Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Eligibility Criteria
2.3. Multimodal Treatment Strategy
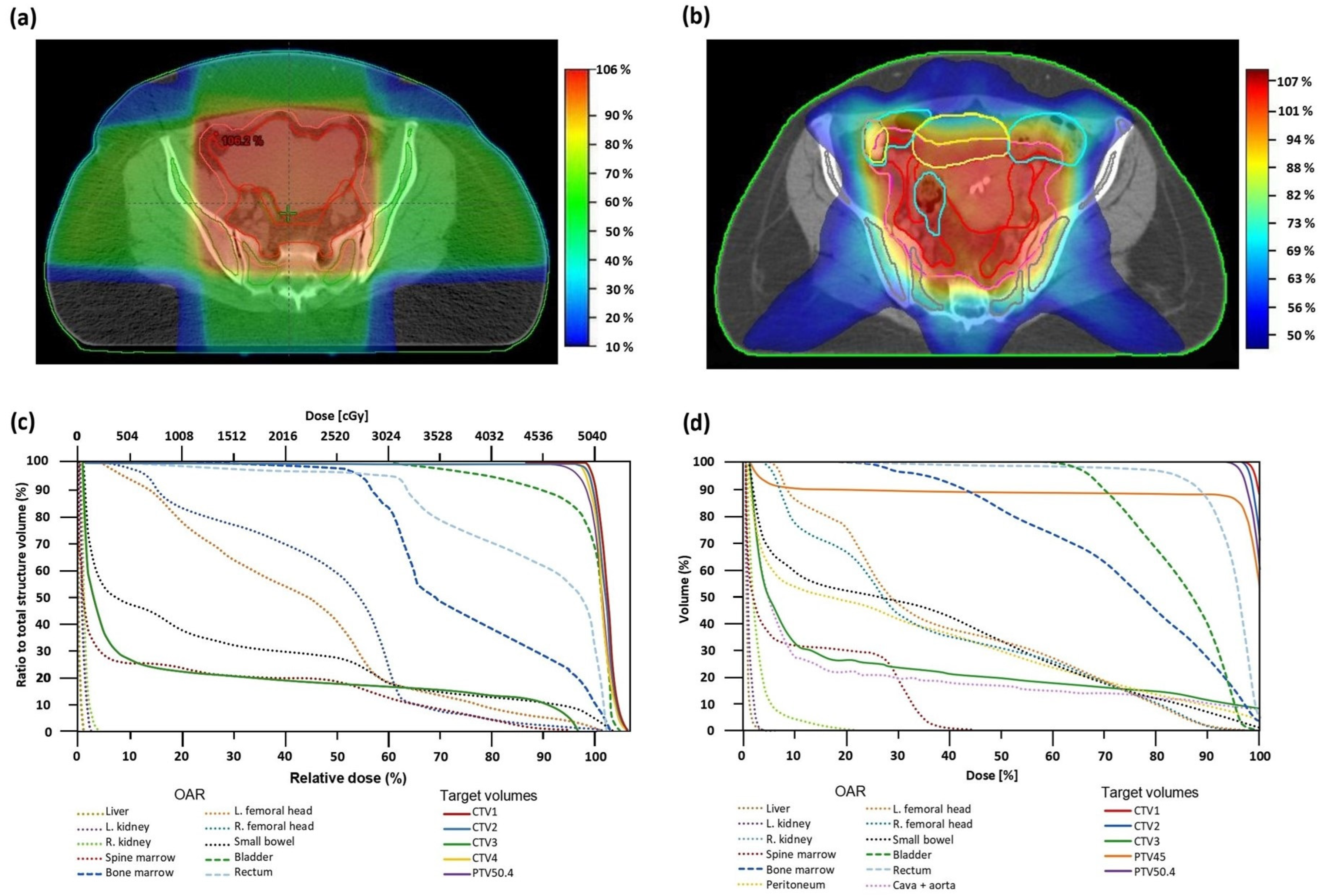
2.4. Radiotherapy Simulation, Contouring, and Planning
2.5. Statistical Analysis
2.5.1. Primary Variables
2.5.2. Variables Statistics
2.5.3. Secondary Variables
2.5.4. Risk Analysis and Logistic Model
3. Results
3.1. Patients
3.2. Therapy Modalities
3.3. Treatment-Related Adverse Events
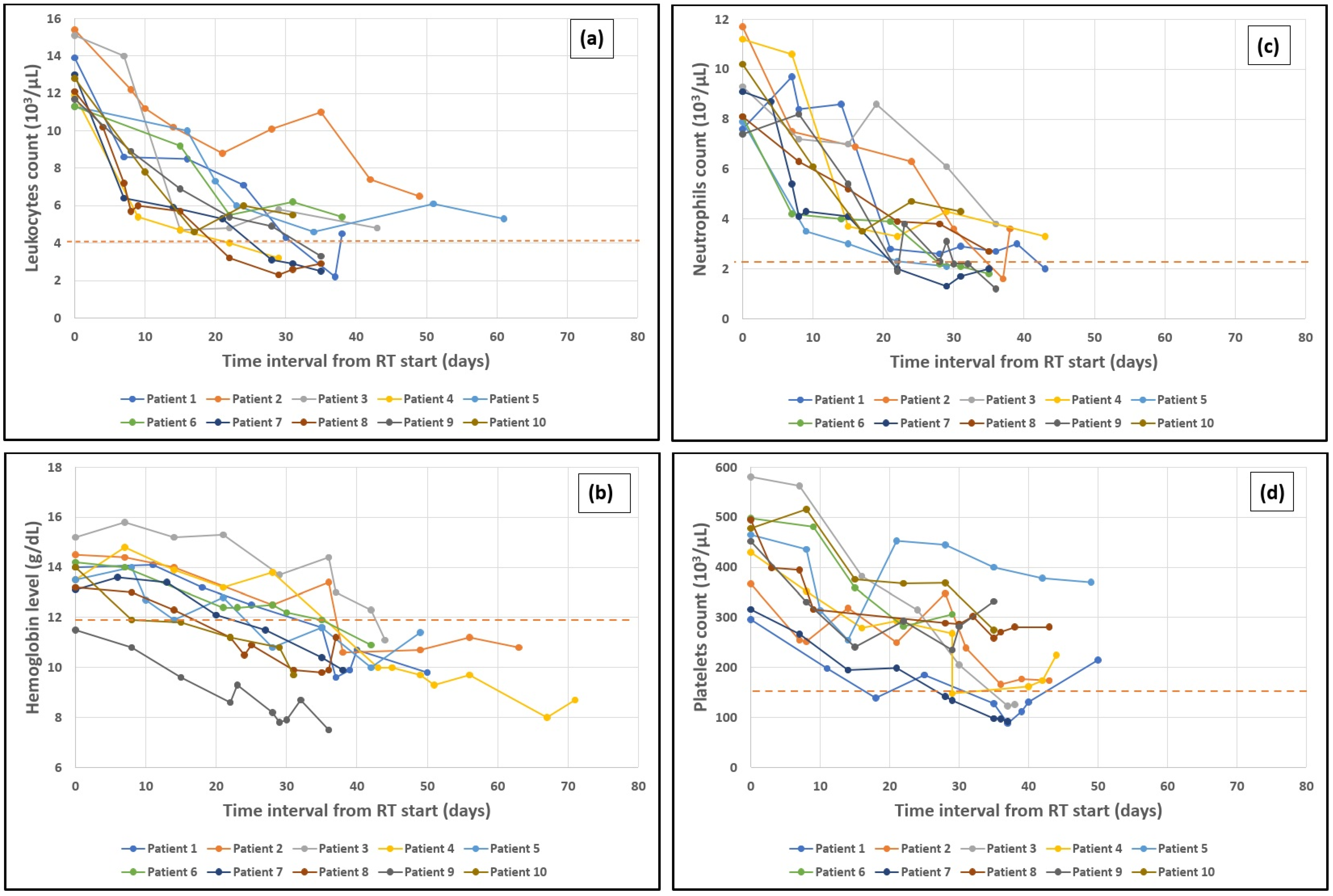
3.4. Time Period to Maximum Drop of Leukocytes and Hemoglobin
3.5. Neutrophils and Platelets Drop
3.6. Hemoglobin Drop over the Course of Treatment
4. Discussion
4.1. Hematological Adverse Events
4.2. Clinical Adverse Events
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| 3D-CRT | 3D-conformal radiotherapy |
| AE | Adverse event |
| BMI | Body mass index |
| CCRT | Concurrent chemoradiotherapy |
| CHT | Chemotherapy |
| CT | Computed tomography |
| CTV | Clinical target volume |
| CV | Cardiovascular |
| DM | Diabetes mellitus |
| DVH | Dose-volume histogram |
| EBRT | External beam radiotherapy |
| EF | Extended field |
| GTV | Gross tumor volume |
| HT | Hematological toxicity |
| IMRT | Intensity-modulated radiotherapy |
| OAR | Organ at risk |
| OR | Odds-ratio |
| OS | Overall survival |
| PFS | Progression-free survival |
| PTV | Planning target volume |
| QoL | Quality of life |
| RT | Radiotherapy |
| RR | Risk-ratio |
| UAIC | Uterine arterial interventional chemoembolization |
| VMAT | Volumetric modulated arc therapy |
References
- Yadav, N.; Parveen, S.; Banerjee, M. Potential of nano-phytochemicals in cervical cancer therapy. Clin. Chim. Acta 2020, 505, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Home—Eurostat. Available online: https://ec.europa.eu/eurostat/ (accessed on 13 February 2023).
- Zou, D.; Guo, M.; Zhou, Q. A clinical study of pegylated recombinant human granulocyte colony stimulating factor (PEG-rhG-CSF) in preventing neutropenia during concurrent chemoradiotherapy of cervical cancer. BMC Cancer 2021, 21, 661. [Google Scholar] [CrossRef]
- Nosaka, K.; Shibata, K.; Utsumi, F.; Yoshida, K.; Niimi, K.; Sekiya, R.; Suzuki, S.; Kajiyama, H.; Kikkawa, F. Feasibility and benefit of concurrent chemoradiotherapy for elderly patients with uterine cervical cancer. Tumori J. 2016, 102, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Trifanescu, O.G.; Gales, L.N.; Serbanescu, G.L.; Zgura, A.F.; Iliescu, L.; Mehedintu, C.; Anghel, R.M. Long-term oncological outcome in patients with cervical cancer after 3 trimodality treatment (radiotherapy, platinum-based chemotherapy, and robotic surgery). Medicine 2021, 100, e25271. [Google Scholar] [CrossRef]
- Bjelic-Radisic, V.; Jensen, P.T.; Vlasic, K.K.; Waldenstrom, A.-C.; Singer, S.; Chie, W.; Nordin, A.; Greimel, E. Quality of life characteristics inpatients with cervical cancer. Eur. J. Cancer 2012, 48, 3009–3018. [Google Scholar] [CrossRef]
- Hui, B.; Zhang, Y.; Shi, F.; Wang, J.; Wang, T.; Wang, J.; Yuan, W.; Li, Y.; Liu, Z. Association Between Bone Marrow Dosimetric Parameters and Acute Hematologic Toxicity in Cervical Cancer Patients Undergoing Concurrent Chemoradiotherapy: Comparison of Three-Dimensional Conformal Radiotherapy and Intensity-Modulated Radiation Therapy. Int. J. Gynecol. Cancer 2014, 24, 1648–1652. [Google Scholar] [CrossRef]
- Andreyev, J. Gastrointestinal symptoms after pelvic radiotherapy: A new understanding to improve management of symptomatic patients. Lancet Oncol. 2007, 8, 1007–1017. [Google Scholar] [CrossRef]
- Wit, E.M.K.; Horenblas, S. Urological complications after treatment of cervical cancer. Nat. Rev. Urol. 2014, 11, 110–117. [Google Scholar] [CrossRef]
- Mell, L.K.; Kochanski, J.D.; Roeske, J.C.; Haslam, J.J.; Mehta, N.; Yamada, S.D.; Hurteau, J.A.; Collins, Y.C.; Lengyel, E.; Mundt, A.J. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Vistad, I.; Fosså, S.; Kristensen, G.; Dahl, A. Chronic fatigue and its correlates in long-term survivors of cervical cancer treated with radiotherapy. BJOG Int. J. Obstet. Gynaecol. 2007, 114, 1150–1158. [Google Scholar] [CrossRef]
- Nara, K.; Taguchi, A.; Tojima, Y.; Miyamoto, Y.; Tanikawa, M.; Sone, K.; Mori, M.; Tsuruga, T.; Yamamoto, T.; Takenaka, R.; et al. History of whole pelvis plus para-aortic radiation is a risk factor associated with febrile neutropenia during chemotherapy for recurrent cervical cancer. Int. J. Clin. Oncol. 2021, 26, 1759–1766. [Google Scholar] [CrossRef]
- Faye, M.D.; Alfieri, J. Advances in Radiation Oncology for the Treatment of Cervical Cancer. Curr. Oncol. 2022, 29, 928–944. [Google Scholar] [CrossRef]
- Lin, Y.; Ouyang, Y.; Chen, K.; Lu, Z.; Liu, Y.; Cao, X. Clinical outcomes of volumetric modulated arc therapy following intracavitary/interstitial brachytherapy in cervical cancer: A single institution retrospective experience. Front. Oncol. 2019, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) American Joint Committee on Cancer (AJCC). In AJCC Cancer Staging Manual; Springer: Cham, Switzerland, 2017; Available online: https://link.springer.com/book/9783319406176 (accessed on 18 February 2023).
- Gaffney, D.K.; Gaillard, S.; Giuntoli, R.I.I.; Glaser, S.; Holmes, J.; Howitt, B.E.; Lea, J.; Landrum, L.; Lee, N.; Mantia-Smaldone, G.; et al. NCCN Guidelines Version 1.2023 Cervical Cancer. 2023. Available online: https://www.nccn.org/home/member- (accessed on 18 February 2023).
- AAPM Publications—Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC). Available online: https://www.aapm.org/pubs/quantec.asp (accessed on 18 February 2023).
- Izmajłowicz, B.; Rusiecka, M.; Sztuder, A.; Stępień, M.; Pacyna, A.I.; Romaniuk, B.S.-.; Mazur, Z.; Kornafel, J. Tolerance of combined radiochemotherapy in cervical cancer patients. Adv. Clin. Exp. Med. 2017, 26, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Dunst, J.; Kuhnt, T.; Strauss, H.G.; Krause, U.; Pelz, T.; Koelbl, H.; Haensgen, G. Anemia in cervical cancers: Impact on survival, patterns of relapse, and association with hypoxia and angiogenesis. Int. J. Radiat. Oncol. 2003, 56, 778–787. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Ni, L.-Q.; Wang, S.-S.; Lv, Q.-L.; Chen, W.-J.; Ying, S.-P. Outcome and prognostic factors in cervical cancer patients treated with surgery and concurrent chemoradiotherapy: A retrospective study. World J. Surg. Oncol. 2018, 16, 18. [Google Scholar] [CrossRef]
- Mell, L.K.; Sirák, I.; Wei, L.; Tarnawski, R.; Mahantshetty, U.; Yashar, C.M.; McHale, M.T.; Xu, R.; Honerkamp-Smith, G.; Carmona, R.; et al. Bone Marrow-sparing Intensity Modulated Radiation Therapy With Concurrent Cisplatin For Stage IB-IVA Cervical Cancer: An International Multicenter Phase II Clinical Trial (INTERTECC-2). Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 536–545. [Google Scholar] [CrossRef]
- Zayed, S.; Nguyen, T.K.; Lin, C.; Boldt, G.; Beriwal, S.; Creutzberg, C.L.; Kamrava, M.; Mendez, L.C.; Velker, V.; Doll, C.; et al. Red Blood Cell Transfusion Practices for Patients With Cervical Cancer Undergoing Radiotherapy. JAMA Netw. Open 2021, 4, e213531. [Google Scholar] [CrossRef]
- Li, D.; Wang, D.; Feng, S.; Chen, Q.; Sheng, X.; Jia, J.; Yan, X.; Zhu, J.; Yin, Y. Comparing dosimetric and cancer control outcomes after intensity-modulated radiation therapy and tomotherapy for advanced cervical cancer. Oncol. Lett. 2022, 24, 239. [Google Scholar] [CrossRef]
- Imafuku, H.; Ebina, Y.; Suzuki, K.; Wakahashi, S.; Miyahara, Y.; Yoshida, K.; Yamada, H. Definitive radiotherapy in elderly patients and patients with locally advanced cervical cancer with complications. Eur. J. Gynaecol. Oncol. 2020, 41, 60–64. [Google Scholar] [CrossRef]
- Mahantshetty, U.; Krishnatry, R.; Chaudhari, S.; Kanaujia, A.; Engineer, R.; Chopra, S.; Shrivastava, S. Comparison of 2 Contouring Methods of Bone Marrow on CT and Correlation With Hematological Toxicities in Non–Bone Marrow–Sparing Pelvic Intensity-Modulated Radiotherapy With Concurrent Cisplatin for Cervical Cancer. Int. J. Gynecol. Cancer 2012, 22, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Kumar, T.; Schernberg, A.; Busato, F.; Laurans, M.; Fumagalli, I.; Dumas, I.; Deutsch, E.; Haie-Meder, C.; Chargari, C. Correlation between pelvic bone marrow radiation dose and acute hematological toxicity in cervical cancer patients treated with concurrent chemoradiation. Cancer Manag. Res. 2019, 11, 6285–6297. [Google Scholar] [CrossRef]
- Qing, H.; Chuanshu, C.; Weijian, Z.; Shaobin, Z.; Jing, L. Low-dose range of pelvic irradiation leads to acute hematological toxicity in early-stage cervical cancer with intermediate risk factors by postoperative intensity-modulated radiotherapy. Eur. J. Gynaecol. Oncol. 2019, 40, 437–442. [Google Scholar] [CrossRef]
- Aishanjiang, D.; Han, L.; Niyazi, M.; Hou, Q. Clinical analysis of uterine arterial interventional chemoembolization combined with radiotherapy in mid-advanced cervical cancer. J. BUON 2021, 26, 656–662. [Google Scholar]
- Kibaara, M.; Degu, A. Assessment of adverse events among cervical cancer patients at Kenyatta National Hospital. J. Oncol. Pharm. Pract. 2021, 29, 326–332. [Google Scholar] [CrossRef]
- Roszak, A.; Wareńczak-Florczak, K.; Bratos, K.; Milecki, P. Incidence of radiation toxicity in cervical cancer and endometrial cancer patients treated with radiotherapy alone versus adjuvant radiotherapy. Rep. Pract. Oncol. Radiother. 2012, 17, 332–338. [Google Scholar] [CrossRef]
- Yang, X.; Ren, H.; Li, Z.; Zhang, L.; Shao, Y.; Li, H.; Sun, Y.; Zhang, X.; Wang, Z.; Fu, J. A phase III randomized, controlled trial of nedaplatin versus cisplatin concurrent chemoradiotherapy in patients with cervical cancer. ESMO Open 2022, 7, 100565. [Google Scholar] [CrossRef]
- Gullo, G.; Cucinella, G.; Chiantera, V.; Dellino, M.; Cascardi, E.; Török, P.; Herman, T.; Garzon, S.; Uccella, S.; Laganà, A.S. Fertility-Sparing Strategies for Early-Stage Endometrial Cancer: Stepping towards Precision Medicine Based on the Molecular Fingerprint. Int. J. Mol. Sci. 2023, 24, 811. [Google Scholar] [CrossRef]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef]
- Giampaolino, P.; Cafasso, V.; Boccia, D.; Ascione, M.; Mercorio, A.; Viciglione, F.; Palumbo, M.; Serafino, P.; Buonfantino, C.; De Angelis, M.C.; et al. Fertility-Sparing Approach in Patients with Endometrioid Endometrial Cancer Grade 2 Stage IA (FIGO): A Qualitative Systematic Review. BioMed Res. Int. 2022, 2022, 4070368. [Google Scholar] [CrossRef]
| Data Class | |
|---|---|
| 1 | Clinical and Demographical |
| 2 | Pre-treatment tumor pathology features |
| 3 | Treatment modalities |
| 4 | Radiotherapy-related adverse events |
| 5 | Post-treatment characteristics |
| Variable | Age (in yrs) | Weight (in kg) | BMI (in kg/m2) | |
|---|---|---|---|---|
| Mean | 54.19 | 69.47 | 26.33 | |
| Range | 48 | 62 | 20.06 | |
| Minimum | 28 | 40 | 15.63 | |
| Maximum | 76 | 102 | 35.69 | |
| Percentiles | 0.25 | 48.50 | 61.00 | 23.23 |
| 0.33 | 50.14 | 63.28 | 24.64 | |
| 0.50 | 53.00 | 70.00 | 26.56 | |
| 0.67 | 59.00 | 75.00 | 27.86 | |
| 0.75 | 62.50 | 76.00 | 28.90 | |
| Variable | Value | N | % | |
| High blood pressure and/or other CV diseases | Yes | 11 | 15.9 | |
| No | 58 | 84.0 | ||
| DM and/or obesity | Yes | 4 | 5.7 | |
| No | 65 | 94.2 | ||
| CV disease and DM or obesity combined | Yes | 10 | 14.4 | |
| No | 59 | 85.5 | ||
| Other diseases or immunity-related | Yes | 4 | 5.7 | |
| No | 65 | 94.2 | ||
| Characteristic | Value | N | % |
|---|---|---|---|
| Histopathological type | Squamous cell carcinoma | 53 | 94.6 |
| Adenocarcinoma | 3 | 5.4 | |
| Histopathological differentiation | G1 | 1 | 2.4 |
| G2 | 24 | 58.5 | |
| G3 | 16 | 39.0 | |
| Presence of invaded lymph nodes | No | 27 | 46.6 |
| Yes | 31 | 53.4 | |
| Pre-treatment tumor (T) staging | T1a2 | 4 | 7.4 |
| T1b | 2 | 3.7 | |
| T1b1 | 1 | 1.9 | |
| T1b2 | 1 | 1.9 | |
| T1b3 | 1 | 1.9 | |
| T2a | 36 | 66.7 | |
| T2a2 | 6 | 11.1 | |
| T2b | 3 | 5.6 | |
| Pre-treatment nodal status (N) | Nx or N0 | 27 | 46.6 |
| N1 | 26 | 44.8 | |
| N2 | 5 | 8.6 | |
| Pre-treatment AJCC Staging | Stage IB1 | 3 | 5.5 |
| Stage IB2 | 1 | 1.8 | |
| Stage IIB | 2 | 3.6 | |
| Stage III | 20 | 36.4 | |
| Stage IIIC1 | 7 | 12.7 | |
| Stage IIIC2 | 17 | 30.9 | |
| Stage IVA | 3 | 5.5 | |
| Stage IVB | 2 | 3.6 |
| Characteristic | Value | N | % |
|---|---|---|---|
| Chemotherapy | No chemotherapy | 1 | 1.8 |
| Cisplatin | 52 | 92.9 | |
| Carboplatin | 3 | 5.4 | |
| NA | 13 | ||
| Radiotherapy technique | 3D-CRT | 41 | 59.4 |
| VMAT | 18 | 26.1 | |
| Brachytherapy fractions | 0 | 4 | 5.8 |
| 2 | 45 | 65.2 | |
| 3 | 9 | 13.0 | |
| 4 | 1 | 1.4 | |
| Duration (in weeks) of radiotherapy breaks | 0 | 49 | 82.5 |
| 1 | 5 | 8.8 | |
| 2 | 4 | 7.0 | |
| 4 | 1 | 1.8 |
| Characteristic | Value | N | % |
|---|---|---|---|
| General | |||
| Fatigue | No | 21 | 36.2 |
| Yes | 37 | 63.8 | |
| Unknown | 10 | ||
| Genitourinary | |||
| Dysuria | No | 41 | 70.7 |
| Yes | 17 | 29.3 | |
| Unknown | 10 | ||
| Digestive | |||
| Nausea and vomiting | No | 24 | 41.4 |
| Yes | 34 | 58.6 | |
| Unknown | 10 | ||
| Diarrhea | No | 23 | 39.7 |
| Yes | 35 | 60.3 | |
| Unknown | 10 | ||
| Hematological | |||
| Anemia | No | 15 | 22.1 |
| Yes | 53 | 77.9 | |
| Thrombocytopenia | No | 43 | 63.2 |
| Yes | 25 | 36.8 | |
| Neutropenia | No | 39 | 57.4 |
| Yes | 29 | 42.6 | |
| Leukopenia | No | 23 | 33.8 |
| Yes | 45 | 66.2 | |
| Variable | OR | 95%CI | p | RR | 95%CI |
|---|---|---|---|---|---|
| Neutropenia with a minimal neutrophils level ≤ 2.2; (VN: 2.0–7.7) (in 103/μL) during follow-up | |||||
| Planned dose for pelvic region volume = 54 Gy | 6.82 | (1.15, 40.41) | 0.034 | 2.56 | (1.53, 4.29) |
| Thrombocytopenia with a minimal platelets level ≤ 131.00; (VN: 150–400) (in 103/μL) during follow-up | |||||
| Planned dose for pelvic region volume = 54 Gy | 6.67 | (1.22, 36.59) | 0.029 | 1.95 | (1.34, 2.83) |
| Neutropenia during follow-up | |||||
| Rectal V45 as planned within VMAT > 63.43% | 18.00 | (1.19, 271.46) | 0.037 | 5.26 | (2.86, 9.67) |
| Bladder V50 as planned within VMAT > 10.69% | 16.50 | (1.09, 250.18) | 0.043 | 4.88 | (2.66, 8.93) |
| Bowel V45 as planned within VMAT > 137.38 cc | 16.50 | (1.09, 250.18) | 0.043 | 4.88 | (2.66, 8.93) |
| Maximum drop of leukocytes level in less than 35 days from RT initiation | |||||
| Radiotherapy technique = 3D-CRT | 4.44 | (1.25, 15.82) | 0.021 | 1.94 | (1.00, 3.78) |
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 4.12 | (1.20, 14.14) | 0.025 | 1.75 | (1.04, 2.93) |
| Planned dose for pelvic region volume = 54 Gy | 4.00 | (1.19, 13.50) | 0.025 | 1.75 | (1.02, 3.00) |
| Variable | OR | 95%CI | p | RR | 95%CI |
|---|---|---|---|---|---|
| Maximum Hb variation > 2.50 g/dL | |||||
| BMI > 23.23 kg/m2 | 8.68 | (1.01, 75.01) | 0.049 | 5.29 | (2.31, 12.14) |
| Age > 53 yrs | 4.60 | (1.10, 19.22) | 0.036 | 2.99 | (1.84, 4.86) |
| Radiotherapy technique 3D-CRT | 7.25 | (1.73, 30.38) | 0.007 | 4.13 | (2.63, 6.49) |
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 4.71 | (1.09, 20.47) | 0.038 | 3.26 | (1.95, 5.44) |
| Per day maximum Hb variation rate > 0.03 g/dL/day | |||||
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 11.81 | (1.35, 103.04) | 0.025 | 1.49 | (1.31, 1.71) |
| Planned dose for pelvic region volume = 54 Gy | 10.56 | (1.22, 91.01) | 0.032 | 1.48 | (1.30, 1.68) |
| Per dose maximum Hb variation rate > 0.04 g/dL/Gy | |||||
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 10.50 | (2.00, 55.03) | 0.005 | 1.82 | (1.52, 2.19) |
| Planned dose for pelvic region volume = 54 Gy | 8.82 | (1.71, 45.52) | 0.009 | 1.75 | (1.47, 2.07) |
| Planned radiotherapy fractions > 28.00 fractions | 8.82 | (1.71, 45.52) | 0.009 | 1.75 | (1.47, 2.07) |
| Per dose maximum Hb variation rate > 0.08 g/dL/fraction | |||||
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 8.89 | (1.69, 46.63) | 0.01 | 1.69 | (1.42, 2.00) |
| Planned dose for pelvic region volume = 54 Gy | 7.60 | (1.47, 39.29) | 0.016 | 1.63 | (1.39, 1.91) |
| Maximum Hb variation in less than 29 days from RT initiation | |||||
| Planned dose for pelvic region volume = 54 Gy | 6.76 | (1.88, 24.29) | 0.003 | 2.85 | (1.27, 6.38) |
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 6.02 | (1.72, 21.10) | 0.005 | 2.61 | (1.24, 5.46) |
| Maximum Hb variation in less than 35 days from the RT initiation | |||||
| Planned dose for pelvic region volume > 50.40 Gy | 11.11 | (2.54, 48.66) | 0.001 | 5.32 | (3.25, 8.71) |
| Total EQD2 > 66.1 Gy(CTV includes lomboaortic lymph nodes) | 8.00 | (1.86, 34.36) | 0.005 | 4.35 | (2.66, 7.11) |
| Radiotherapy technique (1—VMAT, 2—3D-CRT) | 3.71 | (1.03, 13.46) | 0.035 | 2.36 | (1.65, 3.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinescu, Ș.A.; Toma, R.-V.; Trifănescu, O.G.; Galeș, L.N.; Folea, A.R.; Sima, A.; Bîlteanu, L.; Anghel, R. Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis. Cancers 2024, 16, 3032. https://doi.org/10.3390/cancers16173032
Marinescu ȘA, Toma R-V, Trifănescu OG, Galeș LN, Folea AR, Sima A, Bîlteanu L, Anghel R. Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis. Cancers. 2024; 16(17):3032. https://doi.org/10.3390/cancers16173032
Chicago/Turabian StyleMarinescu, Șerban Andrei, Radu-Valeriu Toma, Oana Gabriela Trifănescu, Laurenția Nicoleta Galeș, Antonia Ruxandra Folea, Adrian Sima, Liviu Bîlteanu, and Rodica Anghel. 2024. "Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis" Cancers 16, no. 17: 3032. https://doi.org/10.3390/cancers16173032
APA StyleMarinescu, Ș. A., Toma, R.-V., Trifănescu, O. G., Galeș, L. N., Folea, A. R., Sima, A., Bîlteanu, L., & Anghel, R. (2024). Predictors of Clinical Hematological Toxicities under Radiotherapy in Patients with Cervical Cancer—A Risk Analysis. Cancers, 16(17), 3032. https://doi.org/10.3390/cancers16173032






