Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
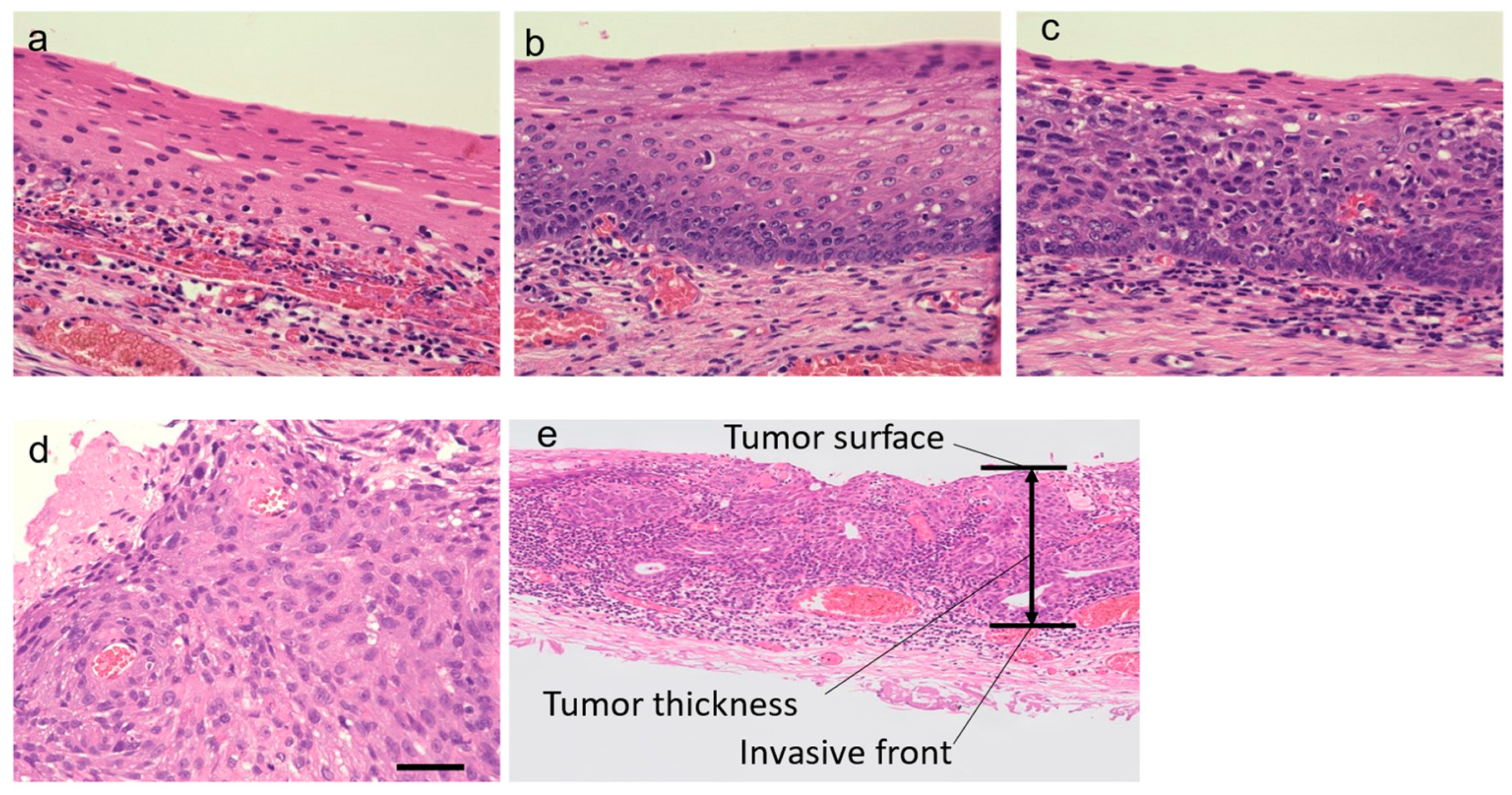
2.2. Histological Evaluation
2.3. Immunofluorescence Analysis
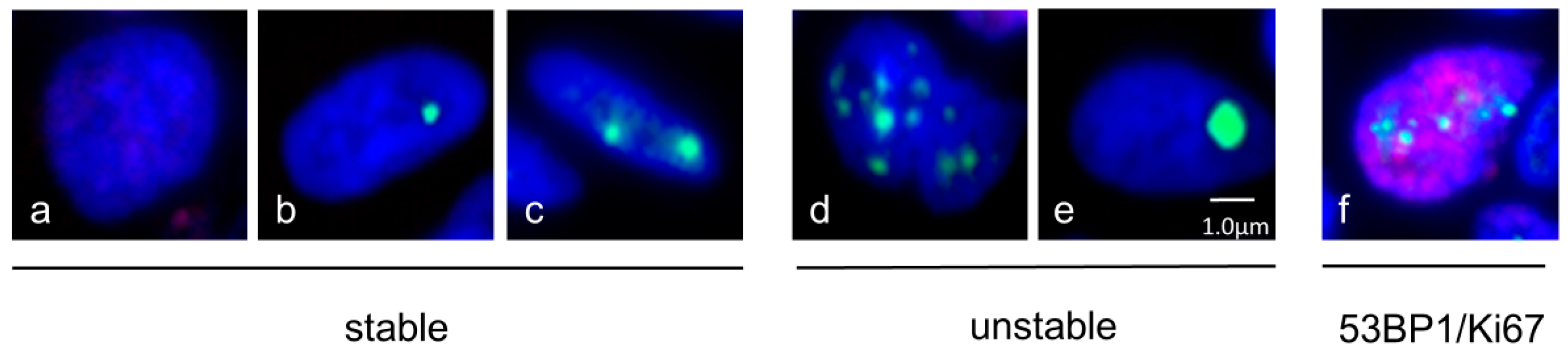
2.4. Quantification of 53BP1 Nuclear Expression Pattern
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
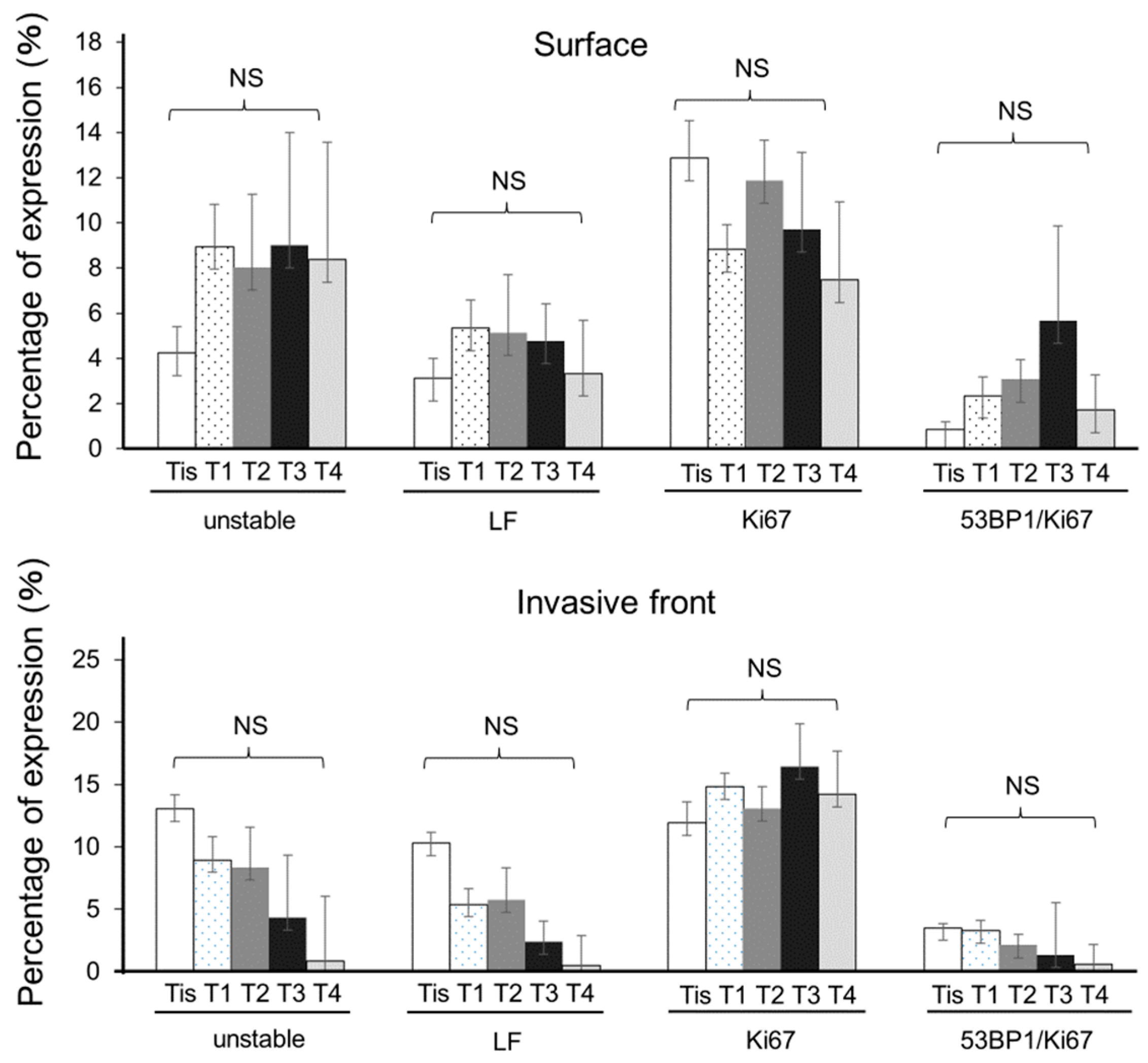
3.2. Progressive Increments in 53BP1 Nuclear Foci at the Tumor Surface during Hypopharyngeal Carcinogenesis
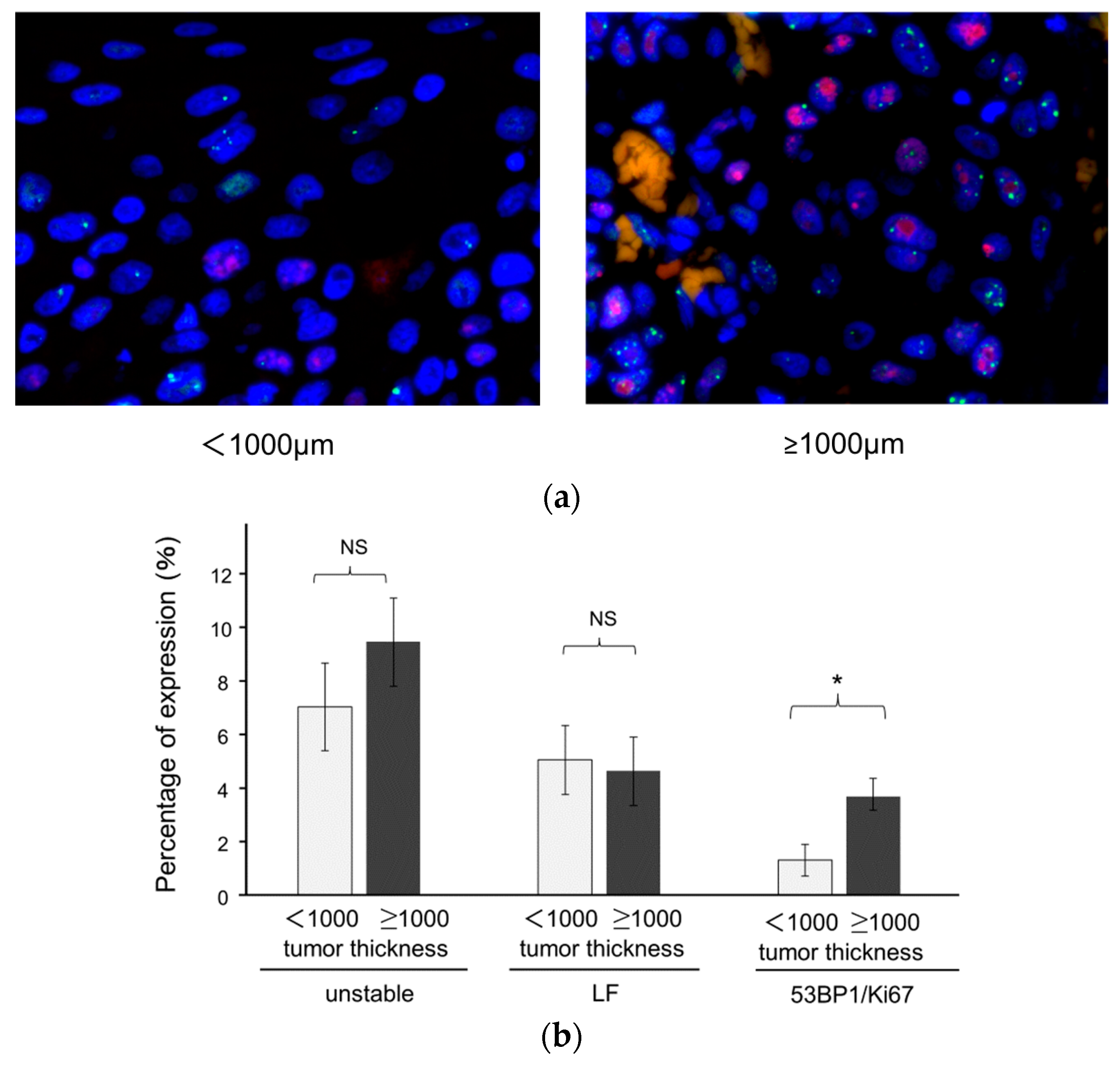
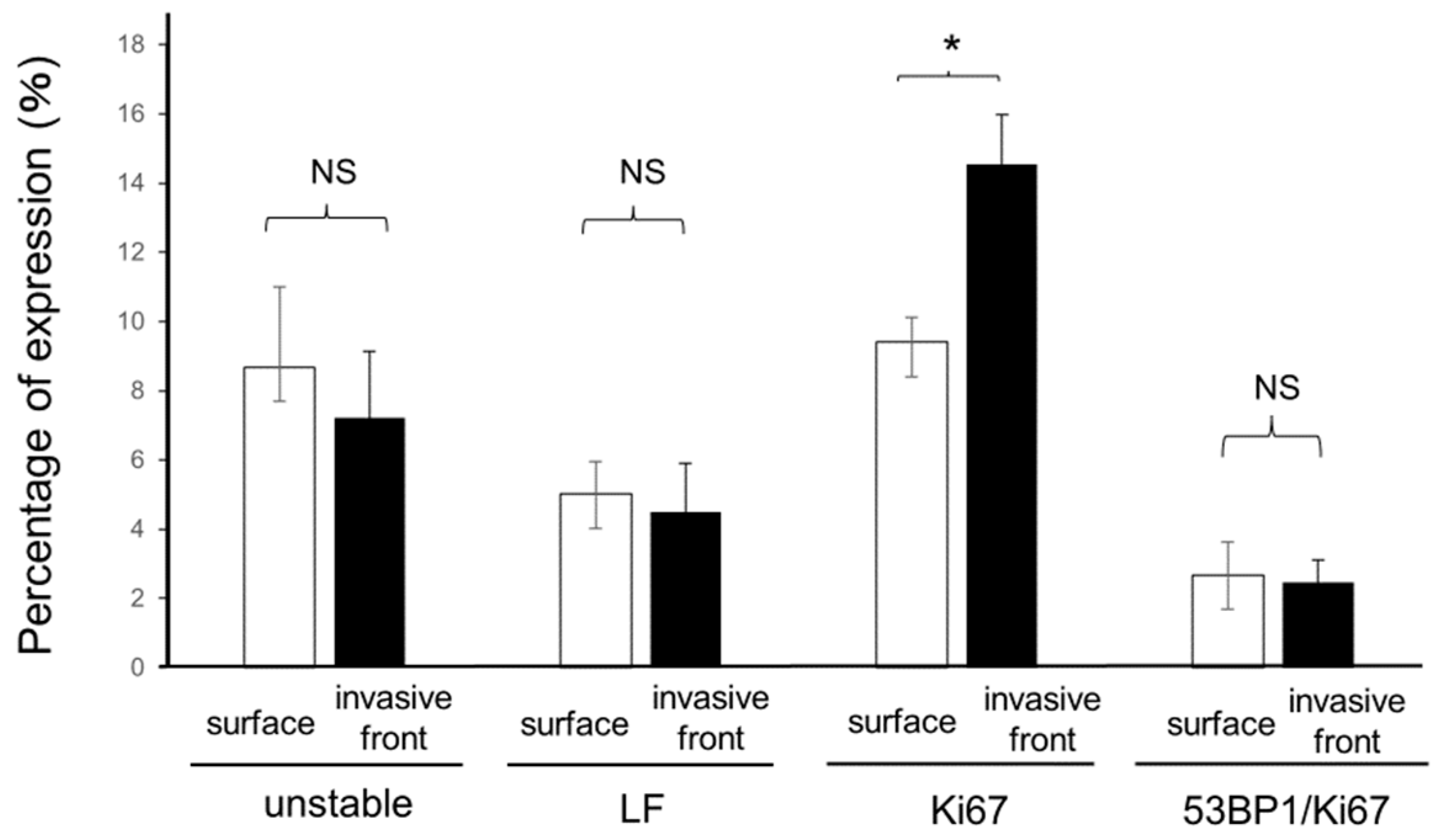
3.3. Association of 53BP1 Nuclear Foci with Tumor Stage and Depth
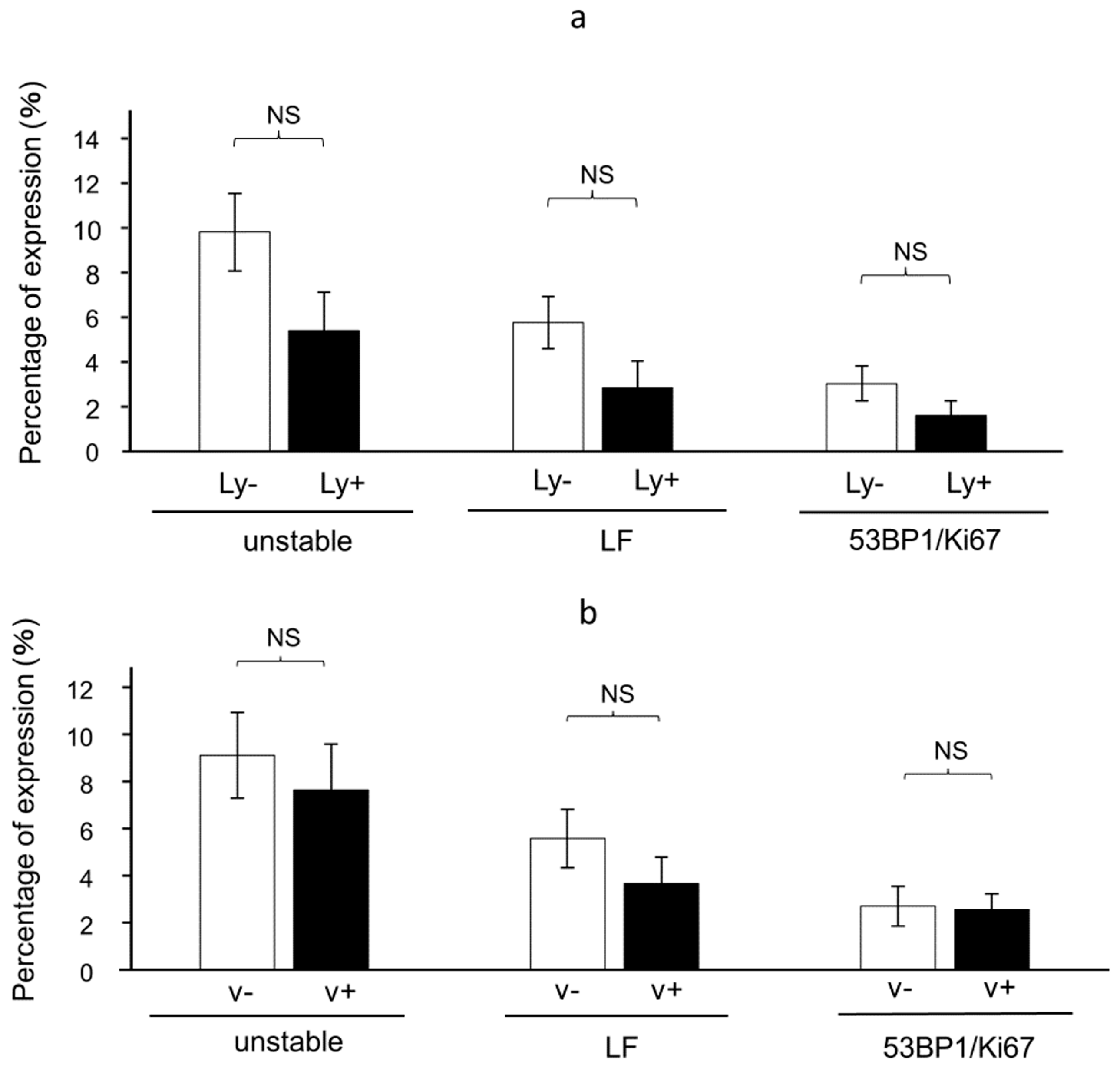
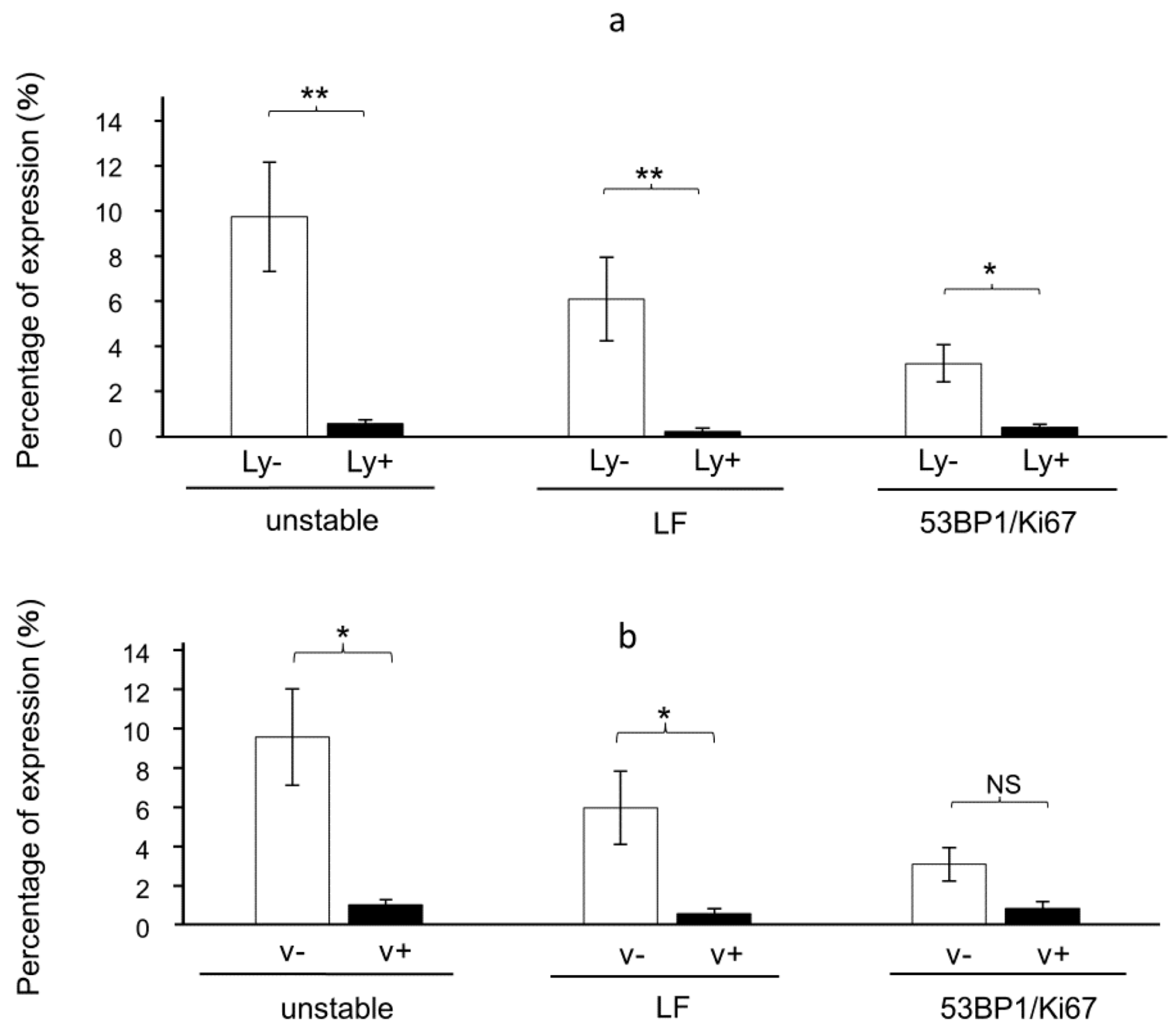
3.4. The Presence of 53BP1 Nuclear Foci at the Invasive Front Is Inversely Correlated with Lymphovascular Invasion in HPSCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and Neck Squamous Cell Carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global Cancer Statistics, 2002. CA A Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Rho, Y.-S.; Choi, E.-C.; Kim, M.-S.; Woo, J.-H.; Lee, D.H.; Chung, E.J.; Park, M.W.; Kim, D.-H.; Joo, Y.-H. Clinicopathological Factors Influencing the Outcomes of Surgical Treatment in Patients with T4a Hypopharyngeal Cancer. BMC Cancer 2017, 17, 904. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Nakane, M.; Katada, C.; Sano, Y.; Ohtsu, A.; Esumi, H.; Ebihara, S.; Yoshida, S. Squamous Cell Carcinoma in Situ at Oropharyngeal and Hypopharyngeal Mucosal Sites. Cancer 2004, 101, 1375–1381. [Google Scholar] [CrossRef]
- Katada, C.; Tanabe, S.; Koizumi, W.; Higuchi, K.; Sasaki, T.; Azuma, M.; Katada, N.; Masaki, T.; Nakayama, M.; Okamoto, M.; et al. Narrow Band Imaging for Detecting Superficial Squamous Cell Carcinoma of the Head and Neck in Patients with Esophageal Squamous Cell Carcinoma. Endoscopy 2010, 42, 185–190. [Google Scholar] [CrossRef]
- Gale, N.; Poljak, M.; Zidar, N. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: What Is New in the 2017 WHO Blue Book for Tumours of the Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol. 2017, 11, 23–32. [Google Scholar] [CrossRef]
- Zidar, N.; Gale, N. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Hypopharynx, Larynx, Trachea and Parapharyngeal Space. Head Neck Pathol. 2022, 16, 31–39. [Google Scholar] [CrossRef]
- Imaizumi, T.; Matsuda, K.; Tanaka, K.; Kondo, H.; Ueki, N.; Kurohama, H.; Otsubo, C.; Matsuoka, Y.; Akazawa, Y.; Miura, S.; et al. Detection of Endogenous DNA Double-Strand Breaks in Oral Squamous Epithelial Lesions by P53-Binding Protein 1. Anticancer. Res. 2021, 41, 4771–4779. [Google Scholar] [CrossRef]
- Schimberg, A.S.; Wellenstein, D.J.; Schutte, H.W.; Honings, J.; van den Hoogen, F.J.A.; Marres, H.A.M.; Takes, R.P.; van den Broek, G.B. Flexible Endoscopic Biopsy: Identifying Factors to Increase Accuracy in Diagnosing Benign and Malignant Laryngopharyngeal Pathology. J. Voice 2022, 36, 128–133. [Google Scholar] [CrossRef]
- Yoshimura, N.; Goda, K.; Tajiri, H.; Yoshida, Y.; Kato, T.; Seino, Y.; Ikegami, M.; Urashima, M. Diagnostic Utility of Narrow-Band Imaging Endoscopy for Pharyngeal Superficial Carcinoma. World J. Gastroenterol. 2011, 17, 4999–5006. [Google Scholar] [CrossRef]
- Dixon, M.F. Gastrointestinal Epithelial Neoplasia: Vienna Revisited. Gut 2002, 51, 130–131. [Google Scholar] [CrossRef]
- Dawsey, S.M.; Lewin, K.J.; Wang, G.Q.; Liu, F.S.; Nieberg, R.K.; Yu, Y.; Li, J.Y.; Blot, W.J.; Li, B.; Taylor, P.R. Squamous Esophageal Histology and Subsequent Risk of Squamous Cell Carcinoma of the Esophagus. A Prospective Follow-up Study from Linxian, China. Cancer 1994, 74, 1686–1692. [Google Scholar] [CrossRef]
- Taniguchi, M.; Watanabe, A.; Tsujie, H.; Tomiyama, T.; Fujita, M.; Hosokawa, M.; Sasaki, S. Predictors of Cervical Lymph Node Involvement in Patients with Pharyngeal Carcinoma Undergoing Endoscopic Mucosal Resection. Auris Nasus Larynx 2011, 38, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Kinjo, Y.; Nonaka, S.; Oda, I.; Abe, S.; Suzuki, H.; Yoshinaga, S.; Maki, D.; Yoshimoto, S.; Taniguchi, H.; Saito, Y. The Short-Term and Long-Term Outcomes of the Endoscopic Resection for the Superficial Pharyngeal Squamous Cell Carcinoma. Endosc. Int. Open 2015, 3, E266–E273. [Google Scholar] [CrossRef]
- Sasaki, T.; Kishimoto, S.; Kawabata, K.; Sato, Y.; Tsuchida, T. Risk Factors for Cervical Lymph Node Metastasis in Superficial Head and Neck Squamous Cell Carcinoma. J. Med. Dent. Sci. 2015, 62, 19–24. [Google Scholar] [CrossRef]
- Zhu, C.; Mills, K.D.; Ferguson, D.O.; Lee, C.; Manis, J.; Fleming, J.; Gao, Y.; Morton, C.C.; Alt, F.W. Unrepaired DNA Breaks in P53-Deficient Cells Lead to Oncogenic Gene Amplification Subsequent to Translocations. Cell 2002, 109, 811–821. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic Instability--an Evolving Hallmark of Cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Tang, M.-S.; Lee, H.-W.; Weng, M.-W.; Wang, H.-T.; Hu, Y.; Chen, L.-C.; Park, S.-H.; Chan, H.-W.; Xu, J.; Wu, X.-R.; et al. DNA Damage, DNA Repair and Carcinogenicity: Tobacco Smoke versus Electronic Cigarette Aerosol. Mutat. Res.-Rev. Mut. Res. 2022, 789, 108409. [Google Scholar] [CrossRef] [PubMed]
- Dylawerska, A.; Barczak, W.; Wegner, A.; Golusinski, W.; Suchorska, W.M. Association of DNA Repair Genes Polymorphisms and Mutations with Increased Risk of Head and Neck Cancer: A Review. Med. Oncol. 2017, 34, 197. [Google Scholar] [CrossRef] [PubMed]
- Tubbs, A.; Nussenzweig, A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell 2017, 168, 644–656. [Google Scholar] [CrossRef]
- Bork, P.; Hofmann, K.; Bucher, P.; Neuwald, A.F.; Altschul, S.F.; Koonin, E.V. A Superfamily of Conserved Domains in DNA Damage-Responsive Cell Cycle Checkpoint Proteins. FASEB J. 1997, 11, 68–76. [Google Scholar] [CrossRef]
- Ward, I.M.; Minn, K.; Jorda, K.G.; Chen, J. Accumulation of Checkpoint Protein 53BP1 at DNA Breaks Involves Its Binding to Phosphorylated Histone H2AX. J. Biol. Chem. 2003, 278, 19579–19582. [Google Scholar] [CrossRef]
- Schultz, L.B.; Chehab, N.H.; Malikzay, A.; Halazonetis, T.D. P53 Binding Protein 1 (53BP1) Is an Early Participant in the Cellular Response to DNA Double-Strand Breaks. J. Cell Biol. 2000, 151, 1381–1390. [Google Scholar] [CrossRef]
- Ueki, N.; Akazawa, Y.; Miura, S.; Matsuda, K.; Kurohama, H.; Imaizumi, T.; Kondo, H.; Nakashima, M. Significant Association between 53 BP1 Expression and Grade of Intraepithelial Neoplasia of Esophagus: Alteration during Esophageal Carcinogenesis. Pathol. Res. Pract. 2019, 215, 152601. [Google Scholar] [CrossRef]
- Matsuda, K.; Miura, S.; Kurashige, T.; Suzuki, K.; Kondo, H.; Ihara, M.; Nakajima, H.; Masuzaki, H.; Nakashima, M. Significance of P53-Binding Protein 1 Nuclear Foci in Uterine Cervical Lesions: Endogenous DNA Double Strand Breaks and Genomic Instability during Carcinogenesis. Histopathology 2011, 59, 441–451. [Google Scholar] [CrossRef]
- Nakashima, M.; Suzuki, K.; Meirmanov, S.; Naruke, Y.; Matsuu-Matsuyama, M.; Shichijo, K.; Saenko, V.; Kondo, H.; Hayashi, T.; Ito, M.; et al. Foci Formation of P53-Binding Protein 1 in Thyroid Tumors: Activation of Genomic Instability during Thyroid Carcinogenesis. Int. J. Cancer 2008, 122, 1082–1088. [Google Scholar] [CrossRef]
- Iritani, K.; Del Mundo, D.A.A.; Iwaki, S.; Masuda, K.; Kanzawa, M.; Furukawa, T.; Teshima, M.; Shinomiya, H.; Morimoto, K.; Otsuki, N.; et al. Prognostic Factors after Transoral Resection of Early Hypopharyngeal Cancer. Laryngoscope Investig. Otolaryngol. 2021, 6, 756–763. [Google Scholar] [CrossRef]
- Akazawa, Y.; Nakashima, R.; Matsuda, K.; Okamaoto, K.; Hirano, R.; Kawasaki, H.; Miuma, S.; Miyaaki, H.; Malhi, H.; Abiru, S.; et al. Detection of DNA Damage Response in Nonalcoholic Fatty Liver Disease via P53-Binding Protein 1 Nuclear Expression. Mod. Pathol. 2019, 32, 997–1007. [Google Scholar] [CrossRef]
- Matsuda, K.; Kawasaki, T.; Akazawa, Y.; Hasegawa, Y.; Kondo, H.; Suzuki, K.; Iseki, M.; Nakashima, M. Expression Pattern of P53-Binding Protein 1 as a New Molecular Indicator of Genomic Instability in Bladder Urothelial Carcinoma. Sci. Rep. 2018, 8, 15477. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017; ISBN 9781119263579. [Google Scholar]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.C.; Gatter, K.C. Monoclonal Antibody Ki-67: Its Use in Histopathology. Histopathology 1990, 17, 489–503. [Google Scholar] [CrossRef]
- Bryne, M.; Jenssen, N.; Boysen, M. Histological Grading in the Deep Invasive Front of T1 and T2 Glottic Squamous Cell Carcinomas Has High Prognostic Value. Virchows Arch. 1995, 427, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Otsubo, C.; Mussazhanova, Z.; Kurohama, H.; Shalgimbayeva, G.; Ueki, N.; Matsuoka, Y.; Madiyeva, M.; Sato, S.; Yamashita, H.; Nakashima, M. A New Indicator to Differentiate Thyroid Follicular Inclusions in Cervical Lymph Nodes from Patients with Thyroid Cancer. Int. J. Mol. Sci. 2022, 24, 490. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.J. Targeting the DNA Damage Response in Cancer. Mol. Cell 2015, 60, 547–560. [Google Scholar] [CrossRef]
- Kong, X.; Ding, X.; Li, X.; Gao, S.; Yang, Q. 53BP1 Suppresses Epithelial-Mesenchymal Transition by Downregulating ZEB1 through MicroRNA-200b/429 in Breast Cancer. Cancer Sci. 2015, 106, 982–989. [Google Scholar] [CrossRef]

| n = 39 | |
|---|---|
| Sex | |
| Male, n (%) | 36 (92%) |
| Female, n (%) | 3 (8%) |
| Age, mean ± SD | 66 ± 7.62 |
| Multifocal, n (%) | 6 (15%) |
| Metachronous, n (%) | 10 (26%) |
| History of pharyngeal/esophagus cancer, n (%) | 26 (67%) |
| MCV, mean ± SD | 91.8 ± 12.0 |
| γGTP, median [95%CI] | 27 [19–41] |
| Alcohol, g/day, median [95%CI] | 50 [62–70] |
| Alcohol, g/year, mean ± SD | 42 ± 14.7 |
| Smoking pack years, mean ± SD | 44 ± 32.1 |
| cTNM, n (%) | |
|---|---|
| cTis/1/2/3/4 | 6 (15)/19 (48)/7 (17)/3 (7)/4 (10) |
| cN 0/1/2b/2c/3b | 32 (82)/3 (7)/1 (2)/2 (5)/1 (2) |
| cM 0/1 | 38 (97)/1 (2) |
| cStage 0/I/II/III/IVA/IVB/IVC | 6 (15)/17 (43)/4 (10)/5 (12)/5 (12)/1 (2)/1 (2) |
| Lympho-vascular invasion | |
| Ly+, n (%) | 9 (23) |
| v+, n (%) | 8 (20) |
| Ly and v, +, n (%) | 5 (13) |
| Follow up rate, n (%) | 22 (56) |
| Logistic Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Factors | OR | 95%CI | p | Sensitivity | Specificity | Cutoff | AUC |
| Unstable expression | 1.495 | 1.26–1.78 | <0.0001 | 0.789 | 0.865 | 3.251 | 0.858 |
| n ≥ 3 | 2.13 | 1.49–3.05 | <0.0001 | 0.816 | 0.769 | 0.787 | 0.837 |
| LF | 1.816 | 1.37–2.40 | <0.0001 | 0.868 | 0.769 | 1.46 | 0.834 |
| Ki67 | 1.084 | 0.99–1.19 | 0.0845 | 0.447 | 0.827 | 10.784 | 0.629 |
| 53BP1/Ki67 | 1.864 | 1.25–2.79 | 0.0025 | 0.605 | 0.808 | 0.96 | 0.747 |
| OR | 95%CI | p-Value | ||
|---|---|---|---|---|
| Unstable 53BP1 (n ≥ 3 + LF) at surface | 1.56107 | 1.276307 | 1.909368 | <0.0001 |
| Sex (male/female) | 0.69938 | 0.093741 | 5.217704 | 0.7273 |
| Age | 1.002435 | 0.922729 | 1.089025 | 0.9541 |
| Multifocal | 1.430849 | 0.273848 | 7.476156 | 0.6711 |
| Metachronous | 2.117932 | 0.525765 | 8.531632 | 0.2911 |
| History of pharyngeal/esophagus cancer | 0.371583 | 0.098147 | 1.406806 | 0.1450 |
| Alcohol, g/day | 0.996389 | 0.988839 | 1.003997 | 0.3513 |
| Smoking pack years | 1.001769 | 0.984427 | 1.019417 | 0.8428 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawasaki-Inomata, H.; Tabuchi, M.; Norimatsu, K.; Honda, T.; Matsuda, K.; Hashiguchi, K.; Yamaguchi, N.; Nishi, H.; Kumai, Y.; Nakashima, M.; et al. Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms. Cancers 2024, 16, 2987. https://doi.org/10.3390/cancers16172987
Kawasaki-Inomata H, Tabuchi M, Norimatsu K, Honda T, Matsuda K, Hashiguchi K, Yamaguchi N, Nishi H, Kumai Y, Nakashima M, et al. Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms. Cancers. 2024; 16(17):2987. https://doi.org/10.3390/cancers16172987
Chicago/Turabian StyleKawasaki-Inomata, Hiroko, Maiko Tabuchi, Kiyuu Norimatsu, Tetsuro Honda, Katsuya Matsuda, Keiichi Hashiguchi, Naoyuki Yamaguchi, Hideaki Nishi, Yoshihiko Kumai, Masahiro Nakashima, and et al. 2024. "Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms" Cancers 16, no. 17: 2987. https://doi.org/10.3390/cancers16172987
APA StyleKawasaki-Inomata, H., Tabuchi, M., Norimatsu, K., Honda, T., Matsuda, K., Hashiguchi, K., Yamaguchi, N., Nishi, H., Kumai, Y., Nakashima, M., Miyaaki, H., Nakao, K., & Akazawa, Y. (2024). Significance of P53-Binding Protein 1 as a Novel Molecular Histological Marker for Hypopharyngeal Squamous Neoplasms. Cancers, 16(17), 2987. https://doi.org/10.3390/cancers16172987






