Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Population
2.2. Statistical Analysis
3. Results
3.1. Treatment Characteristics and Responses
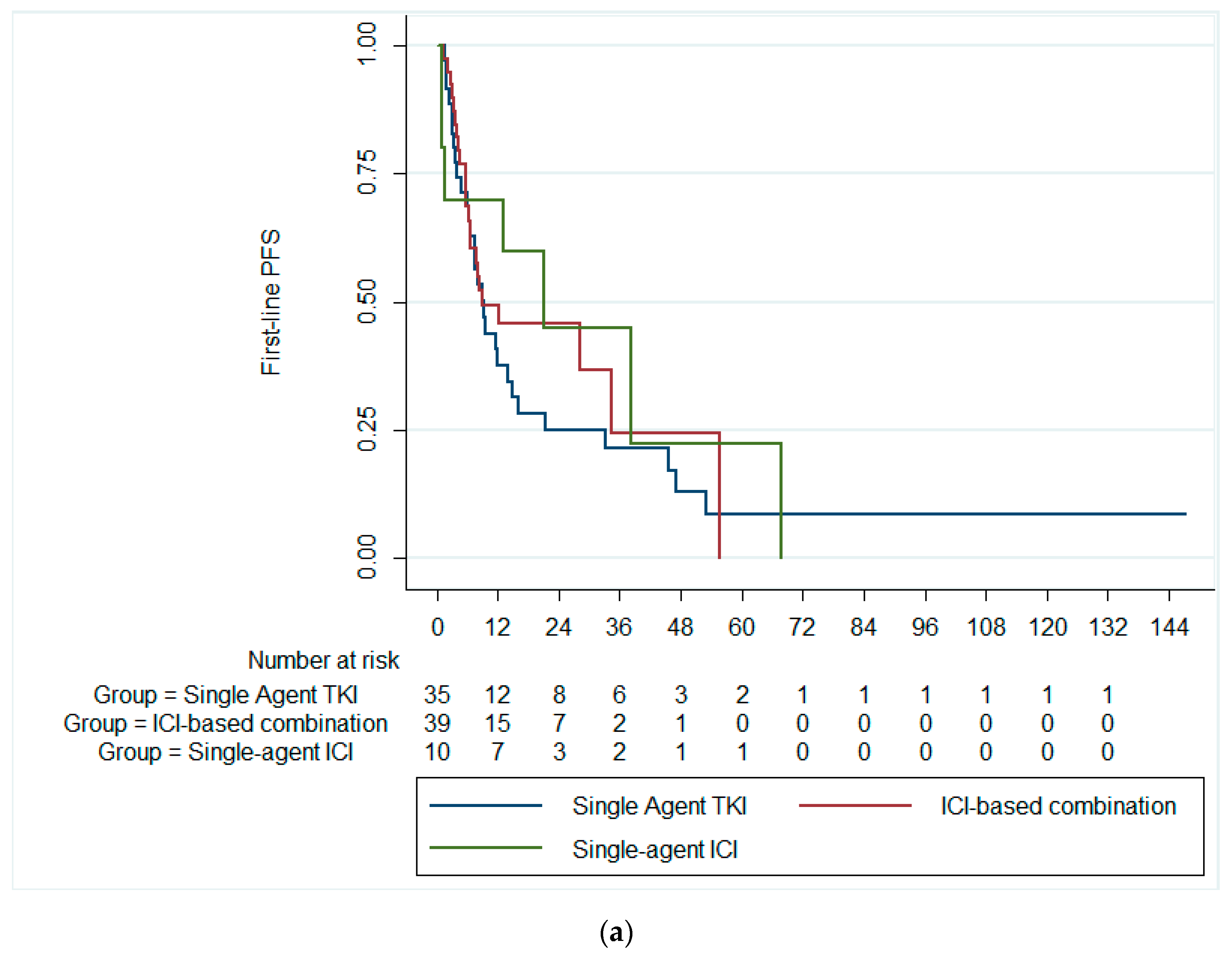
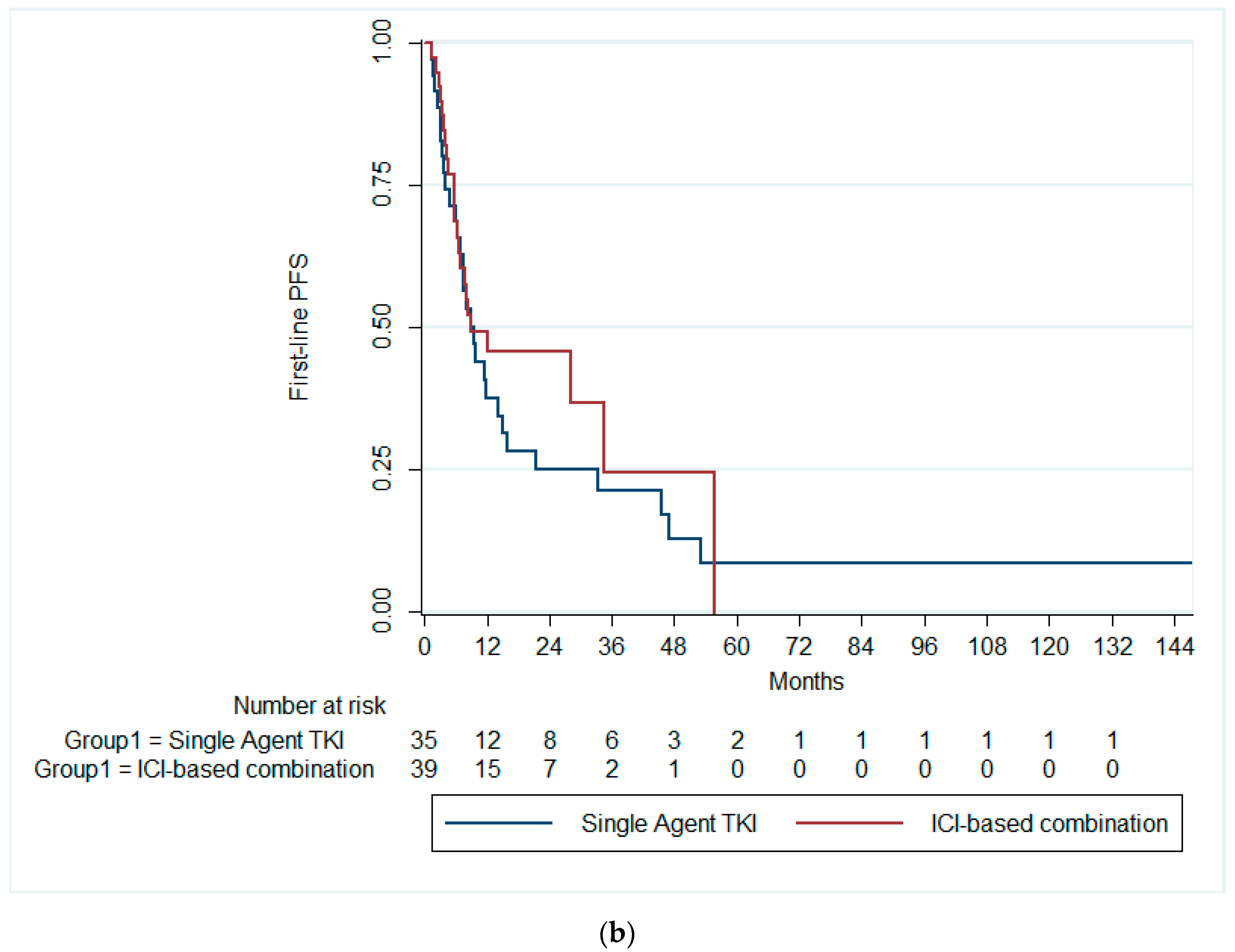
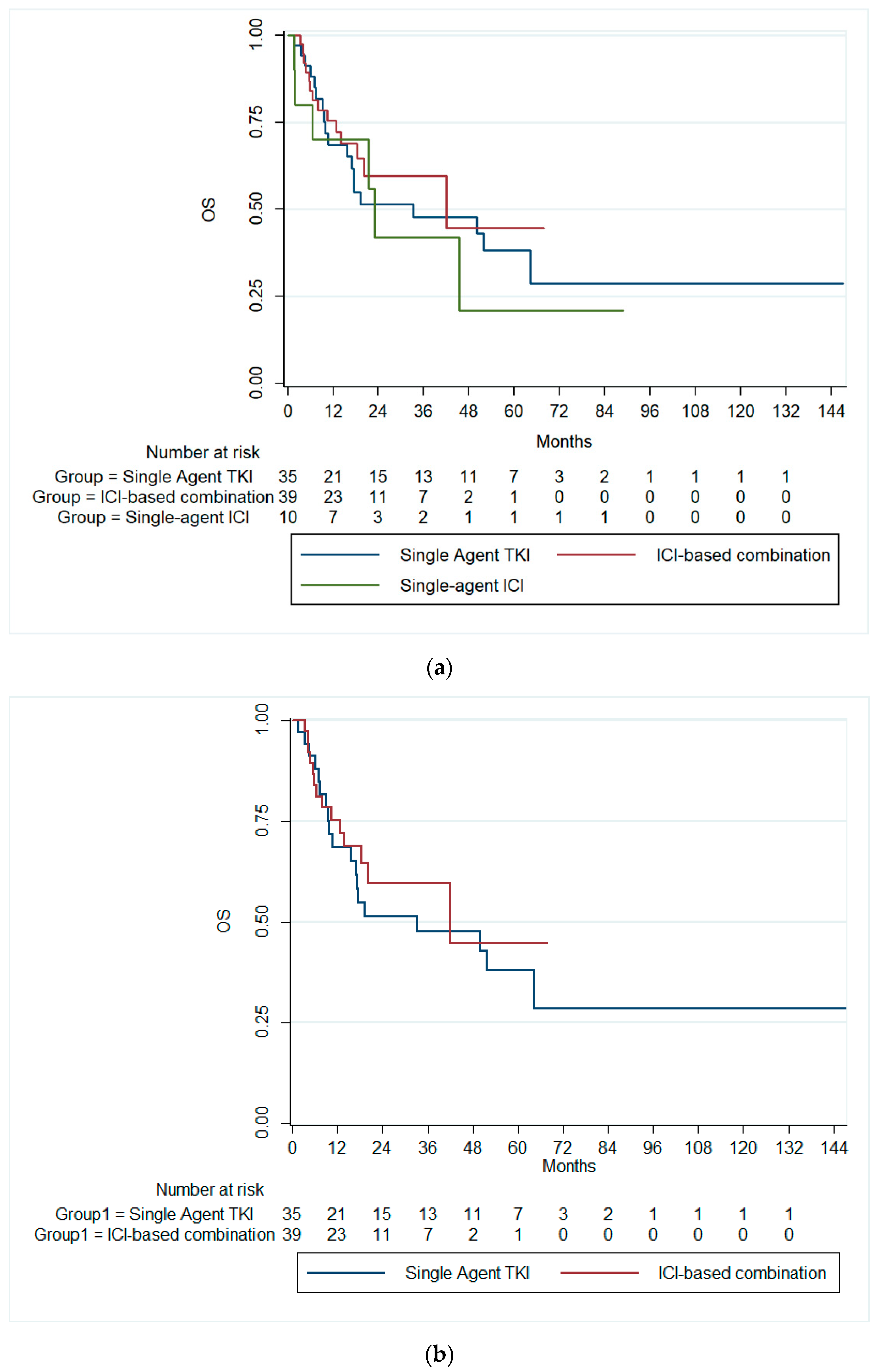
3.2. Survival Outcomes
3.3. Predictors of Survival Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Padala, S.A.; Barsouk, A.; Thandra, K.C.; Saginala, K.; Mohammed, A.; Vakiti, A.; Rawla, P.; Barsouk, A. Epidemiology of renal cell carcinoma. World J. Oncol. 2020, 11, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Chen, Z.; Zhong, Q.; Chen, Y.; Shangguan, W.; Xie, W. Efficacy and safety of immunological checkpoint inhibitors combined with anti-angiogenic drugs in first-line treatment of metastatic renal cell carcinoma: A systematic review and meta-analysis. Transl. Androl. Urol. 2021, 10, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; Feuer, E.J. SEER Cancer Statistics Review, 1975–2016. Based November. 2018 SEER Data Submission. Available online: https://seer.cancer.gov/archive/csr/1975_2016/index.html (accessed on 20 September 2024).
- Vaishampayan, U.; Vankayala, H.; Vigneau, F.D.; Quarshie, W.; Dickow, B.; Chalasani, S.; Schwartz, K. The effect of targeted therapy on overall survival in advanced renal cancer: A study of the national surveillance epidemiology and end results registry database. Clin. Genitourin. Cancer 2014, 12, 124–129. [Google Scholar] [CrossRef]
- Kane, R.C.; Farrell, A.T.; Saber, H.; Tang, S.; Williams, G.; Jee, J.M.; Liang, C.; Booth, B.; Chidambaram, N.; Morse, D.; et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin. Cancer Res. An Off. J. Am. Assoc. Cancer Res. 2006, 12, 7271–7278. [Google Scholar] [CrossRef]
- Ito, K. Recent advances in the systemic treatment of metastatic non-clear cell renal cell carcinomas. Int. J. Urol. 2019, 26, 868–877. [Google Scholar] [CrossRef]
- Iacovelli, R.; Sternberg, C.; Porta, C.; Verzoni, E.; Braud, F.; Escudier, B.; Procopio, G. Inhibition of the VEGF/VEGFR Pathway Improves Survival in Advanced Kidney Cancer: A Systematic Review and Meta-Analysis. Curr. Drug Targets 2015, 16, 164–170. [Google Scholar] [CrossRef]
- Raman, R.; Vaena, D. Immunotherapy in Metastatic Renal Cell Carcinoma: A Comprehensive Review. BioMed Res. Int. 2015, 2015, 367354. [Google Scholar] [CrossRef]
- Rijnders, M.; de Wit, R.; Boormans, J.L.; Lolkema, M.P.J.; van der Veldt, A.A.M. Systematic Review of Immune Checkpoint Inhibition in Urological Cancers. Eur. Urol. 2017, 72, 411–423. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Garje, R.; An, J.; Greco, A.; Vaddepally, R.K.; Zakharia, Y. The future of immunotherapy-based combination therapy in metastatic renal cell carcinoma. Cancers 2020, 12, 143. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef]
- Kathuria-Prakash, N.; Drolen, C.; Hannigan, C.A.; Drakaki, A. Immunotherapy and metastatic renal cell carcinoma: A review of new treatment approaches. Life 2022, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.F.; Lee, J.L.; Bjarnason, G.A.; Larkin, J.M.G.; Gafanov, R.A.; Kochenderfer, M.D.; Jensen, N.V.; Donskov, F.; Malik, J.; Poprach, A.; et al. Open-Label, Single-Arm Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients with Advanced Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1020–1028. [Google Scholar] [CrossRef]
- Nazha, B.; Mishra, M.; Pentz, R.; Owonikoko, T.K. Enrollment of Racial Minorities in Clinical Trials: Old Problem Assumes New Urgency in the Age of Immunotherapy. Am. Soc. Clin. Oncol. Educ. B. 2019, 39, 3–10. [Google Scholar] [CrossRef]
- Olsen, T.A.; Martini, D.J.; Goyal, S.; Liu, Y.; Evans, S.T.; Magod, B.; Brown, J.T.; Yantorni, L.; Russler, G.A.; Caulfield, S.; et al. Racial Differences in Clinical Outcomes for Metastatic Renal Cell Carcinoma Patients Treated with Immune-Checkpoint Blockade. Front. Oncol. 2021, 11, 701345. [Google Scholar] [CrossRef]
- Chow, R.D.; Long, J.B.; Hassan, S.; Wheeler, S.B.; Spees, L.P.; Leapman, M.S.; Hurwitz, M.E.; McManus, H.D.; Gross, C.P.; Dinan, M.A. Disparities in immune and targeted therapy utilization for older US patients with metastatic renal cell carcinoma. JNCI Cancer Spectr. 2023, 7, pkad036. [Google Scholar] [CrossRef]
- Bazarbashi, S.; Al Eid, H.; Minguet, J. Cancer incidence in Saudi Arabia: 2012 data from the Saudi cancer registry. Asian Pac. J. Cancer Prev. 2017, 18, 2437–2444. [Google Scholar] [CrossRef]
- Mahasin, S.Z.; Aloudah, N.; Al-Surimi, K.; Alkhateeb, S. Epidemiology profile of renal cell carcinoma: A 10-year patients’ experience at King Abdulaziz Medical City, National Guard Health Affairs, Saudi Arabia. Urol. Ann. 2018, 10, 59–64. [Google Scholar] [CrossRef]
- Motzer, R.J.; Mazumdar, M.; Bacik, J.; Berg, W.; Amsterdam, A.; Ferrara, J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J. Clin. Oncol. 1999, 17, 2530–2540. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.N.; Xie, G.Y.; Xiao, L.; Mo, D.C.; Huang, J.F.; Luo, P.H.; Liang, X.J. Severe and fatal adverse events of immune checkpoint inhibitor combination therapy in patients with metastatic renal cell carcinoma: A systematic review and meta-analysis. Front. Immunol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Wu, Z.; Zou, X.; Liu, H.; Wu, Y.; Xiong, L.; Yu, C.; Guo, S.; Han, H.; Zhou, F.; et al. Immune checkpoint inhibitors further aggravate proteinuria in patients with metastatic renal cell carcinoma after long-term targeted therapy. Transl. Androl. Urol. 2022, 11, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Q.; Qu, L.; Li, M.; Wang, L.; Mir, M.C.; Carbonara, U.; Pandolfo, S.D.; Black, P.C.; Paul, A.K.; et al. Adverse Events of Immune Checkpoint Inhibitors Therapy for Urologic Cancer Patients in Clinical Trials: A Collaborative Systematic Review and Meta-analysis. Eur. Urol. 2022, 81, 414–425. [Google Scholar] [CrossRef]
- McDermott, D.F.; Lee, J.-L.; Ziobro, M.; Suarez, C.; Langiewicz, P.; Matveev, V.B.; Wiechno, P.; Gafanov, R.A.; Tomczak, P.; Pouliot, F.; et al. Open-Label, Single-Arm, Phase II Study of Pembrolizumab Monotherapy as First-Line Therapy in Patients With Advanced Non–Clear Cell Renal Cell Carcinoma. J. Clin. Oncol. 2021, 39, 1029–1039. [Google Scholar] [CrossRef]
- Que, Y.; Liang, Y.; Zhao, J.; Ding, Y.; Peng, R.; Guan, Y.; Zhang, X. Treatment-related adverse effects with pazopanib, sorafenib and sunitinib in patients with advanced soft tissue sarcoma: A pooled analysis. Cancer Manag. Res. 2018, 10, 2141–2150. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Iinuma, K.; Yamada, T.; Kameyama, K.; Taniguchi, T.; Kawada, K.; Ishida, T.; Nagai, S.; Enomoto, T.; Ueda, S.; Takagi, K.; et al. The Efficacy and Safety of Immune Checkpoint Inhibitor and Tyrosine Kinase Inhibitor Combination Therapy for Advanced or Metastatic Renal Cell Carcinoma: A Multicenter Retrospective Real-World Cohort Study. Cancers 2023, 15, 947. [Google Scholar] [CrossRef]
- Zakharia, Y.; Thomaidou, D.; Li, B.; Siu, G.; Levin, R.; Vlahiotis, A.; Rao, D.; Zanotti, G. Real-World Therapy Management and Outcomes of First-Line Axitinib Plus Pembrolizumab in Patients with Advanced Renal Cell Carcinoma in the United States. Front. Oncol. 2022, 12, 861189. [Google Scholar] [CrossRef]
| Characteristics | Total (n = 84) | Single-Agent TKI (n = 35) | Combination Therapy (n = 39) | Single-Agent ICI (n = 10) | p-Value | |
|---|---|---|---|---|---|---|
| Age | Median (IQR) | 60.5 (54–68.8) | 61 (56–69) | 58 (52–65) | 67.5 (60.8–79.3) | 0.019 |
| Gender | Female | 26 (31.0%) | 12 (34.3%) | 10 (25.6%) | 4 (40.0%) | 0.583 |
| Male | 58 (69.0%) | 23 (65.7%) | 29 (74.4%) | 6 (60.0%) | ||
| BMI | Median (IQR) | 26.3 (22.7–28.8) | 23.5 (20.4–31.1) | 25.7 (22.8–36.4) | 0.166 | |
| Karnofsky performance status score | >80 | 61 (72.6%) | 25 (71.4%) | 30 (76.9%) | 6 (60.0%) | 0.552 |
| <80 | 23 (27.4%) | 10 (28.6%) | 9 (23.1%) | 4 (40.0%) | ||
| MSKCC Prognostic risk group | Favourable | 4 (4.8%) | 2 (5.7%) | 2 (5.1%) | 0 (0.0%) | 0.960 |
| Intermediate | 50 (59.5%) | 21 (60.0%) | 23 (59.0%) | 6 (60.0%) | ||
| High | 30 (35.7%) | 12 (34.3%) | 14 (35.9%) | 4 (40.0%) | ||
| IMDC prognostic risk group | Favourable | 5 (6.0%) | 2 (5.7%) | 3 (7.7%) | 0 (0.0%) | 0.875 |
| Intermediate | 46 (54.8%) | 20 (57.1%) | 21 (53.8%) | 5 (50.0%) | ||
| Poor | 33 (39.3%) | 13 (37.1%) | 15 (38.5%) | 5 (50.0%) | ||
| Initial staging of RCC | Stage 1 | 1 (1.2%) | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) | 0.332 |
| Stage 2 | 6 (7.1%) | 5 (14.3%) | 1 (2.6%) | 0 (0.0%) | ||
| Stage 3 | 14 (16.7%) | 6 (17.1%) | 7 (17.9%) | 1 (10.0%) | ||
| Stage 4 | 63 (75.0%) | 23 (65.7%) | 31 (79.5%) | 9 (90.0%) | ||
| Location | Unilateral | 82 (97.6%) | 34 (97.1%) | 38 (97.4%) | 10 (100.0%) | 0.868 |
| Bilateral | 2 (2.4%) | 1 (2.9%) | 1 (2.6%) | 0 (0.0%) | ||
| Number of sites of metastasis | 1 | 31 (36.9%) | 13 (37.1%) | 13 (33.3%) | 5 (50.0%) | 0.420 |
| 2 or more | 53 (63.1%) | 22 (62.9%) | 26 (66.7%) | 5 (50.0%) | ||
| Nephrectomy | No Prior Nephrectomy | 40 (47.6%) | 11 | 22 | 7 | 0.100 |
| Previous Nephrectomy | 43 (51.2%) | 23 | 17 | 3 | ||
| N/R | 1 (1.2%) | 1 | 0 | 0 | ||
| Type of surgery | Partial Nephrectomy | 2 (2.4%) | 0 | 2 | 0 | 0.090 |
| Radical Nephrectomy | 41 (48.8%) | 23 | 15 | 3 | ||
| N/A | 40 (47.6%) | 11 | 22 | 7 | ||
| N/R | 1 (1.2%) | 1 | 0 | 0 | ||
| Adjuvant therapy (Sunitinib) | No Prior Adjuvant Therapy | 83 (98.8%) | 35 (100.0%) | 39 (100.0%) | 9 (90.0%) | 0.024 |
| Prior Adjuvant Therapy | 1 (1.2%) | 0 (0.0%) | 0 (0.0%) | 1 (10.0%) | ||
| Metastatic site | Lung | 59 (70.2%) | 22 (62.9%) | 30 (76.9%) | 7 (70.0%) | 0.418 |
| Liver | 23 (27.4%) | 10 (28.6%) | 11 (28.2%) | 2 (20.0%) | 0.855 | |
| Lymph Node | 32 (38.1%) | 11 (31.4%) | 17 (43.6%) | 4 (40.0%) | 0.556 | |
| Brain | 9 (10.7%) | 2 (5.7%) | 6 (15.4%) | 1 (10.0%) | 0.405 | |
| Bone | 34 (40.5%) | 15 (42.9%) | 14 (35.9%) | 5 (50.0%) | 0.671 | |
| Other Site | 25 (29.8%) | 13 (37.1%) | 9 (23.1%) | 3 (30.0%) | 0.418 | |
| Follow-up (months) | Median (IQR) | 16.5 (7–36.8) | 17.4 (7.2–51) | 16 (7.5–24.6) | 19 (8.9–28.8) | 0.529 |
| Variables | Single-Agent TKI (n = 35) | Combination Therapy (n = 39) | Single-Agent ICI (n = 10) | |
|---|---|---|---|---|
| Lines of therapy | 1 | 14 (40.0%) | 25 (64.1%) | 8 (80.0%) |
| 2 | 15 (42.9%) | 6 (15.4%) | 1 (10.0%) | |
| 3 | 3 (8.6%) | 4 (10.3%) | 1 (10.0%) | |
| 4 | 3 (8.6%) | 4 (10.3%) | 0 (0.0%) | |
| First-line drug | Cabozantinib | 1 (2.9%) | 0 (0.0%) | 0 (0.0%) |
| Ipilimumab and Nivolumab | 0 (0.0%) | 15 (38.5%) | 0 (0.0%) | |
| Nivolumab | 0 (0.0%) | 0 (0.0%) | 5 (50.0%) | |
| Nivolumab and Cabozantinib | 0 (0.0%) | 4 (10.3%) | 0 (0.0%) | |
| Pazopanib | 10 (28.6%) | 0 (0.0%) | 0 (0.0%) | |
| Pembrolizumab | 0 (0.0%) | 0 (0.0%) | 5 (50.0%) | |
| Pembrolizumab and Axitinib | 0 (0.0%) | 8 (20.5%) | 0 (0.0%) | |
| Pembrolizumab and Lenvatinib | 0 (0.0%) | 12 (30.8%) | 0 (0.0%) | |
| Sunitinib | 24 (68.6%) | 0 (0.0%) | 0 (0.0%) | |
| First-line response | CR | 3 (8.6%) | 0 (0.0%) | 0 (0.0%) |
| PR | 3 (8.6%) | 16 (41.0%) | 2 (20.0%) | |
| SD | 4 (11.4%) | 2 (5.1%) | 3 (30.0%) | |
| PD | 25 (71.4%) | 20 (51.3%) | 5 (50.0%) | |
| Second-line drug | n | =21 | =14 | =2 |
| Cabozantinib | 0 (0.0%) | 6 (42.9%) | 0 (0.0%) | |
| Ipilimumab and Nivolumab | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) | |
| Nivolumab | 16 (76.2%) | 0 (0.0%) | 0 (0.0%) | |
| Nivolumab and Cabozantinib | 2 (9.5%) | 1 (7.1%) | 0 (0.0%) | |
| Pazopanib | 1 (4.8%) | 1 (7.1%) | 2 (100%) | |
| Pembrolizumab | 1 (4.8%) | 0 (0.0%) | 0 (0.0%) | |
| Pembrolizumab and Lenvatinib | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) | |
| Sunitinib | 1 (4.8%) | 4 (28.6%) | 0 (0.0%) | |
| Second-line response | n | =20 | =14 | =2 |
| CR | 1 (5%) | 0 (0.0%) | 0 (0.0%) | |
| PR | 5 (25%) | 3 (21.4%) | 0 (0.0%) | |
| SD | 2 (10%) | 1 (7.1%) | 0 (0.0%) | |
| PD | 12 (60%) | 10 (71.4%) | 2 (100%) | |
| Third-line drug | n | =6 | =8 | =1 |
| Cabozantinib | 2 (33.3%) | 1 (12.5%) | 1 (100.0%) | |
| Ipilimumab and Nivolumab | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | |
| Lenvatinib and Everolimus | 1 (16.7%) | 1 (12.5%) | 0 (0.0%) | |
| Nivolumab | 2 (33.3%) | 0 (0.0%) | 0 (0.0%) | |
| Nivolumab and Cabozantinib | 0 (0.0%) | 2 (25%) | 0 (0.0%) | |
| Pazopanib | 1 (16.7%) | 1 (12.5%) | 0 (0.0%) | |
| Pembrolizumab | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | |
| Pembrolizumab and Lenvatinib | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | |
| Third-line response | n | =6 | =8 | =1 |
| CR | 1 (16.7%) | 2 (25%) | 0 (0.0%) | |
| PR | 0 (0.0%) | 1 (12.5%) | 0 (0.0%) | |
| SD | 5 (83.3%) | 5 (62.5%) | 1 (100.0%) | |
| Fourth-line drug | n | =3 | =4 | =0 |
| Cabozantinib | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | |
| Lenvatinib and Everolimus | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | |
| Everolimus | 0 (0.0%) | 1 (25%) | 0 (0.0%) | |
| Nivolumab | 0 (0.0%) | 1 (25%) | 0 (0.0%) | |
| Pembrolizumab and Axitinib | 1 (33.3%) | 0 (0.0%) | 0 (0.0%) | |
| Pembrolizumab and Lenvatinib | 0 (0.0%) | 1 (25%) | 0 (0.0%) | |
| Sunitinib | 0 (0.0%) | 1 (25%) | 0 (0.0%) | |
| Fourth-line response | n | =3 | =2 | =0 |
| CR | 2 (66.7%) | 0 (0.0%) | 0 (0.0%) | |
| PR | 1 (33.3%) | 1 (50%) | 0 (0.0%) | |
| SD | 0 (0.0%) | 1 (50%) | 0 (0.0%) |
| Variables | Univariate Analysis | Adjusted Model | |||
|---|---|---|---|---|---|
| No. of Events | HR (95.0% CI) | p-Value | HR (95.0% CI) | p-Value | |
| Age ≥ 65 years old | 22 | 1.1 (0.62–1.8) | 0.824 | ||
| Male gender | 41 | 1.1 (0.64–1.9) | 0.697 | ||
| BMI ≥ 30 Kg/m2 | 11 | 0.79 (0.41–1.5) | 0.490 | ||
| Karnofsky performance status score < 80 | 19 | 2.4 (1.4–4.3) | 0.002 | 1.3 (0.67–2.5) | 0.444 |
| MSKCC prognostic risk group (Ref: favourable) | |||||
| Intermediate | 31 | 3 (0.69–13.2) | 0.140 | NE | 0.913 |
| High | 26 | 11.1 (2.4–50.9) | 0.002 | NE | 0.925 |
| IMDC prognostic risk group (Ref: favourable) | |||||
| Intermediate | 28 | 3.1 (0.73–13.7) | 0.125 | NE | 0.900 |
| High | 29 | 10.7 (2.4–48.4) | 0.002 | NE | 0.893 |
| Bilateral location | 1 | 1.66 (0.22–12.2) | 0.646 | ||
| Prior nephrectomy | 31 | 0.59 (0.35–1.1) | 0.057 | 0.98 (0.54–1.77) | 0.961 |
| Radical nephrectomy | 30 | 0.95 (0.13–7) | 0.958 | ||
| Adjuvant therapy (Sunitinib) | 1 | 0.34 (0.043–2.6) | 0.224 | ||
| First-line treatment (Ref: Single-agent TKI) | |||||
| ICI combination | 23 | 0.79 (0.46–1.4) | 0.428 | ||
| Single-agent ICI | 7 | 0.7 (0.3–1.61) | 0.400 | ||
| Lung metastasis | 41 | 1.3 (0.7–2.2) | 0.359 | ||
| Liver metastasis | 18 | 2 (1.2–3.5) | 0.015 | 1.8 (1.1–3.3) | 0.043 |
| Lymph node metastasis | 23 | 1.1 (0.63–1.8) | 0.839 | ||
| Brain metastasis | 5 | 0.83 (0.33–2.1) | 0.676 | ||
| Bone metastasis | 25 | 1.34 (0.79–2.3) | 0.270 | ||
| Variables | Univariate Analysis | Adjusted Model | |||
|---|---|---|---|---|---|
| No. of Events | HR (95.0% CI) | p-Value | HR (95.0% CI) | p-Value | |
| Age ≥ 65 years old | 17 | 1.48 (0.79–2.8) | 0.228 | ||
| Male gender | 27 | 1.1 (0.56–2.2) | 0.776 | ||
| BMI ≥ 30 Kg/m2 | 6 | 0.72 (0.3–1.7) | 0.449 | ||
| Karnofsky performance status score < 80 | 17 | 4.2 (2.1–8.1) | <0.001 | 3.5 (0.16–7.4) | <0.001 |
| MSKCC prognostic risk Group (Ref: favourable) | |||||
| Intermediate | 19 | NE | 0.916 | ||
| High | 20 | NE | 0.904 | ||
| IMDC prognostic risk Group (Ref: favourable) | |||||
| Intermediate | 17 | NE | 0.913 | ||
| High | 22 | NE | 0.900 | ||
| Bilateral location | 1 | 3.9 (0.5–30.5) | 0.192 | ||
| Prior nephrectomy | 18 | 0.49 (0.25–0.94) | 0.032 | 1.2 (0.27–5.54) | 0.794 |
| Radical nephrectomy | 16 | 0.26 (0.06–1.17) | 0.080 | 0.55 (0.11–2.74) | 0.467 |
| Adjuvant therapy (Sunitinib) | 1 | ||||
| First-line treatment (Ref: Single-agent TKI) | |||||
| ICI combination | 14 | 0.84 (0.41–1.69) | 0.624 | ||
| Single-agent ICI | 6 | 1.22 (0.49–0.31) | 0.668 | ||
| Lung metastasis | 25 | 1.13 (0.58–2.2) | 0.717 | ||
| Liver metastasis | 12 | 1.8 (0.92–3.61) | 0.086 | 1.89 (0.93–3.87) | 0.078 |
| Lymph node metastasis | 15 | 1.1 (0.56–2.1) | 0.832 | ||
| Brain metastasis | 5 | 1.4 (0.56–3.79) | 0.433 | ||
| Bone metastasis | 22 | 2.01 (1.07–3.79) | 0.031 | 1.26 (0.62–2.56) | 0.516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mansour, M.M.; Aga, S.S.; Alharbi, H.A.; Alsulami, M.N.; Fallatah, H.A.; Albedaiwi, T.B.; Anbari, L.K.; Surrati, T.R.; Algethami, A.A.; Althubaiti, A.; et al. Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia. Cancers 2024, 16, 3234. https://doi.org/10.3390/cancers16183234
Al-Mansour MM, Aga SS, Alharbi HA, Alsulami MN, Fallatah HA, Albedaiwi TB, Anbari LK, Surrati TR, Algethami AA, Althubaiti A, et al. Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia. Cancers. 2024; 16(18):3234. https://doi.org/10.3390/cancers16183234
Chicago/Turabian StyleAl-Mansour, Mubarak M., Syed Sameer Aga, Hanin A. Alharbi, Maria N. Alsulami, Halah A. Fallatah, Tarfah B. Albedaiwi, Lujain K. Anbari, Taleen R. Surrati, Ashwag A. Algethami, Alaa Althubaiti, and et al. 2024. "Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia" Cancers 16, no. 18: 3234. https://doi.org/10.3390/cancers16183234
APA StyleAl-Mansour, M. M., Aga, S. S., Alharbi, H. A., Alsulami, M. N., Fallatah, H. A., Albedaiwi, T. B., Anbari, L. K., Surrati, T. R., Algethami, A. A., Althubaiti, A., Alfayea, T. M., & Alolayan, A. (2024). Real-World Survival Outcomes of First-Line Therapies in Patients with Metastatic Clear Cell Renal Cell Carcinoma: A Retrospective Analysis from Two Centres in Saudi Arabia. Cancers, 16(18), 3234. https://doi.org/10.3390/cancers16183234







