Sentinel Lymph Node Assessment in Endometrial Cancer: A Review
Abstract
Simple Summary
Abstract
1. Introduction
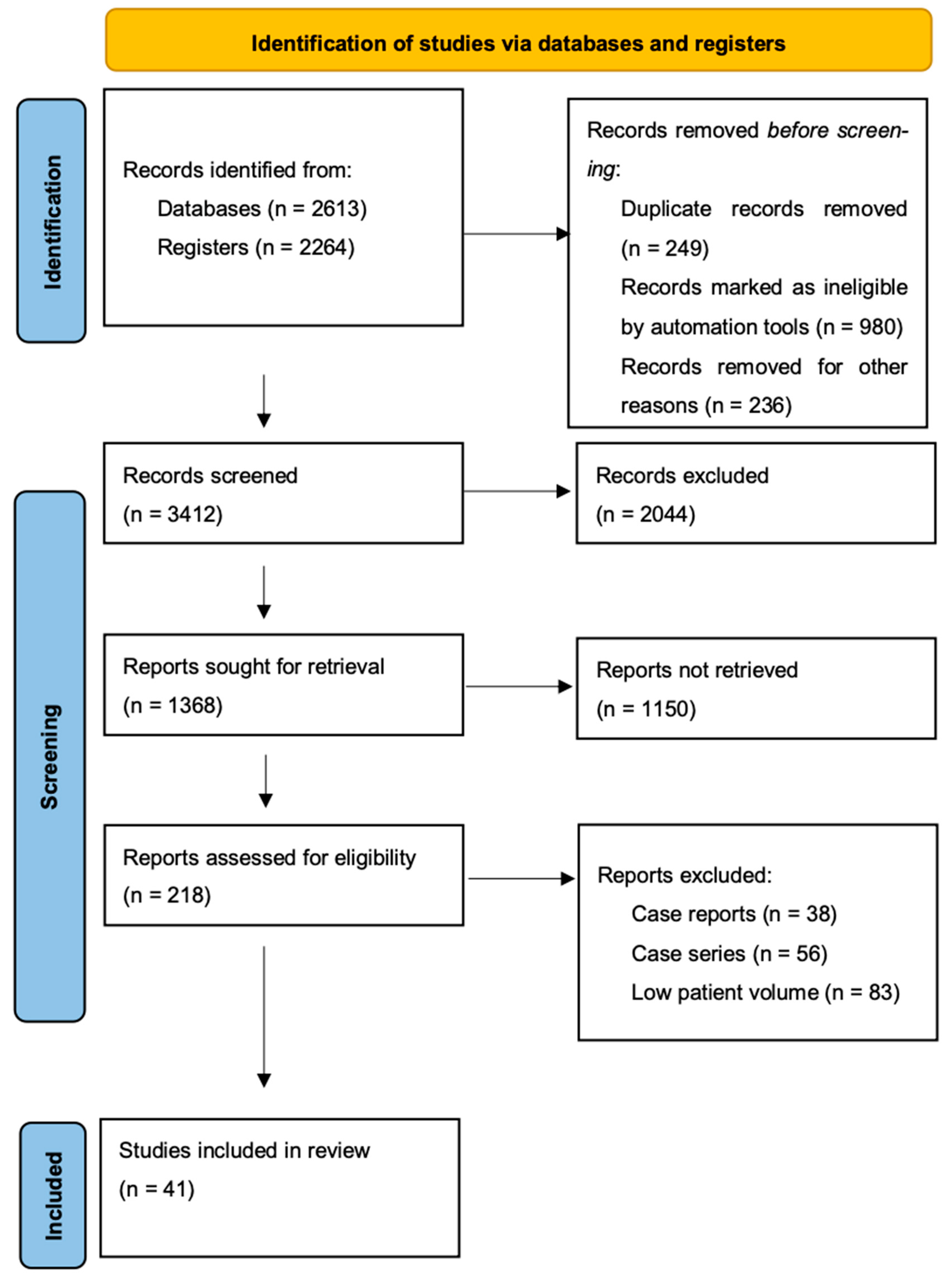
2. Materials and Methods
3. Results
3.1. SLN Mapping and Failure Predictors
3.2. SLN Ultrastaging Techniques
3.3. Management of Positive SLN, ITC, Micrometastases, and Macrometastases
3.4. Sentinel Lymph Node Evaluation in Atypical Endometrial Hyperplasia
3.5. Sentinel Lymph Node in Apparently Early-Stage Endometrial Cancer
3.6. Sentinel Lymph Node Biopsy in High-Grade Endometrial Carcinoma
3.7. Further Perspectives and Ongoing Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2024 [Internet]. American Cancer Society: Atlanta. 2024. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2024/2024-cancer-facts-and-figures-acs.pdf (accessed on 1 August 2024).
- Rungruang, B.; Olawaiye, A.B. Comprehensive surgical staging for endometrial cancer. Rev. Obstet. Gynecol. 2012, 5, 28–34. [Google Scholar] [PubMed]
- Gould, E.A.; Winship, T.; Philbin, P.H.; Kerr, H.H. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960, 13, 77–78. [Google Scholar] [CrossRef]
- Daniilidis, A.; Margioula-Siarkou, C.; Margioula-Siarkou, G.; Papandreou, P.; Papanikolaou, A.; Dinas, K.; Petousis, S. Sentinel lymph node mapping in endometrial cancer to reduce surgical morbidity: Always, sometimes, or never. Prz Menopauzalny 2022, 21, 207–213. [Google Scholar] [CrossRef]
- Akrivos, N.; Rodolakis, A.; Vlachos, G.; Sotiropoulou, M.; Papantoniou, V.; Biliatis, I.; Haidopoulos, D.; Thomakos, N.; Simou, M.; Antsaklis, A. Detection and credibility of sentinel node in vulvar cancer: A single institutional study and short review of literature. Arch. Gynecol. Obstet. 2011, 284, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Lin, H.; Ding, Z.; Kota, V.G.; Zhang, X.; Zhou, J. Sentinel lymph node mapping in endometrial cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 46601–46610. [Google Scholar] [CrossRef] [PubMed]
- López-De la Manzanara, C.C.; Cordero García, J.M.; Martín-Francisco, C.; Pascual-Ramírez, J.; Parra, C.P.; Céspedes Casas, C. ATL: A Prospective Study. Inl. J. Gynecol. Cancer 2014, 24, 1048–1053. [Google Scholar] [CrossRef]
- Ballester, M.; Dubernard, G.; Lécuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Torné, A.; Pahisa, J.; Vidal-Sicart, S.; Martínez-Roman, S.; Paredes, P.; Puerto, B.; Albela, S.; Fusté, P.; Perisinotti, A.; Ordi, J.; et al. Transvaginal ultrasound-guided myometrial injection of radiotracer (TUMIR): A new method for sentinel lymph node detection in endometrial cancer. Gynecol. Oncol. 2013, 128, 88–94. [Google Scholar] [CrossRef]
- Biliatis, I.; Thomakos, N.; Koutroumpa, I.; Haidopoulos, D.; Sotiropoulou, M.; Antsaklis, A.; Vlachos, G.; Akrivos, N.; Rodolakis, A. Subserosal uterine injection of blue dye for the identification of the sentinel node in patients with endometrial cancer: A feasibility study. Arch. Gynecol. Obstet. 2017, 296, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.; Casarin, I.; Pinelli, C.; Perrone, A.M.; Scollo, P.; Martinelli, F.; Bogani, G.; Leone Roberti Maggiore, U.; Signorelli, M.; Chiappa, V.; et al. Hysteroscopic versus cervical injection for sentinel node detection in endometrial cancer: A multicenter prospective randomised controlled trial from the Multicenter Italian Trials in Ovarian cancer (MITO) study group. Eur. J. Cancer 2020, 140, 1–10, Correction in Eur. J. Cancer 2021, 144, 399. [Google Scholar] [CrossRef] [PubMed]
- Levenback, C.F. Status of sentinel lymph node biopsy in gynecological cancers. Ann. Surg. Oncol. 2008, 15, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Teng, F.; Yu, H.; Wang, B.; Yan, Y.; Gao, C.; Guo, F.; Gao, J.; Tian, W.; Wang, Y.; Xue, F.; et al. Patterns of lymph node metastases and their implications in individualized radiotherapeutic clinical target volume delineation of regional lymph nodes in patients with endometrial cancer. J. Cancer 2022, 13, 3575–3583. [Google Scholar] [CrossRef]
- Kumar, S.; Podratz, K.C.; Bakkum-Gamez, J.N.; Dowdy, S.C.; Weaver, A.L.; McGree, M.E.; Cliby, W.A.; Keeney, G.L.; Thomas, G.; Mariani, A. Prospective assessment of the prevalence of pelvic, paraaortic and high paraaortic lymph node metastasis in endometrial cancer. Gynecol. Oncol. 2014, 132, 38–43. [Google Scholar] [CrossRef]
- Taşkın, S.; Sarı, M.E.; Altın, D.; Ersöz, C.C.; Gökçe, A.; Yüksel, S.; Kankaya, D.; Ortaç, F. Risk factors for failure of sentinel lymph node mapping using indocyanine green/near-infrared fluorescent imaging in endometrial cancer. Arch. Gynecol. Obstet. 2019, 299, 1667–1672. [Google Scholar] [CrossRef]
- Tortorella, L.; Casarin, J.; Multinu, F.; Cappuccio, S.; McGree, M.E.; Weaver, A.L.; Langstraat, C.L.; Keeney, G.L.; Kumar, A.; Melis, G.B.; et al. Sentinel lymph node biopsy with cervical injection of indocyanine green in apparent early-stage endometrial cancer: Predictors of unsuccessful mapping. Gynecol. Oncol. 2019, 155, 34–38. [Google Scholar] [CrossRef]
- Mauro, J.; Raimondo, D.; Di Martino, G.; Gasparri, M.L.; Restaino, S.; Neola, D.; Clivio, L.; Calidona, C.; Fruscio, R.; Vizzielli, G.; et al. Assessment of sentinel Lymph node mapping with different volumes of Indocyanine green in early-stage ENdometrial cancer: The ALIEN study. Int. J. Gynecol. Cancer 2024, 34, 824–829. [Google Scholar] [CrossRef]
- Body, N.; Grégoire, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Plante, M. Tips and tricks to improve sentinel lymph node mapping with Indocyanin green in endometrial cancer. Gynecol. Oncol. 2018, 150, 267–273. [Google Scholar] [CrossRef]
- Sozzi, G.; Fanfani, F.; Berretta, R.; Capozzi, V.A.; Uccella, S.; Buono, N.; Giallombardo, V.; Catello Di Donna, M.; Monterossi, G.; Restaino, S.; et al. Laparoscopic sentinel node mapping with intracervical indocyanine green injection for endometrial cancer: The SENTIFAIL study—A multicentric analysis of predictors of failed mapping. Int. J. Gynecol. Cancer 2020, 30, 1713–1718. [Google Scholar] [CrossRef]
- Zhang-Yin, J.; Mauel, E.; Talpe, S. Update on Sentinel Lymph Node Methods and Pathology in Breast Cancer. Diagnostics 2024, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.; Tessier-Cloutier, B.; Klein, E.; Ardon, O.; Mueller, J.J.; Leitao, M.M.; Abu-Rustum, N.R.; Ellenson, L.H. Establishing guidelines for sentinel lymph node ultrastaging in endometrial cancer. Int. J. Gynecol. Cancer 2024, 34, 681–688. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285, Correction in Lancet Oncol. 2019, 20, e468. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer. 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. 2023, 162, 383–394, Correction in Int. J. Gynaecol. Obstet. 2024, 166, 1374. [Google Scholar] [CrossRef] [PubMed]
- Ignatov, A.; Lebius, C.; Ignatov, T.; Ivros, S.; Knueppel, R.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Lymph node micrometastases and outcome of endometrial cancer. Gynecol. Oncol. 2019, 154, 475–479. [Google Scholar] [CrossRef]
- Backes, F.J.; Felix, A.S.; Plante, M.; Grégoire, J.; Sullivan, S.A.; Rossi, E.C.; Tanner, E.J.; Stewart, K.I.; Soliman, P.T.; Holloway, R.W.; et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Gynecol. Oncol. 2021, 161, 347–352. [Google Scholar] [CrossRef]
- Bourdel, N.; Chauvet, P.; Tognazza, E.; Pereira, B.; Botchorishvili, R.; Canis, M. Sampling in Atypical Endometrial Hyperplasia: Which Method Results in the Lowest Underestimation of Endometrial Cancer? A Systematic Review and Meta-analysis. J. Minim Invasive Gynecol. 2016, 23, 692–701. [Google Scholar] [CrossRef]
- Giede, K.C.; Yen, T.W.; Chibbar, R.; Pierson, R.A. Significance of concurrent endometrial cancer in women with a preoperative diagnosis of atypical endometrial hyperplasia. J. Obstet. Gynaecol. Can. 2008, 30, 896–901. [Google Scholar] [CrossRef]
- Rosati, A.; Vargiu, V.; Capozzi, V.A.; Giannarelli, D.; Palmieri, E.; Baroni, A.; Perrone, E.; Berretta, R.; Cosentino, F.; Scambia, G.; et al. Concurrent endometrial cancer in atypical endometrial hyperplasia and the role of sentinel lymph nodes: Clinical insights from a multicenter experience. Int. J. Gynecol. Cancer 2024, 34, 1011–1019. [Google Scholar] [CrossRef]
- Mueller, J.J.; Pedra Nobre, S.; Braxton, K.; Alektiar, K.M.; Leitao, M.M.; Aghajanian, C.; Ellenson, L.H.; Abu-Rustum, N.R. Incidence of pelvic lymph node metastasis using modern FIGO staging and sentinel lymph node mapping with ultrastaging in surgically staged patients with endometrioid and serous endometrial carcinoma. Gynecol. Oncol. 2020, 157, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, P.B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60, 2035–2041. [Google Scholar] [CrossRef] [PubMed]
- De Vitis, L.A.; Fumagalli, D.; Schivardi, G.; Capasso, I.; Grcevich, L.; Multinu, F.; Cucinella, G.; Occhiali, T.; Betella, I.; Guillot, B.E.; et al. Incidence of sentinel lymph node metastases in apparent early-stage endometrial cancer: A multicenter observational study. Int. J. Gynecol. Cancer 2024, 34, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Matanes, E.; Eisenberg, N.; Amajoud, Z.; Gupta, V.; Yasmeen, A.; Ismail, S.; Racovitan, F.; Raban, O.; Lau, S.; Salvador, S.; et al. Sentinel Lymph Node Sampling as an Alternative to Lymphadenectomy in Patients With Endometrial Cancer and Obesity. J. Obstet. Gynaecol. Can. 2021, 43, 1136–1144.e1. [Google Scholar] [CrossRef] [PubMed]
- Raimond, E.; Ballester, M.; Hudry, D.; Bendifallah, S.; Daraï, E.; Graesslin, O.; Coutant, C. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol. Oncol. 2014, 133, 506–511. [Google Scholar] [CrossRef]
- Obermair, A.; Nicklin, J.; Gebski, V.; Hayes, S.C.; Graves, N.; Mileshkin, L.; Lin, M.Y.; Beale, P.; Baxter, E.; Robledo, K.; et al. A phase III randomized clinical trial comparing sentinel node biopsy with no retroperitoneal node dissection in apparent early-stage endometrial cancer—ENDO-3: ANZGOG trial 1911/2020. Int. J. Gynecol. Cancer 2021, 31, 1595–1601. [Google Scholar] [CrossRef]
- Grassi, T.; Mariani, A.; Cibula, D.; Soliman, P.T.; Suman, V.J.; Weaver, A.L.; Pedra Nobre, S.; Weigelt, B.; Glaser, G.E.; Cappuccio, S.; et al. A prospective multicenter international single-arm observational study on the oncological safety of the sentinel lymph node algorithm in stage I intermediate-risk endometrial cancer (SELECT, SEntinel Lymph node Endometrial Cancer Trial). Int. J. Gynecol. Cancer 2020, 30, 1627–1632. [Google Scholar] [CrossRef]
- Dick, A.; Perri, T.; Kogan, L.; Brandt, B.; Meyer, R.; Levin, G. Sentinel lymph node mapping in endometrial cancer: A comparison of main national and international guidelines. Int. J. Gynecol. Obstet. 2023, 160, 220–225. [Google Scholar] [CrossRef]
- Abu-Rustum, N.; Yashar, C.; Arend, R.; Barber, E.; Bradley, K.; Brooks, R.; Campos, S.M.; Chino, J.; Chon, H.S.; Chu, C.; et al. Uterine Neoplasms, Version 1.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2023, 21, 181–209. [Google Scholar] [CrossRef]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Jeffrey Lowery, W.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, M.; Lefor, A.T.; Hozumi, Y.; Kurihara, K.; Sata, N.; Yasuda, Y.; Kusakabe, M. Sentinel lymph node biopsy in patients with breast cancer using superparamagnetic iron oxide and a magnetometer. Breast Cancer 2013, 20, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.; Argáez, C. Magnetic Localization System for Sentinel Lymph Node Biopsy: A Review of the Diagnostic Accuracy, Cost-Effectiveness, and Guidelines [Internet]. Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 26 February 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562944/ (accessed on 1 August 2024).
- Murakami, K.; Kotani, Y.; Suzuki, A.; Takaya, H.; Nakai, H.; Matsuki, M.; Sato, T.; Mandai, M.; Matsumura, N. Superparamagnetic iron oxide as a tracer for sentinel lymph node detection in uterine cancer: A pilot study. Sci. Rep. 2020, 10, 7945. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Di Donato, V.; Papadia, A.; Buda, A.; Casarin, J.; Multinu, F.; Plotti, F.; Gasparri, M.L.; Pinelli, C.; Perrone, A.M.; et al. Hysterectomy alone vs. hysterectomy plus sentinel node mapping in endometrial cancer: Perioperative and long-term results from a propensity-score based study. Eur. J. Surg. Oncol. 2023, 49, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Schivardi, G.; deVitis, L.A.; Fumagalli, C.; Raviele, P.R.; Achilarre, M.T.; Aloisi, A.; Garbi, A.; Lapresa, M.; Parma, G.; Zanagnolo, V.; et al. 2022-RA-659-ESGO The role of molecular classification in endometrial cancers with lymph nodes metastasis. Int. J. Gynecol. Cancer 2022, 32, A103–A104. [Google Scholar]
- Liu, X.F.; Yan, B.C.; Li, Y.; Ma, F.H.; Qiang, J.W. Radiomics Nomogram in Assisting Lymphadenectomy Decisions by Predicting Lymph Node Metastasis in Early-Stage Endometrial Cancer. Front. Oncol. 2022, 12, 894918. [Google Scholar] [CrossRef]
| Tracer | Overall Detection Rate | Sensitivity | Negative Predictive Value | Disadvantages |
|---|---|---|---|---|
| ICG | 93% † | 97.2% * | 99.6% * | High costs, long learning curve, needs near-infrared light source for detection, mapping failure due to prolonged operating times and inexperienced surgeons |
| Blue Dyes (BD) + 99mTc | 89% ** | 84% § | 100% ** | Patient discomfort, higher rates of adverse reactions, exposure to radiation, longer operating time |
| NCT | Title | Study Type | Intervention | Status |
|---|---|---|---|---|
| NCT04492995 | Sentinel Node in Endometrial Cancer (HYBRIDENDONOD) | Interventional |
| Recruiting |
| NCT06163963 | Sentinel Lymph Node in Early-Stage Endometrium Cancer | Interventional | Diagnostic Test: Sentinel Lymph Node Mapping With Double Tracer and Double Injection Sites in Early-Stage Endometrium Cancer | Recruiting |
| NCT05191212 | The Role of Real-time Appearance of Lymphatic Flow in Lymphatic Mapping in Endometrial Cancer | Interventional | Procedure: indocyanine green | Recruiting |
| NCT | Title | Study Type | Intervention | Status |
|---|---|---|---|---|
| NCT04845828 | Randomized Comparison Between Sentinel Lymph Node Biopsy and Lymph Node Dissection in Early-Stage Endometrial Cancer (SELYE) | Interventional |
| Recruiting |
| NCT03366051 | Sentinel Node Mapping in High-Risk Endometrial Cancer (ALICE) | Interventional |
| Recruiting |
| NCT | Title | Study Type | Groups and Intervention | Status |
|---|---|---|---|---|
| NCT05707312 | Staging Endometrial caNcer Based on molEcular ClAssification | Observational | POLE mutant endometrial cancer patients Mismatch repair endometrial cancer patients No specific mutational profile endometrial cancer patients to NSMP P53 abnormal endometrial cancer patients | Recruiting |
| NCT04604613 | Prediction of Recurrence Among Low-Risk Endometrial Cancer Patients | Observational | Bilateral Salpingectomy with Oophorectomy Biospecimen Collection Hysterectomy Other: Laboratory Biomarker Analysis Correlative studies Lymph Node Mapping Sentinel Lymph Node Biopsy | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, C.; Loizzi, V.; Cormio, G.; Lopez, S. Sentinel Lymph Node Assessment in Endometrial Cancer: A Review. Cancers 2024, 16, 3202. https://doi.org/10.3390/cancers16183202
Clark C, Loizzi V, Cormio G, Lopez S. Sentinel Lymph Node Assessment in Endometrial Cancer: A Review. Cancers. 2024; 16(18):3202. https://doi.org/10.3390/cancers16183202
Chicago/Turabian StyleClark, Christopher, Vera Loizzi, Gennaro Cormio, and Salvatore Lopez. 2024. "Sentinel Lymph Node Assessment in Endometrial Cancer: A Review" Cancers 16, no. 18: 3202. https://doi.org/10.3390/cancers16183202
APA StyleClark, C., Loizzi, V., Cormio, G., & Lopez, S. (2024). Sentinel Lymph Node Assessment in Endometrial Cancer: A Review. Cancers, 16(18), 3202. https://doi.org/10.3390/cancers16183202







