, F.A.; Tinker, L.; Vitolins, M.; Rajkovic, A.; Bueche, M.; et al. Vasomotor symptoms, adoption of a low-fat dietary pattern, and risk of invasive breast cancer: A secondary analysis of the Women’s Health Initiative randomized controlled dietary modification trial. J. Clin. Oncol. 2009, 27, 4500–4507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Campbell, K.L.; Foster-Schubert, K.E.; Alfano, C.M.; Wang, C.C.; Wang, C.Y.; Duggan, C.R.; Mason, C.; Imayama, I.; Kong, A.; Xiao, L.; et al. Reduced-calorie dietary weight loss, exercise, and sex hormones in postmenopausal women: Randomized controlled trial. J. Clin. Oncol. 2012, 30, 2314–2326. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Blackburn, G.L.; Thomson, C.A.; Nixon, D.W.; Shapiro, A.; Hoy, M.K.; Goodman, M.T.; Giuliano, A.E.; Karanja, N.; McAndrew, P.; et al. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women’s Intervention Nutrition Study. J. Natl. Cancer Inst. 2006, 98, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Thomson, C.A.; Manson, J.E.; Simon, M.S.; Howard, B.V.; Rohan, T.E.; Snetselar, L.; Lane, D.; et al. Low-Fat Dietary Pattern and Breast Cancer Mortality in the Women’s Health Initiative Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Chlebowski, R.T.; Anderson, G.L.; Manson, J.E.; Prentice, R.L.; Aragaki, A.K.; Snetselaar, L.; Beresford, S.A.A.; Kuller, L.H.; Johnson, K.; Lane, D.; et al. Low-Fat Dietary Pattern and Cancer Mortality in the Women’s Health Initiative (WHI) Randomized Controlled Trial. JNCI Cancer Spectr. 2018, 2, pky065. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Aragaki, A.K.; Anderson, G.L.; Simon, M.S.; Manson, J.E.; Neuhouser, M.L.; Pan, K.; Stefanic, M.L.; Rohan, T.E.; Lane, D.; et al. Association of Low-Fat Dietary Pattern with Breast Cancer Overall Survival: A Secondary Analysis of the Women’s Health Initiative Randomized Clinical Trial. JAMA Oncol. 2018, 4, e181212. [Google Scholar] [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J.-L.; Monjaud, I.; Boucher, P.; Mamelle, N. Mediterranean Dietary Pattern in a Randomized Trial: Prolonged Survival and Possible Reduced Cancer Rate. Arch. Intern. Med. 1998, 158, 1181–1187. [Google Scholar] [CrossRef]
- Duggan, C.; de Dieu Tapsoba, J.; Mason, C.; Imayama, I.; Korde, L.; Wang, C.Y.; McTiernan, A. Effect of Vitamin D3 Supplementation in Combination with Weight Loss on Inflammatory Biomarkers in Postmenopausal Women: A Randomized Controlled Trial. Cancer Prev. Res. 2015, 8, 628–635. [Google Scholar] [CrossRef]
- Duggan, C.; Tapsoba Jde, D.; Wang, C.Y.; McTiernan, A. Dietary Weight Loss and Exercise Effects on Serum Biomarkers of Angiogenesis in Overweight Postmenopausal Women: A Randomized Controlled Trial. Cancer Res. 2016, 76, 4226–4235. [Google Scholar] [CrossRef]
- Duggan, C.; Tapsoba, J.D.; Shivappa, N.; Harris, H.R.; Hébert, J.R.; Wang, C.Y.; McTiernan, A. Changes in Dietary Inflammatory Index Patterns with Weight Loss in Women: A Randomized Controlled Trial. Cancer Prev. Res. 2021, 14, 85–94. [Google Scholar] [CrossRef]
- Emond, J.A.; Patterson, R.E.; Natarajan, L.; Laughlin, G.A.; Gold, E.B.; Pierce, J.P. Sex hormone concentrations and the risk of breast cancer recurrence in postmenopausal women without hot flashes. Cancer Epidemiol. Biomark. Prev. 2011, 20, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Flood, A.; Mai, V.; Pfeiffer, R.; Kahle, L.; Remaley, A.T.; Rosen, C.J.; Lanza, E.; Schatzkin, A. The effects of a high-fruit and -vegetable, high-fiber, low-fat dietary intervention on serum concentrations of insulin, glucose, IGF-I and IGFBP-3. Eur. J. Clin. Nutr. 2008, 62, 186–196. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fontana, L.; Villareal, D.T.; Das, S.K.; Smith, S.R.; Meydani, S.N.; Pittas, A.G.; Klein, S.; Bhapkar, M.; Rochon, J.; Ravussin, E.; et al. Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: A randomized clinical trial. Aging Cell 2016, 15, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Gamba, C.S.; Stefanick, M.L.; Shikany, J.M.; Larson, J.; Linos, E.; Sims, S.T.; Marshall, J.; Van Horn, L.; Zeitouni, N.; Tang, J.Y. Low-fat diet and skin cancer risk: The women’s health initiative randomized controlled dietary modification trial. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Gann, P.H.; Chatterton, R.T.; Gapstur, S.M.; Liu, K.; Garside, D.; Giovanazzi, S.; Thedford, K.; Van Horn, L. The effects of a low-fat/high-fiber diet on sex hormone levels and menstrual cycling in premenopausal women: A 12-month randomized trial (the diet and hormone study). Cancer 2003, 98, 1870–1879. [Google Scholar] [CrossRef]
- Habermann, N.; Makar, K.W.; Abbenhardt, C.; Xiao, L.; Wang, C.Y.; Utsugi, H.K.; Alfano, C.M.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; et al. No effect of caloric restriction or exercise on radiation repair capacity. Med. Sci. Sports Exerc. 2015, 47, 896–904. [Google Scholar] [CrossRef]
- Imayama, I.; Ulrich, C.M.; Alfano, C.M.; Wang, C.; Xiao, L.; Wener, M.H.; Campbell, K.L.; Duggan, C.; Foster-Schubert, K.E.; Kong, A.; et al. Effects of a caloric restriction weight loss diet and exercise on inflammatory biomarkers in overweight/obese postmenopausal women: A randomized controlled trial. Cancer Res. 2012, 72, 2314–2326. [Google Scholar] [CrossRef]
- Jiao, L.; Chen, L.; White, D.L.; Tinker, L.; Chlebowski, R.T.; Van Horn, L.V.; Richardson, P.; Lane, D.; Sangi-Haghpeykar, H.; El-Serag, H.B. Low-fat Dietary Pattern and Pancreatic Cancer Risk in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 49–56. [Google Scholar] [CrossRef]
- Lanza, E.; Yu, B.; Murphy, G.; Albert, P.S.; Caan, B.; Marshall, J.R.; Lance, P.; Paskett, E.D.; Weissfeld, J.; Slattery, M.; et al. The polyp prevention trial continued follow-up study: No effect of a low-fat, high-fiber, high-fruit, and -vegetable diet on adenoma recurrence eight years after randomization. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1745–1752. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, L.L.; Li, J.W.; Jin, Y.S.; An, R.H. A Randomized Study on the Effect of Metformin Combined with Intensive-Exercise Diet Therapy on Glucose and Lipid Metabolism and Islet Function in Patients with Renal Cell Carcinoma and Diabetes. Dis. Markers 2022, 2022, 7383745. [Google Scholar] [CrossRef]
- Martin, L.J.; Li, Q.; Melnichouk, O.; Greenberg, C.; Minkin, S.; Hislop, G.; Boyd, N.F. A randomized trial of dietary intervention for breast cancer prevention. Cancer Res. 2011, 71, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Masala, G.; Bendinelli, B.; Della Bella, C.; Assedi, M.; Tapinassi, S.; Ermini, I.; Occhini, D.; Castaldo, M.; Saieva, C.; Caini, S.; et al. Inflammatory marker changes in a 24-month dietary and physical activity randomised intervention trial in postmenopausal women. Sci. Rep. 2020, 10, 21845. [Google Scholar] [CrossRef] [PubMed]
- Mason, C.; Xiao, L.; Duggan, C.; Imayama, I.; Foster-Schubert, K.E.; Kong, A.; Campbell, K.L.; Wang, C.Y.; Alfano, C.M.; Blackburn, G.L.; et al. Effects of dietary weight loss and exercise on insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in postmenopausal women: A randomized controlled trial. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1457–1463. [Google Scholar] [CrossRef]
- McKeown-Eyssen, G.E.; Bright-See, E.; Bruce, W.R.; Jazmaji, V.; Cohen, L.B.; Pappas, S.C.; Saibil, F.G. A randomized trial of a low fat high fibre diet in the recurrence of colorectal polyps. Toronto Polyp Prevention Group. J. Clin. Epidemiol. 1994, 47, 525–536. [Google Scholar] [CrossRef]
- Pan, K.; Luo, J.; Aragaki, A.K.; Chlebowski, R.T. Weight loss, diet composition and breast cancer incidence and outcome in postmenopausal women. Oncotarget 2019, 10, 3088–3092. [Google Scholar] [CrossRef]
- Pan, K.; Aragaki, A.K.; Neuhouser, M.L.; Simon, M.S.; Luo, J.; Caan, B.; Snetselaar, L.; Mortimer, J.E.; Manson, J.E.; Kroenke, C.; et al. Low-fat dietary pattern and breast cancer mortality by metabolic syndrome components: A secondary analysis of the Women’s Health Initiative (WHI) randomised trial. Br. J. Cancer 2021, 125, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Peila, R.; Chlebowski, R.; Manson, J.E.; Crane, T.E.; Lane, D.S.; Saquib, N.; Shadyab, A.H.; Tabung, F.K.; Barac, A.; Zhang, Z.; et al. Low-Fat Dietary Modification and Risk of Ductal Carcinoma In Situ of the Breast in the Women’s Health Initiative Dietary Modification Trial. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1753–1756. [Google Scholar] [CrossRef]
- Pierce, J.P.; Natarajan, L.; Caan, B.J.; Parker, B.A.; Greenberg, E.R.; Flatt, S.W.; Rock, C.L.; Kealey, S.; Al-Delaimy, W.K.; Bardwell, W.A.; et al. Influence of a diet very high in vegetables, fruit, and fiber and low in fat on prognosis following treatment for breast cancer: The Women’s Healthy Eating and Living (WHEL) randomized trial. JAMA 2007, 298, 289–298. [Google Scholar] [CrossRef]
- Prentice, R.L.; Caan, B.; Chlebowski, R.T.; Patterson, R.; Kuller, L.H.; Ockene, J.K.; Margolis, K.L.; Limacher, M.C.; Manson, J.E.; Parker, L.M.; et al. Low-Fat Dietary Pattern and Risk of Invasive Breast CancerThe Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006, 295, 629–642. [Google Scholar] [CrossRef]
- Prentice, R.L.; Thomson, C.A.; Caan, B.; Hubbell, F.A.; Anderson, G.L.; Beresford, S.A.; Pettinger, M.; Lane, D.S.; Lessin, L.; Yasmeen, S.; et al. Low-fat dietary pattern and cancer incidence in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2007, 99, 1534–1543. [Google Scholar] [CrossRef]
- Prentice, R.L.; Aragaki, A.K.; Howard, B.V.; Chlebowski, R.T.; Thomson, C.A.; Van Horn, L.; Tinker, L.F.; Manson, J.E.; Anderson, G.L.; Kuller, L.E.; et al. Low-Fat Dietary Pattern among Postmenopausal Women Influences Long-Term Cancer, Cardiovascular Disease, and Diabetes Outcomes. J. Nutr. 2019, 149, 1565–1574. [Google Scholar] [CrossRef]
- Rana, B.K.; Flatt, S.W.; Health, D.D.; Pakiz, B.; Quintana, E.L.; Natarajan, L.; Rock, C.L. The IL-6 Gene Promoter SNP and Plasma IL-6 in Response to Diet Intervention. Nutrients 2017, 9, 552. [Google Scholar] [CrossRef] [PubMed]
- Reeves, M.M.; Terranova, C.O.; Winkler, E.A.H.; McCarthy, N.; Hickman, I.J.; Ware, R.S.; Lawler, S.P.; Eakin, E.G.; Demark-Wahnefried, W. Effect of a Remotely Delivered Weight Loss Intervention in Early-Stage Breast Cancer: Randomized Controlled Trial. Nutrients 2021, 13, 4091. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Thomson, C.A.; Stefanick, M.L.; Newman, V.A.; Jones, L.A.; Natarajan, L.; Ritenbaugh, C.; Hollenbach, K.A.; Pierce, J.P.; et al. Effects of a high-fiber, low-fat diet intervention on serum concentrations of reproductive steroid hormones in women with a history of breast cancer. J. Clin. Oncol. 2004, 22, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L.; Flatt, S.W.; Pakiz, B.; Quintana, E.L.; Heath, D.D.; Rana, B.K.; Natarajan, L. Effects of diet composition on weight loss, metabolic factors and biomarkers in a 1-year weight loss intervention in obese women examined by baseline insulin resistance status. Metabolism 2016, 65, 1605–1613. [Google Scholar] [CrossRef]
- Rohan, T.E.; Negassa, A.; Caan, B.; Chlebowski, R.T.; Curb, J.D.; Ginsberg, M.; Lane, D.S.; Neuhouser, M.L.; Shikany, J.M.; Wassertheil-Smoller, S.; et al. Low-fat dietary pattern and risk of benign proliferative breast disease: A randomized, controlled dietary modification trial. Cancer Prev. Res. 2008, 1, 275–284. [Google Scholar] [CrossRef]
- Sansbury, L.B.; Wanke, K.; Albert, P.S.; Kahle, L.; Schatzkin, A.; Lanza, E. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am. J. Epidemiol. 2009, 170, 576–584. [Google Scholar] [CrossRef]
- Schatzkin, A.; Lanza, E.; Corle, D.; Lance, P.; Iber, F.; Caan, B.; Shike, M.; Weissfeld, J.; Burt, R.; Cooper, M.R.; et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N. Engl. J. Med. 2000, 342, 1149–1155. [Google Scholar] [CrossRef]
- Thomson, C.A.; Van Horn, L.; Caan, B.J.; Aragaki, A.K.; Chlebowski, R.T.; Manson, J.E.; Rohan, T.E.; Tinker, L.F.; Kuller, L.H.; Hou, L.; et al. Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative dietary modification trial. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2924–2935. [Google Scholar] [CrossRef]
- Toledo, E.; Salas-Salvadó, J.; Donat-Vargas, C.; Buil-Cosiales, P.; Estruch, R.; Ros, E.; Corella, D.; Fitó, M.; Hu, F.B.; Arós, F.; et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern. Med. 2015, 175, 1752–1760. [Google Scholar] [CrossRef]
- Vitale, S.; Palumbo, E.; Polesel, J.; Hebert, J.R.; Shivappa, N.; Montagnese, C.; Porciello, G.; Calabrese, I.; Luongo, A.; Prete, M.; et al. One-year nutrition counselling in the context of a Mediterranean diet reduced the dietary inflammatory index in women with breast cancer: A role for the dietary glycemic index. Food Funct. 2023, 14, 1560–1572. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Buffington, C. Early weight loss outcomes from a newly established hospital-affiliated specialized obesity care delivery model in Central Florida. Int. J. Obes. 2019, 43, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.A.; Serbetci, D.; O’Brien, K.; Alexi, N. The Validated Features of Psychological Interventions for Weight Loss: An Integration. Behav. Med. 2022, 48, 147–161. [Google Scholar] [CrossRef]
- Williamson, D.A.; Anton, S.D.; Han, H.; Champagne, C.M.; Allen, R.; Leblanc, E.; Ryan, D.H.; Rood, J.; McManus, K.; Laranjo, N.; et al. Early behavioral adherence predicts short and long-term weight loss in the POUNDS LOST study. J. Behav. Med. 2010, 33, 305–314. [Google Scholar] [CrossRef]
- Unick, J.L.; Neiberg, R.H.; Hogan, P.E.; Cheskin, L.J.; Dutton, G.R.; Jeffery, R.; Nelson, J.A.; Pi-Sunyer, X.; West, D.S.; Wing, R.R.; et al. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity 2015, 23, 1353–1356. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement:an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, J.R.; Pingault, J.B.; Schoeler, T.; Sallis, H.M.; Munafo, M.R. Protecting against researcher bias in secondary data analysis: Challenges and potential solutions. Eur. J. Epidemiol. 2022, 37, 1–10. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER: Cancer Stat Facts: Cancer of Any Site. Available online: https://seer.cancer.gov/statfacts/html/all.html (accessed on 19 September 2024).
- National Cancer Institute. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics#:~:text=The%20cancer%20mortality%20rate%20is,on%202017%E2%80%932019%20data). (accessed on 19 September 2024).
- Rubin, J.B.; Lagas, J.S.; Broestl, L.; Sponagel, J.; Rockwell, N.; Rhee, G.; Rosen, S.F.; Chen, S.; Klein, R.S.; Imoukhuede, P.; et al. Sex differences in cancer mechanisms. Biol. Sex Differ. 2020, 11, 17. [Google Scholar] [CrossRef]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; LeBlanc, M.; Minasian, L.M.; Gotay, C.C.; Henry, N.L.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex Differences in Risk of Severe Adverse Events in Patients Receiving Immunotherapy, Targeted Therapy, or Chemotherapy in Cancer Clinical Trials. J. Clin. Oncol. 2022, 40, 1474–1486. [Google Scholar] [CrossRef]
- Kammula, A.V.; Schaffer, A.A.; Rajagopal, P.S.; Kurzrock, R.; Ruppin, E. Outcome differences by sex in oncology clinical trials. Nat. Commun. 2024, 15, 2608. [Google Scholar] [CrossRef]
- Jackson, S.S.; Pfeiffer, R.M.; Hsieh, M.C.; Li, J.; Madeleine, M.M.; Pawlish, K.S.; Zeng, Y.; Yu, K.J.; Engels, E.A. Sex differences in cancer incidence among solid organ transplant recipients. J. Natl. Cancer Inst. 2024, 116, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Hendryx, M.; Manson, J.E.; Figueiredo, J.C.; LeBlanc, E.S.; Barrington, W.; Rohan, T.E.; Howard, B.V.; Reding, K.; Ho, G.Y.; et al. Intentional Weight Loss and Obesity-Related Cancer Risk. JNCI Cancer Spectr. 2019, 3, pkz054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Luo, Y.; Dai, H.; Deng, Z. Effects of Bariatric Surgery on Cancer Risk: Evidence from Meta-analysis. Obes. Surg. 2020, 30, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Sjoholm, K.; Carlsson, L.M.S.; Svensson, P.A.; Andersson-Assarsson, J.C.; Kristensson, F.; Jacobson, P.; Peltonen, M.; Taube, M. Association of Bariatric Surgery with Cancer Incidence in Patients with Obesity and Diabetes: Long-term Results from the Swedish Obese Subjects Study. Diabetes Care 2022, 45, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.M.S.; Sjoholm, K.; Jacobson, P.; Andersson-Assarsson, J.C.; Svensson, P.A.; Taube, M.; Carlsson, B.; Peltonen, M. Life Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. N. Engl. J. Med. 2020, 383, 1535–1543. [Google Scholar] [CrossRef]
- Ryan, D.H.; Lingvay, I.; Deanfield, J.; Kahn, S.E.; Barros, E.; Burguera, B.; Colhoun, H.M.; Cercato, C.; Dicker, D.; Horn, D.B.; et al. Long-term weight loss effects of semaglutide in obesity without diabetes in the SELECT trial. Nat. Med. 2024, 30, 2049–2057. [Google Scholar] [CrossRef]
- Pagano, S.L.; Appelhans, B.M. A call for an end to the diet debates. JAMA 2013, 310, 687–688. [Google Scholar]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; McVay, M.A.; Brinkworth, G.D. Adherence to diets for weight loss. JAMA 2013, 310, 2676. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.J.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).