Head-to-Head Comparison of [18F]FDG PET Imaging and MRI for the Detection of Recurrence or Residual Tumor in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search and Inclusion Criteria
2.2. Study Selection
2.3. Data Extraction
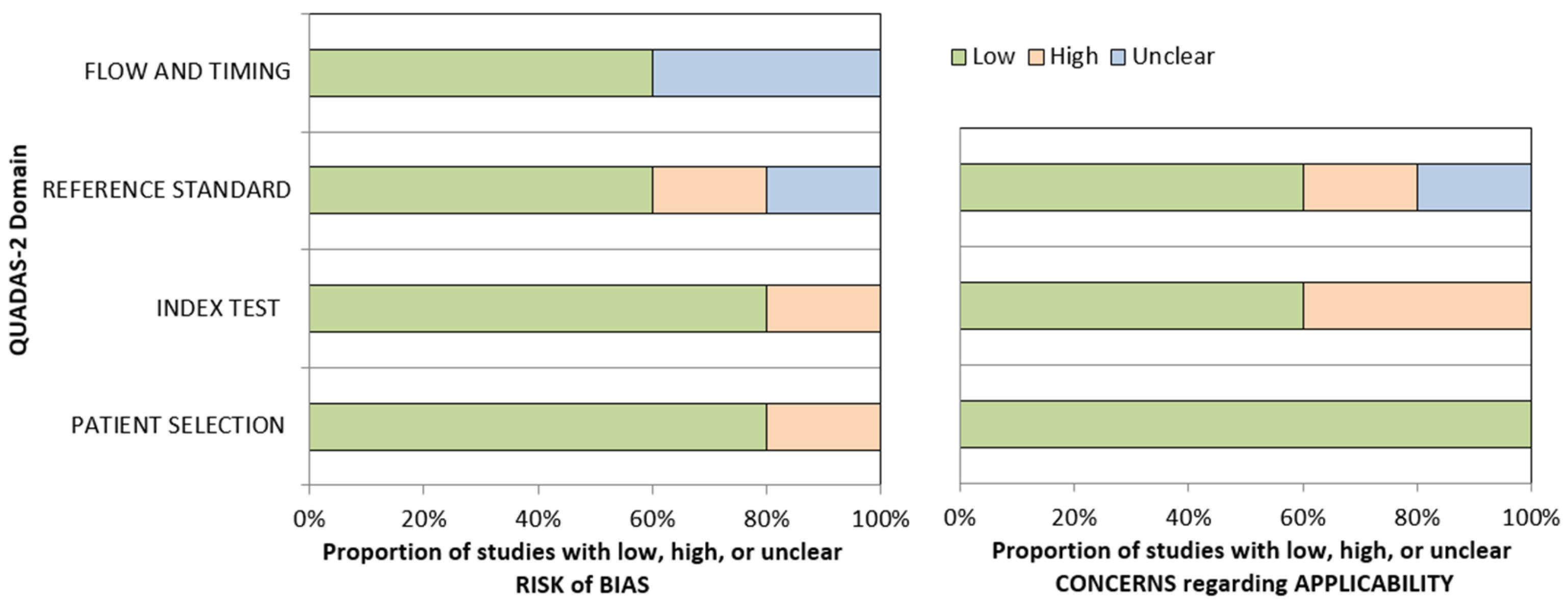
2.4. Methodological Quality Assessment
2.5. Statistical Analysis
3. Results
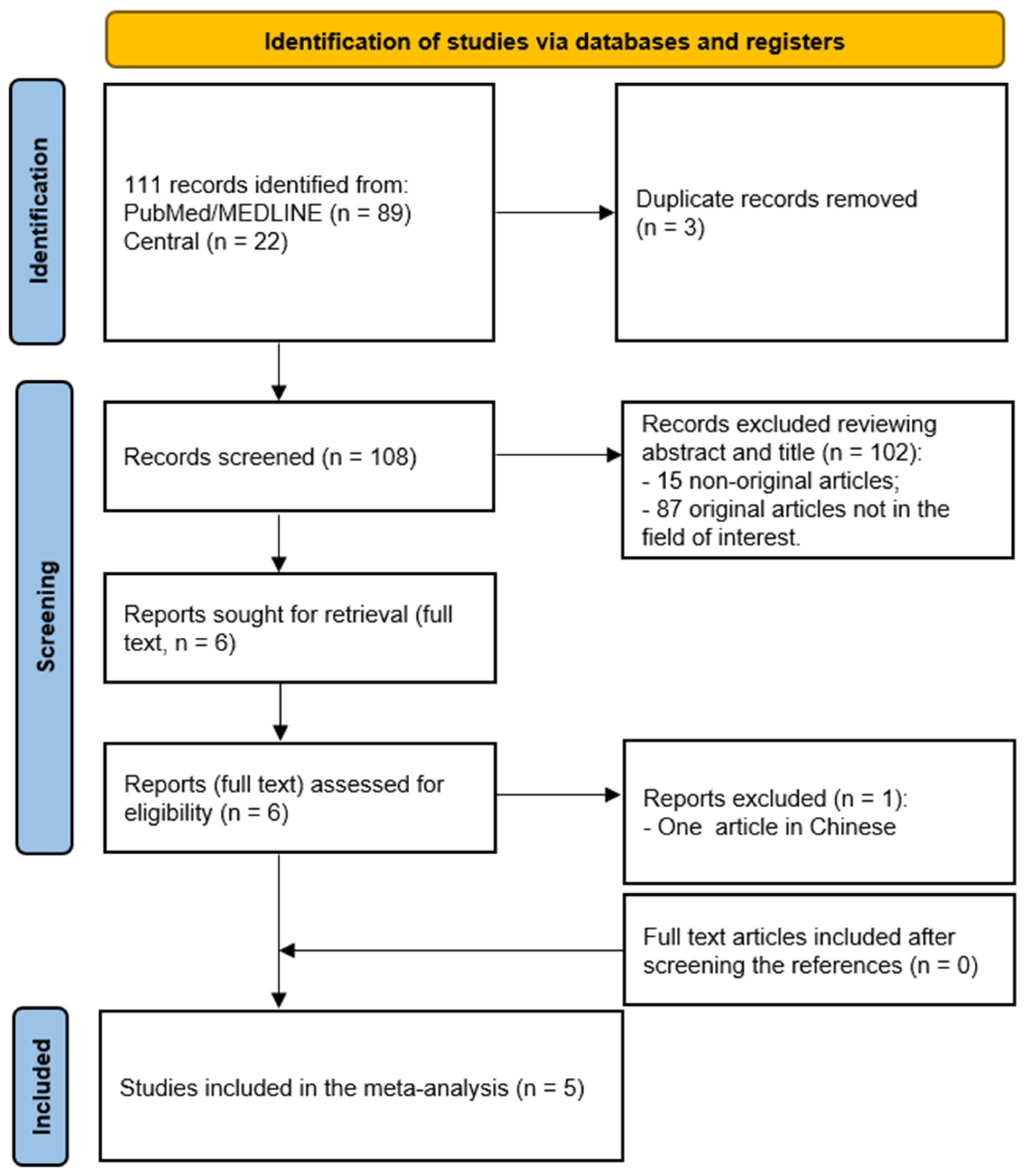
3.1. Literature Search
3.2. Qualitative Analysis of the Studies
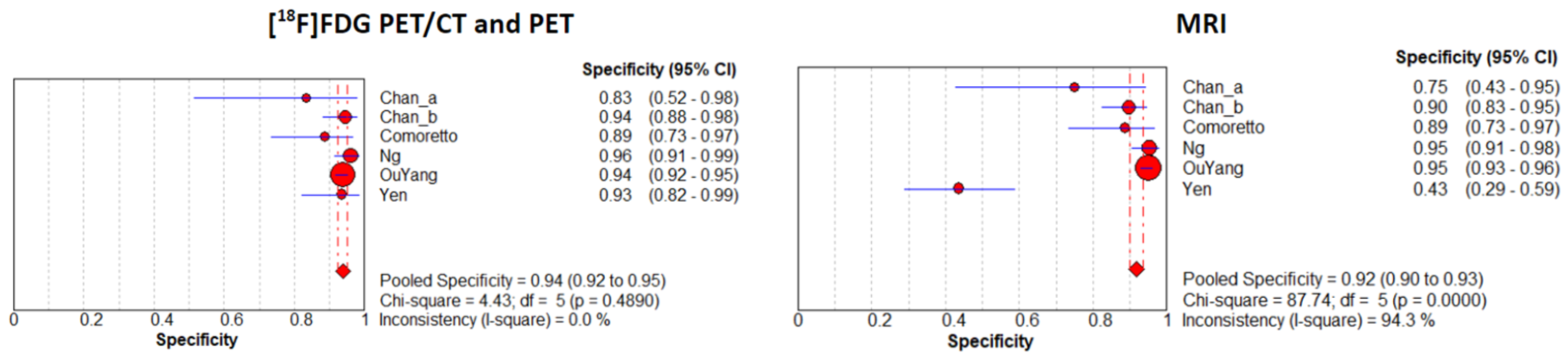
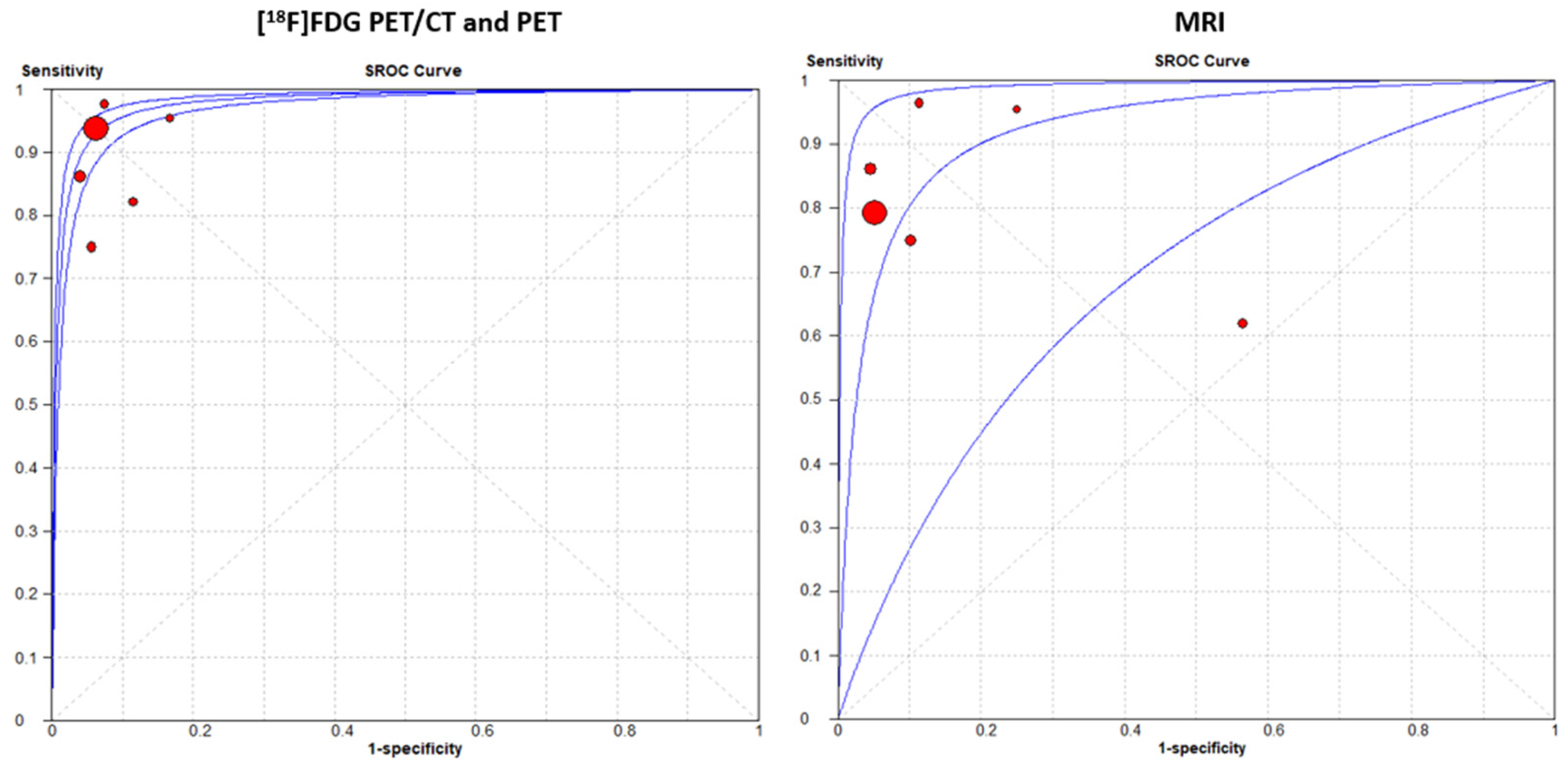
3.3. Meta-Analysis: Diagnostic Performance of PET Imaging and MRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, W.S.; Li, J.J.; Hong, L.; Xing, Z.B.; Wang, F.; Li, C.Q. Comparison of mri, ct and 18f-fdg pet/ct in the diagnosis of local and metastatic of nasopharyngeal carcinomas: An updated meta analysis of clinical studies. Am. J. Transl. Res. 2016, 8, 4532–4547. [Google Scholar] [PubMed]
- Chan, A.T.; Teo, P.M.; Johnson, P.J. Nasopharyngeal carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2002, 13, 1007–1015. [Google Scholar] [CrossRef]
- Sabarimurugan, S.; Kumarasamy, C.; Baxi, S.; Devi, A.; Jayaraj, R. Systematic review and meta-analysis of prognostic microrna biomarkers for survival outcome in nasopharyngeal carcinoma. PLoS ONE 2019, 14, e0209760. [Google Scholar] [CrossRef]
- Juarez-Vignon Whaley, J.J.; Afkhami, M.; Onyshchenko, M.; Massarelli, E.; Sampath, S.; Amini, A.; Bell, D.; Villaflor, V.M. Recurrent/metastatic nasopharyngeal carcinoma treatment from present to future: Where are we and where are we heading? Curr. Treat. Options Oncol. 2023, 24, 1138–1166. [Google Scholar] [CrossRef]
- Lee, A.W.; Sze, W.M.; Au, J.S.; Leung, S.F.; Leung, T.W.; Chua, D.T.; Zee, B.C.; Law, S.C.; Teo, P.M.; Tung, S.Y.; et al. Treatment results for nasopharyngeal carcinoma in the modern era: The hong kong experience. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 1107–1116. [Google Scholar] [CrossRef]
- Xu, T.; Tang, J.; Gu, M.; Liu, L.; Wei, W.; Yang, H. Recurrent nasopharyngeal carcinoma: A clinical dilemma and challenge. Curr. Oncol. 2013, 20, e406–e419. [Google Scholar] [CrossRef]
- Li, J.X.; Lu, T.X.; Huang, Y.; Han, F. Clinical characteristics of recurrent nasopharyngeal carcinoma in high-incidence area. Sci. World J. 2012, 2012, 719754. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Fan, R.; Gao, K.; Xie, S.; Wang, F.; Zhang, J.; Zhang, H.; He, Y.; Xie, Z.; et al. Treatment of recurrent nasopharyngeal carcinoma: A sequential challenge. Cancers 2022, 14, 4111. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.T.; Wong, E.C.Y.; Cheung, A.K.W.; Chow, J.C.H.; Poon, D.M.C.; Lai, J.W.Y.; Chiang, C.L.; Choi, H.C.W.; Chau, T.C.; Lee, V.H.F.; et al. Patterns of care and treatment outcomes for local recurrence of npc after definite imrt-a study by the hknpcsg. Head Neck 2019, 41, 3661–3669. [Google Scholar] [CrossRef]
- Jones, T.; Townsend, D. History and future technical innovation in positron emission tomography. J. Med. Imaging 2017, 4, 011013. [Google Scholar] [CrossRef]
- Chikui, T.; Obara, M.; Simonetti, A.W.; Ohga, M.; Koga, S.; Kawano, S.; Matsuo, Y.; Kamintani, T.; Shiraishi, T.; Kitamoto, E.; et al. The principal of dynamic contrast enhanced mri, the method of pharmacokinetic analysis, and its application in the head and neck region. Int. J. Dent. 2012, 2012, 480659. [Google Scholar] [CrossRef]
- Chan, S.C.; Ng, S.H.; Chang, J.T.; Lin, C.Y.; Chen, Y.C.; Chang, Y.C.; Hsu, C.L.; Wang, H.M.; Liao, C.T.; Yen, T.C. Advantages and pitfalls of 18f-fluoro-2-deoxy-d-glucose positron emission tomography in detecting locally residual or recurrent nasopharyngeal carcinoma: Comparison with magnetic resonance imaging. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1032–1040. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. De Cardiol. (English ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. Quadas-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Comoretto, M.; Balestreri, L.; Borsatti, E.; Cimitan, M.; Franchin, G.; Lise, M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: Comparison of mr imaging and fdg pet/ct. Radiology 2008, 249, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.H.; Chan, S.C.; Yen, T.C.; Liao, C.T.; Chang, J.T.; Ko, S.F.; Wang, H.M.; Lin, C.Y.; Chang, K.P.; Lin, Y.C. Comprehensive imaging of residual/recurrent nasopharyngeal carcinoma using whole-body mri at 3 t compared with fdg-pet-ct. Eur. Radiol. 2010, 20, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- OuYang, P.Y.; Liu, Z.Q.; Lin, Q.G.; He, Y.; Guo, Z.X.; Yao, W.Y.; Xu, S.K.; Peng, Q.H.; Xiao, S.M.; Li, J.; et al. Benefit of [(18)f] fdg pet/ct in the diagnosis and salvage treatment of recurrent nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 881–891. [Google Scholar] [CrossRef]
- Yen, R.F.; Hung, R.L.; Pan, M.H.; Wang, Y.H.; Huang, K.M.; Lui, L.T.; Kao, C.H. 18-fluoro-2-deoxyglucose positron emission tomography in detecting residual/recurrent nasopharyngeal carcinomas and comparison with magnetic resonance imaging. Cancer 2003, 98, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.H.; Joseph, C.T.; Chan, S.C.; Ko, S.F.; Wang, H.M.; Liao, C.T.; Chang, Y.C.; Lin, W.J.; Fu, Y.K.; Yen, T.C. Clinical usefulness of 18f-fdg pet in nasopharyngeal carcinoma patients with questionable mri findings for recurrence. J. Nucl. Med. 2004, 45, 1669–1676. [Google Scholar]
- Gatta, G. Epidemiological aspects in nasopharyngeal cancer. In Critical Issues in Head and Neck Oncology; Vermorken, J.B., Budach, V., Leemans, C.R., Machiels, J.-P., Nicolai, P., O’Sullivan, B., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 319–325. [Google Scholar]
- Boussen, H.; Bouaouina, N.; Mokni-Baizig, N.; Gamoudi, A.; Chouchane, L.; Benna, F.; Ladgham, A. [Nasopharyngeal carcinoma. Recent data]. Pathol. Biol. 2005, 53, 45–51. [Google Scholar] [CrossRef]
- Okekpa, S.I.; Mydin, R.B.S.M.N.; Mangantig, E.; Azmi, N.S.A.; Zahari, S.N.S.; Kaur, G.; Musa, Y. Nasopharyngeal carcinoma (npc) risk factors: A systematic review and meta-analysis of the association with lifestyle, diets, socioeconomic and sociodemographic in asian region. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3505–3514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Shen, G.; Zhang, W.; Cai, H.; Zhou, Y.; Li, L. 18f-fdg pet/ct for the diagnosis of residual or recurrent nasopharyngeal carcinoma after radiotherapy: A metaanalysis. J. Nucl. Med. 2016, 57, 342–347. [Google Scholar] [CrossRef]
- Cammaroto, G.; Quartuccio, N.; Sindoni, A.; Di Mauro, F.; Caobelli, F.; Young, A.W.G. The role of pet/ct in the management of patients affected by head and neck tumors: A review of the literature. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 1961–1973. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Master, Z.; Kannan, S.; Agarwal, J.P.; Ghsoh-Laskar, S.; Rangarajan, V.; Murthy, V.; Budrukkar, A. Diagnostic performance of post-treatment fdg pet or fdg pet/ct imaging in head and neck cancer: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 2083–2095. [Google Scholar] [CrossRef] [PubMed]

| Authors | Year | Country | Journal | Study Design | Number Patients | Interval from Completion of Therapy to Imaging (Months) | Mean Age | Sex (% Male) |
|---|---|---|---|---|---|---|---|---|
| Chan_a [12] | 2006 | China | Eur J Nucl Med | P | 34 | 3 | 48 ± 11 | 22/34 |
| Chan_b [12] | 112 | 3 | 48 ± 12 | 81/112 | ||||
| Comoretto [15] | 2008 | Italy | Radiology | R | 63 | 2–14 | 52 | 44/63 |
| Ng [16] | 2010 | Taiwan | Eur Radiol | P | 179 | 3–56 | 47.9 | 136/179 |
| OuYang [17] | 2023 | China | Eur J Nucl Med | R | 1453 | >6 | 47 | 1104/1453 |
| Yen [18] | 2003 | China | Cancer | R | 67 | 4–70 | 46.6 ± 12.5 | 53/67 |
| Authors | Setting | PET or PET/CT | MRI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| TP | TN | FP | FN | TP | TN | FP | FN | ||
| Chan_a [12] | Detection of recurrence | 21 | 10 | 2 | 1 | 21 | 9 | 3 | 1 |
| Chan_b [12] | Response assessment 3 months after therapy | 3 | 102 | 6 | 1 | 3 | 97 | 11 | 1 |
| Comoretto [15] | Detection of recurrence or residual tumor | 23 | 31 | 4 | 5 | 27 | 31 | 4 | 1 |
| Ng [16] | Detection of recurrence or residual tumor | 25 | 144 | 6 | 4 | 25 | 143 | 7 | 4 |
| OuYang [17] | Detection of recurrence | 658 | 705 | 47 | 43 | 556 | 713 | 39 | 145 |
| Yen [18] | Detection of recurrence or residual tumor | 21 | 43 | 3 | 0 | 13 | 20 | 26 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quartuccio, N.; Pulizzi, S.; Modica, D.M.; Nicolosi, S.; D’Oppido, D.; Moreci, A.M.; Ialuna, S. Head-to-Head Comparison of [18F]FDG PET Imaging and MRI for the Detection of Recurrence or Residual Tumor in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. Cancers 2024, 16, 3011. https://doi.org/10.3390/cancers16173011
Quartuccio N, Pulizzi S, Modica DM, Nicolosi S, D’Oppido D, Moreci AM, Ialuna S. Head-to-Head Comparison of [18F]FDG PET Imaging and MRI for the Detection of Recurrence or Residual Tumor in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. Cancers. 2024; 16(17):3011. https://doi.org/10.3390/cancers16173011
Chicago/Turabian StyleQuartuccio, Natale, Sabina Pulizzi, Domenico Michele Modica, Stefania Nicolosi, Dante D’Oppido, Antonino Maria Moreci, and Salvatore Ialuna. 2024. "Head-to-Head Comparison of [18F]FDG PET Imaging and MRI for the Detection of Recurrence or Residual Tumor in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis" Cancers 16, no. 17: 3011. https://doi.org/10.3390/cancers16173011
APA StyleQuartuccio, N., Pulizzi, S., Modica, D. M., Nicolosi, S., D’Oppido, D., Moreci, A. M., & Ialuna, S. (2024). Head-to-Head Comparison of [18F]FDG PET Imaging and MRI for the Detection of Recurrence or Residual Tumor in Patients with Nasopharyngeal Carcinoma: A Meta-Analysis. Cancers, 16(17), 3011. https://doi.org/10.3390/cancers16173011








