Volumetric Modulated Arc Therapy for High-Risk and Very High-Risk Locoregional Prostate Cancer in the Modern Era: Real-World Experience from an Asian Cohort
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Radiotherapy
2.3. Follow-Up
2.4. Endpoints and Statistical Analysis
3. Results
3.1. Clinicopathological and Treatment Characteristics
3.2. Treatment Efficacy, Failure Patterns and Toxicity
3.3. Role of WPRT on Clinical Outcomes and Toxicity
3.4. Assessment of Prognostic Factors on Clinical Outcomes
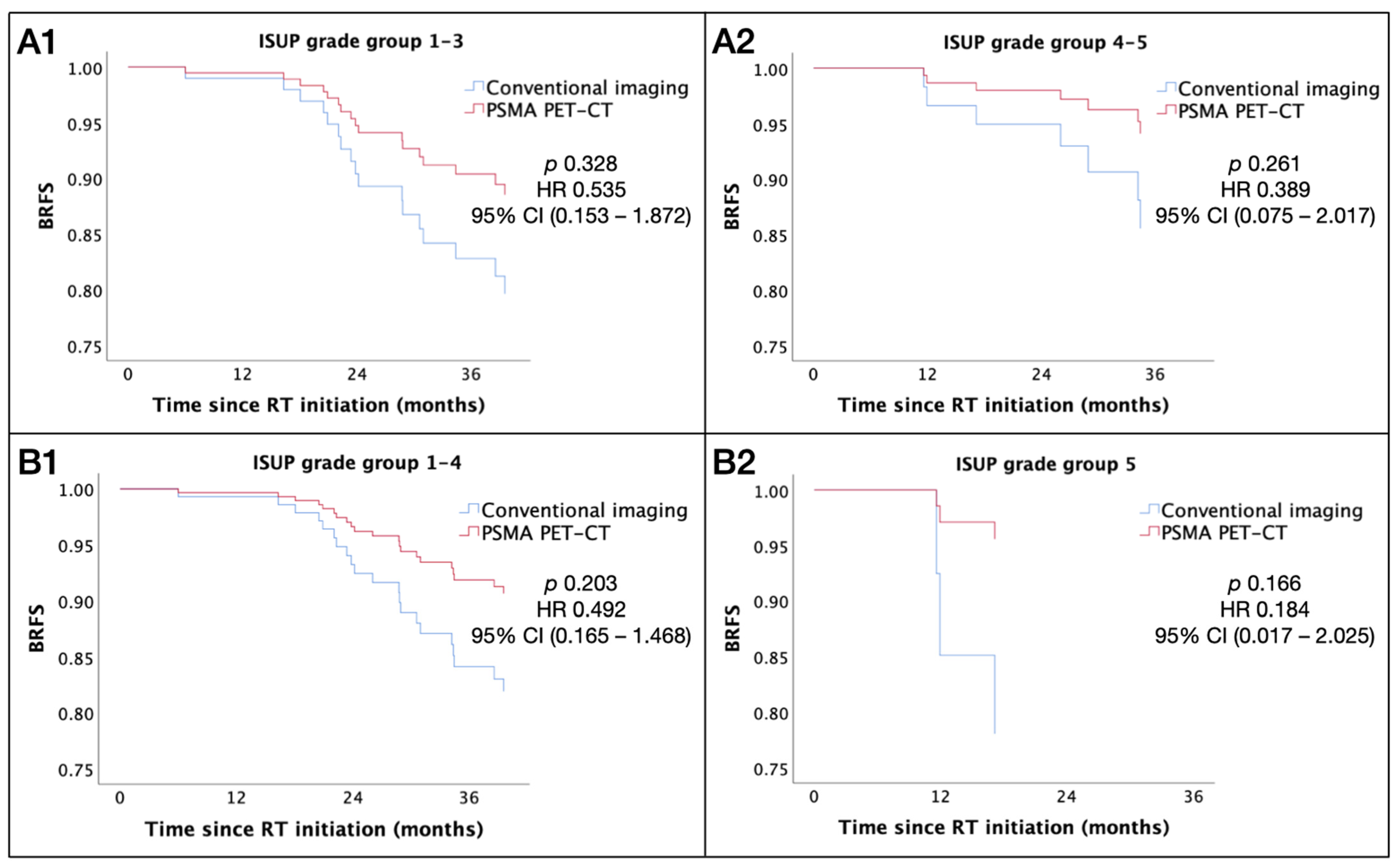
3.5. Impact of PSMA PET-CT Staging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Planning Objectives and Dose Specifications for Tumor Targets and Organs at Risk
| PTV | D2% ≤ 105% (variation acceptable: ≤ 107%) |
| D99% ≥ 95% (variation acceptable: ≥ 93%) | |
| D98% ≥ 100% (variation acceptable: ≥ 95%) | |
| CTV | D99% ≥ 100% (variation acceptable: ≥ 95%) |
| PTVnx | D98% ≥ 95% |
| CTVnx | D98% ≥ 100% |
| Rectum | V70 ≤ 20% |
| V50 ≤ 50% | |
| Bladder | V70 ≤ 30% |
| V55 ≤ 50% | |
| Bowel | D0.03cc ≤ 52.5 Gy (variation acceptable: ≤ 54 Gy) |
| Femoral heads | V50 ≤ 5% |
List of Abbreviations:
| Androgen deprivation therapy | ADT |
| American Joint Committee on Cancer | AJCC |
| American Society for Radiation Oncology | ASTRO |
| Biochemical failure-free survival | BFFS |
| Biochemical relapse-free survival | BRFS |
| Confidence interval | CI |
| Disease-free survival | DFS |
| Equivalent dose at 2 Gy per fraction | EQD2 |
| Eastern Cooperative Oncology Group | ECOG |
| Event-free survival | EFS |
| Food and Drug Administration | FDA |
| Genitourinary | GU |
| Gastrointestinal | GI |
| Hazard ratio | HR |
| High dose rate | HDR |
| Intensity-modulated radiation therapy | IMRT |
| Image-guided radiotherapy | IGRT |
| Interquartile range | IQR |
| International Society of Urological Pathology | ISUP |
| Institutional Review Board | IRB |
| Luteinizing hormone-releasing hormone analogue | LHRHa |
| Metastasis-free survival | MFS |
| Magnetic resonance imaging | MRI |
| National Comprehensive Cancer Network | NCCN |
| Overall survival | OS |
| Organs at risk | OARs |
| Overall treatment time | OTT |
| Prostate cancer-specific survival | PCSS |
| Prostate-only radiotherapy | PORT |
| Prostate-specific membrane antigen positron emission tomography–computed tomography | PSMA PET-CT |
| Primary Tumor Clinical Target Volume | CTVp |
| Prostate-specific antigen | PSA |
| Progression-free survival | PFS |
| Radiotherapy | RT |
| Radiation Therapy Oncology Group | RTOG |
| Radiographic relapse-free survival | RRFS |
| Simultaneous integrated boost | SIB |
| Stereotactic body radiation therapy | SBRT |
| Transperineal | TP |
| Volumetric modulated arc therapy | VMAT |
| Whole pelvic radiotherapy | WPRT |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Cancer Online Resource Hub. Available online: https://www.cancer.gov.hk/en/hong_kong_cancer/common_cancers_in_hong_kong/prostate_cancer.html (accessed on 20 June 2024).
- Prostate Cancer in 2021. Available online: https://www3.ha.org.hk/cancereg/pdf/factsheet/2021/prostate_2021.pdf (accessed on 3 July 2024).
- Chang, A.J.; Autio, K.A.; Roach, M., 3rd; Scher, H.I. High-risk prostate cancer-classification and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Zapatero, A.; Guerrero, A.; Maldonado, X.; Alvarez, A.; Gonzalez San Segundo, C.; Cabeza Rodriguez, M.A.; Macias, V.; Pedro Olive, A.; Casas, F.; Boladeras, A.; et al. High-dose radiotherapy with short-term or long-term androgen deprivation in localised prostate cancer (DART01/05 GICOR): A randomised, controlled, phase 3 trial. Lancet Oncol. 2015, 16, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Cornford, P.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Brunckhorst, O.; Darraugh, J.; Eberli, D.; De Meerleer, G.; De Santis, M.; Farolfi, A.; et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG Guidelines on Prostate Cancer-2024 Update. Part I: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2024, 86, 148–163. [Google Scholar] [CrossRef]
- Zietman, A.L.; DeSilvio, M.L.; Slater, J.D.; Rossi, C.J., Jr.; Miller, D.W.; Adams, J.A.; Shipley, W.U. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: A randomized controlled trial. JAMA 2005, 294, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Peeters, S.T.; Heemsbergen, W.D.; Koper, P.C.; van Putten, W.L.; Slot, A.; Dielwart, M.F.; Bonfrer, J.M.; Incrocci, L.; Lebesque, J.V. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J. Clin. Oncol. 2006, 24, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Dearnaley, D.P.; Sydes, M.R.; Graham, J.D.; Aird, E.G.; Bottomley, D.; Cowan, R.A.; Huddart, R.A.; Jose, C.C.; Matthews, J.H.; Millar, J.; et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: First results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007, 8, 475–487. [Google Scholar] [CrossRef]
- Kuban, D.A.; Tucker, S.L.; Dong, L.; Starkschall, G.; Huang, E.H.; Cheung, M.R.; Lee, A.K.; Pollack, A. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 67–74. [Google Scholar] [CrossRef]
- Beckendorf, V.; Guerif, S.; Le Prise, E.; Cosset, J.M.; Bougnoux, A.; Chauvet, B.; Salem, N.; Chapet, O.; Bourdain, S.; Bachaud, J.M.; et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1056–1063. [Google Scholar] [CrossRef]
- Kishan, A.U.; Cook, R.R.; Ciezki, J.P.; Ross, A.E.; Pomerantz, M.M.; Nguyen, P.L.; Shaikh, T.; Tran, P.T.; Sandler, K.A.; Stock, R.G.; et al. Radical Prostatectomy, External Beam Radiotherapy, or External Beam Radiotherapy With Brachytherapy Boost and Disease Progression and Mortality in Patients With Gleason Score 9-10 Prostate Cancer. JAMA 2018, 319, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Hennequin, C.; Sargos, P.; Roca, L.; Silva, M.; Latorzeff, I.; Peiffert, D.; Cozzi, S.; Benyoucef, A.; Hasbini, A.; Supiot, S.; et al. Long-term results of dose escalation (80 vs 70 Gy) combined with long-term androgen deprivation in high-risk prostate cancers: GETUG-AFU 18 randomized trial. J. Clin. Oncol. 2024, 42 (Suppl. 4), LBA259. [Google Scholar] [CrossRef]
- Roach, M., 3rd; Marquez, C.; Yuo, H.S.; Narayan, P.; Coleman, L.; Nseyo, U.O.; Navvab, Z.; Carroll, P.R. Predicting the risk of lymph node involvement using the pre-treatment prostate specific antigen and Gleason score in men with clinically localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 1994, 28, 33–37. [Google Scholar] [CrossRef]
- Roach, M., 3rd; DeSilvio, M.; Lawton, C.; Uhl, V.; Machtay, M.; Seider, M.J.; Rotman, M.; Jones, C.; Asbell, S.O.; Valicenti, R.K.; et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J. Clin. Oncol. 2003, 21, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Lawton, C.A.; DeSilvio, M.; Roach, M., 3rd; Uhl, V.; Kirsch, R.; Seider, M.; Rotman, M.; Jones, C.; Asbell, S.; Valicenti, R.; et al. An update of the phase III trial comparing whole pelvic to prostate only radiotherapy and neoadjuvant to adjuvant total androgen suppression: Updated analysis of RTOG 94-13, with emphasis on unexpected hormone/radiation interactions. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.; Moughan, J.; Lawton, C.A.F.; Dicker, A.P.; Zeitzer, K.L.; Gore, E.M.; Kwok, Y.; Seider, M.J.; Hsu, I.C.; Hartford, A.C.; et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Maitre, P.; Kannan, S.; Panigrahi, G.; Krishnatry, R.; Bakshi, G.; Prakash, G.; Pal, M.; Menon, S.; Phurailatpam, R.; et al. Prostate-Only Versus Whole-Pelvic Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer (POP-RT): Outcomes From Phase III Randomized Controlled Trial. J. Clin. Oncol. 2021, 39, 1234–1242. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidlines in Oncology Prostate Cancer Version 4. 2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 20 June 2024).
- De Meerleer, G.; Berghen, C.; Briganti, A.; Vulsteke, C.; Murray, J.; Joniau, S.; Leliveld, A.M.; Cozzarini, C.; Decaestecker, K.; Rans, K.; et al. Elective nodal radiotherapy in prostate cancer. Lancet Oncol. 2021, 22, e348–e357. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, C.G.; Carlson, J.A.; Waxweiler, T.V.; Raben, D.; Dewitt, P.E.; Crawford, E.D.; Maroni, P.D.; Kavanagh, B.D. The impact of definitive local therapy for lymph node-positive prostate cancer: A population-based study. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 1064–1073. [Google Scholar] [CrossRef]
- Lin, C.C.; Gray, P.J.; Jemal, A.; Efstathiou, J.A. Androgen deprivation with or without radiation therapy for clinically node-positive prostate cancer. J. Natl. Cancer Inst. 2015, 107, djv119. [Google Scholar] [CrossRef] [PubMed]
- Bryant, A.K.; Kader, A.K.; McKay, R.R.; Einck, J.P.; Mell, L.K.; Mundt, A.J.; Kane, C.J.; Efstathiou, J.A.; Murphy, J.D.; Rose, B.S. Definitive Radiation Therapy and Survival in Clinically Node-Positive Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Seisen, T.; Vetterlein, M.W.; Karabon, P.; Jindal, T.; Sood, A.; Nocera, L.; Nguyen, P.L.; Choueiri, T.K.; Trinh, Q.D.; Menon, M.; et al. Efficacy of Local Treatment in Prostate Cancer Patients with Clinically Pelvic Lymph Node-positive Disease at Initial Diagnosis. Eur. Urol. 2018, 73, 452–461. [Google Scholar] [CrossRef]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; Mason, M.D.; Parker, C.C.; Ritchie, A.W.; Russell, J.M.; Schiavone, F.; Attard, G.; et al. Failure-Free Survival and Radiotherapy in Patients With Newly Diagnosed Nonmetastatic Prostate Cancer: Data From Patients in the Control Arm of the STAMPEDE Trial. JAMA Oncol. 2016, 2, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Pommier, P.; Chabaud, S.; Lagrange, J.L.; Richaud, P.; Le Prise, E.; Wagner, J.P.; Azria, D.; Beckendorf, V.; Suchaud, J.P.; Bernier, V.; et al. Is There a Role for Pelvic Irradiation in Localized Prostate Adenocarcinoma? Update of the Long-Term Survival Results of the GETUG-01 Randomized Study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Sini, C.; Noris Chiorda, B.; Gabriele, P.; Sanguineti, G.; Morlino, S.; Badenchini, F.; Cante, D.; Carillo, V.; Gaetano, M.; Giandini, T.; et al. Patient-reported intestinal toxicity from whole pelvis intensity-modulated radiotherapy: First quantification of bowel dose-volume effects. Radiother. Oncol. 2017, 124, 296–301. [Google Scholar] [CrossRef]
- Tharmalingam, H.; Tsang, Y.; Choudhury, A.; Alonzi, R.; Wylie, J.; Ahmed, I.; Henry, A.; Heath, C.; Hoskin, P.J. External Beam Radiation Therapy (EBRT) and High-Dose-Rate (HDR) Brachytherapy for Intermediate and High-Risk Prostate Cancer: The Impact of EBRT Volume. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 525–533. [Google Scholar] [CrossRef]
- Pollack, A.; Karrison, T.G.; Balogh, A.G.; Gomella, L.G.; Low, D.A.; Bruner, D.W.; Wefel, J.S.; Martin, A.G.; Michalski, J.M.; Angyalfi, S.J.; et al. The addition of androgen deprivation therapy and pelvic lymph node treatment to prostate bed salvage radiotherapy (NRG Oncology/RTOG 0534 SPPORT): An international, multicentre, randomised phase 3 trial. Lancet 2022, 399, 1886–1901. [Google Scholar] [CrossRef]
- Michalski, J.M.; Yan, Y.; Watkins-Bruner, D.; Bosch, W.R.; Winter, K.; Galvin, J.M.; Bahary, J.P.; Morton, G.C.; Parliament, M.B.; Sandler, H.M. Preliminary toxicity analysis of 3-dimensional conformal radiation therapy versus intensity modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group 0126 prostate cancer trial. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 932–938. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Harrison, A.S.; Trabulsi, E.J.; Gomella, L.G.; Showalter, T.N.; Hurwitz, M.D.; Dicker, A.P.; Den, R.B. Evolution of advanced technologies in prostate cancer radiotherapy. Nat. Rev. Urol. 2013, 10, 565–579. [Google Scholar] [CrossRef]
- Yamazaki, H.; Nakamura, S.; Nishimura, T.; Yoshida, K.; Yoshioka, Y.; Koizumi, M.; Ogawa, K. Transitioning from conventional radiotherapy to intensity-modulated radiotherapy for localized prostate cancer: Changing focus from rectal bleeding to detailed quality of life analysis. J. Radiat. Res. 2014, 55, 1033–1047. [Google Scholar] [CrossRef]
- Viani, G.A.; Viana, B.S.; Martin, J.E.; Rossi, B.T.; Zuliani, G.; Stefano, E.J. Intensity-modulated radiotherapy reduces toxicity with similar biochemical control compared with 3-dimensional conformal radiotherapy for prostate cancer: A randomized clinical trial. Cancer 2016, 122, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Kawamura, H.; Murata, K.; Inoue, T.; Murata, H.; Takakusagi, Y.; Okonogi, N.; Ohkubo, Y.; Okamoto, M.; Kaminuma, T.; et al. Intensity-Modulated Radiation Therapy with Simultaneous Integrated Boost for Clinically Node-Positive Prostate Cancer: A Single-Institutional Retrospective Study. Cancers 2021, 13, 3868. [Google Scholar] [CrossRef] [PubMed]
- Fink, C.A.; Wegener, D.; Sauer, L.D.; Jakel, C.; Zips, D.; Debus, J.; Herfarth, K.; Koerber, S.A. Whole-pelvic irradiation with boost to involved nodes and prostate in node-positive prostate cancer-long-term data from the prospective PLATIN-2 trial. Strahlenther. Onkol. 2024, 200, 202–207. [Google Scholar] [CrossRef]
- Maurer, T.; Gschwend, J.E.; Rauscher, I.; Souvatzoglou, M.; Haller, B.; Weirich, G.; Wester, H.J.; Heck, M.; Kubler, H.; Beer, A.J.; et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016, 195, 1436–1443. [Google Scholar] [CrossRef]
- Gupta, M.; Choudhury, P.S.; Hazarika, D.; Rawal, S. A Comparative Study of (68)Gallium-Prostate Specific Membrane Antigen Positron Emission Tomography-Computed Tomography and Magnetic Resonance Imaging for Lymph Node Staging in High Risk Prostate Cancer Patients: An Initial Experience. World J. Nucl. Med. 2017, 16, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Hope, T.A.; Eiber, M.; Armstrong, W.R.; Juarez, R.; Murthy, V.; Lawhn-Heath, C.; Behr, S.C.; Zhang, L.; Barbato, F.; Ceci, F.; et al. Diagnostic Accuracy of 68Ga-PSMA-11 PET for Pelvic Nodal Metastasis Detection Prior to Radical Prostatectomy and Pelvic Lymph Node Dissection: A Multicenter Prospective Phase 3 Imaging Trial. JAMA Oncol. 2021, 7, 1635–1642. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Woythal, N.; Arsenic, R.; Kempkensteffen, C.; Miller, K.; Janssen, J.C.; Huang, K.; Makowski, M.R.; Brenner, W.; Prasad, V. Immunohistochemical Validation of PSMA Expression Measured by (68)Ga-PSMA PET/CT in Primary Prostate Cancer. J. Nucl. Med. 2018, 59, 238–243. [Google Scholar] [CrossRef]
- Lawton, C.A.; Michalski, J.; El-Naqa, I.; Buyyounouski, M.K.; Lee, W.R.; Menard, C.; O’Meara, E.; Rosenthal, S.A.; Ritter, M.; Seider, M. RTOG GU Radiation oncology specialists reach consensus on pelvic lymph node volumes for high-risk prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 383–387. [Google Scholar] [CrossRef]
- Cox, J.D.; Grignon, D.J.; Kaplan, R.S.; Parsons, J.T.; Schellhammer, P.F. Consensus statement: Guidelines for PSA following radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1997, 37, 1035–1041. [Google Scholar]
- Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50 (accessed on 4 July 2024).
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef]
- Bekelman, J.E.; Rumble, R.B.; Chen, R.C.; Pisansky, T.M.; Finelli, A.; Feifer, A.; Nguyen, P.L.; Loblaw, D.A.; Tagawa, S.T.; Gillessen, S.; et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J. Clin. Oncol. 2018, 36, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 2018, 199, 990–997. [Google Scholar] [CrossRef]
- Pommier, P.; Chabaud, S.; Lagrange, J.L.; Richaud, P.; Lesaunier, F.; Le Prise, E.; Wagner, J.P.; Hay, M.H.; Beckendorf, V.; Suchaud, J.P.; et al. Is there a role for pelvic irradiation in localized prostate adenocarcinoma? Preliminary results of GETUG-01. J. Clin. Oncol. 2007, 25, 5366–5373. [Google Scholar] [CrossRef] [PubMed]
- Andruska, N.; Fischer-Valuck, B.W.; Waters, M.; Juarez Diaz, E.; Agabalogun, T.; Kim, E.H.; Smith, Z.L.; Brenneman, R.J.; Gay, H.A.; Andriole, G.L.; et al. Survival Outcomes in Men with Unfavorable Intermediate-Risk and High-Risk Prostate Cancer Treated with Prostate-Only versus Whole Pelvic Radiation Therapy. J. Urol. 2022, 207, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- RTOG-0924. Available online: https://www.nrgoncology.org/Clinical-Trials/Protocol/rtog-0924?filter=rtog-0924 (accessed on 4 July 2024).
- Syndikus, I.; Cruickshank, C.; Staffurth, J.; Tree, A.; Henry, A.; Naismith, O.; Mayles, H.; Snelson, N.; Hassan, S.; Brown, S.; et al. PIVOTALboost: A phase III randomised controlled trial of prostate and pelvis versus prostate alone radiotherapy with or without prostate boost (CRUK/16/018). Clin. Transl. Radiat. Oncol. 2020, 25, 22–28. [Google Scholar] [CrossRef]
- Tsuchida, K.; Inaba, K.; Kashihara, T.; Murakami, N.; Okuma, K.; Takahashi, K.; Igaki, H.; Nakayama, Y.; Maejima, A.; Shinoda, Y.; et al. Clinical outcomes of definitive whole pelvic radiotherapy for clinical lymph node metastatic prostate cancer. Cancer Med. 2020, 9, 6629–6637. [Google Scholar] [CrossRef]
- Draulans, C.; van der Heide, U.A.; Haustermans, K.; Pos, F.J.; van der Voort van Zyp, J.; De Boer, H.; Groen, V.H.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; et al. Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiother. Oncol. 2020, 147, 92–98. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Cheung, E.S.; Law, F.C.; Fung, N.T.; Soong, I.S.; Hung, R.H.; Tse, T.K.; Wong, K.K.; Wu, P.Y. Simultaneous Integrated Boost for Dose Escalation in Node-Positive Cervical Cancer: 5-Year Experience in a Single Institution. Cancers 2023, 15, 4647. [Google Scholar] [CrossRef]
- Kishan, A.U.; Sun, Y.; Hartman, H.; Pisansky, T.M.; Bolla, M.; Neven, A.; Steigler, A.; Denham, J.W.; Feng, F.Y.; Zapatero, A.; et al. Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: An individual patient data meta-analysis. Lancet Oncol 2022, 23, 304–316. [Google Scholar] [CrossRef]
- Attard, G.; Murphy, L.; Clarke, N.W.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; Cook, A.; Brawley, C.; Amos, C.L.; et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022, 399, 447–460. [Google Scholar] [CrossRef] [PubMed]
- GETUG-AFU-23. Available online: https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/getug-afu-23-uc-01601202/ (accessed on 4 July 2024).
- Nguyen, P.L.; Huang, H.R.; Spratt, D.E.; Davicioni, E.; Sandler, H.M.; Shipley, W.U.; Efstathiou, J.A.; Simko, J.P.; Pollack, A.; Dicker, A.P.; et al. Analysis of a Biopsy-Based Genomic Classifier in High-Risk Prostate Cancer: Meta-Analysis of the NRG Oncology/Radiation Therapy Oncology Group 9202, 9413, and 9902 Phase 3 Randomized Trials. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 521–529. [Google Scholar] [CrossRef] [PubMed]
- NRG-GU009. Available online: https://www.nrgoncology.org/Clinical-Trials/Protocol/nrg-gu009-1?filter=nrg-gu009-1 (accessed on 20 June 2024).
- FDA Approves First PSMA-Targeted PET Imaging Drug for Men with Prostate Cancer. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-psma-targeted-pet-imaging-drug-men-prostate-cancer (accessed on 20 June 2024).


| Overall (N = 209) | Roach estimated nodal risk, %; median (IQR) | 31.0 (22.3, 43.9) | ||
| Patient characteristics | NCCN risk group; N (%) | |||
| Age, years; median (IQR) | 72 (67, 77) | High-risk cN0 | 97 (46.4) | |
| ECOG performance status; N (%) | Very high-risk cN0 | 96 (45.9) | ||
| 0–1 | 184 (88.0) | cN1 | 16 (7.7) | |
| 2 | 25 (12.0) | Investigations | ||
| Charlson comorbidity index; N (%) | Prostate biopsy method; N (%) | |||
| ≤2 | 36 (17.2) | Systematic biopsy | 163 (78.0) | |
| >2 | 173 (82.8) | Systematic + targeted biopsy | 28 (13.4) | |
| Clinicopathological characteristics | Not available | 18 (8.6) | ||
| Positive core/total core ratio; median (IQR) | 0.50 (0.25, 0.80) | Prostate biopsy approach; N (%) | ||
| ISUP grade group 4–5 core/total core ratio; median (IQR) | 0.00 (0.00, 0.17) | Transrectal | 166 (79.4) | |
| PNI; N (%) | Transperineal | 27 (12.9) | ||
| Negative | 131 (62.7) | Others # | 16 (7.7) | |
| Positive | 36 (17.2) | Staging imaging; N (%) | ||
| Not available | 42 (20.1) | Conventional imaging | 120 (57.4) | |
| ISUP grade group; N (%) | PSMA PET-CT | 89 (42.6) | ||
| 1–3 | 118 (56.5) | Treatment characteristics | ||
| 4 | 58 (27.8) | ADT; N (%) | ||
| 5 | 33 (15.8) | 2 years | 10 (4.8) | |
| T stage; N (%) | 3 years | 187 (89.5) | ||
| 1–2 | 87 (41.6) | >3 years ^ | 10 (4.8) | |
| 3a | 71 (34.0) | Others | 2 (1.0) | |
| 3b-4 | 51 (24.4) | RT coverage; N (%) | ||
| N stage; N (%) | Prostate/SV | 48 (23.0) | ||
| 0 | 193 (92.3) | Prostate/SV + elective pelvic LN | 149 (71.3) | |
| 1 | 16 (7.7) | Prostate/SV + elective pelvic LN + involved LN boost * | 12 (5.7) | |
| Baseline PSA, ng/mL; median (IQR) | 22.6 (11.4, 42.7) | RT OTT, days; median (IQR) | 55 (52, 56) |
| BRFS | RRFS | MFS | PCSS | |
|---|---|---|---|---|
| 24 months | 93.8% | 99.5% | 99.5% | 100% |
| 36 months | 86.9% | 99.5% | 99.5% | 100% |
| 48 months | 85.2% | 96.8% | 96.8% | 100% |
| 60 months | 85.2% | 95.4% | 96.8% | 98.8% |
| Initial Staging Imaging | Clinical Stage | Baseline PSA, ng/mL | Gleason Score | Positive/ Total Core Ratio | ISUP Group 4–5/Total Core Ratio | ADT Duration, Years | RT Coverage | Nadir PSA, ng/mL | Relapse Pattern | Time to Failure, Months | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Conventional | T3aN0M0 | 27.2 | 4 + 3 | 0.67 | 0 | 3 | Prostate/SV + elective pelvic LN | 0.06 | Prostate | 55.6 |
| 2 | Conventional | T3aN0M0 | 4.5 | 4 + 5 | 0.5 | 0.42 | 3 | Prostate/SV + elective pelvic LN | <0.02 | Pelvic LN, distant LN, bone metastasis | 38.8 |
| 3 | PSMA PET-CT | T3bN1M0 | 40.9 | 4 + 3 | 1 | 0 | 3 | Prostate/SV + elective pelvic LN | 0.08 | Bone metastasis | 47.6 |
| 4 | Conventional | T3bN1M0 | 83.9 | 4 + 5 | 0.62 | 0.15 | 3 | Prostate/SV + elective pelvic LN + involved LN boost | 0.23 | Bone metastasis | 36.3 |
| 5 | PSMA PET-CT | T2cN1M0 | 21.7 | 5 + 4 | 0.85 | 0.85 | >3 | Prostate/SV + elective pelvic LN + involved LN boost | 0.29 | Bone metastasis | 19.5 |
| Overall (N = 209) | RT Coverage | |||||
|---|---|---|---|---|---|---|
| Prostate/SV (N = 48) | Prostate/SV + Pelvis (N = 149) | Prostate/SV + Pelvis + Boost (N = 12) | ||||
| Acute toxicity; N (%) | GU | Grade 1 | 29 (13.9) | 10 (20.8) | 17 (11.4) | 2 (16.7) |
| Grade 2 | 49 (23.4) | 12 (25.0) | 37 (24.8) | 0 (0.0) | ||
| Grade 3 | 15 (7.2) | 4 (8.3) | 9 (6.0) | 2 (16.7) | ||
| GI | Grade 1 | 75 (35.9) | 13 (27.1) | 57 (38.3) | 5 (41.7) | |
| Grade 2 | 18 (8.6) | 1 (2.1) | 17 (11.4) * | 0 (0.0) | ||
| Grade 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Late toxicity; N (%) | GU | Grade 1 | 11 (5.3) | 4 (8.3) | 5 (3.4) | 2 (16.7) |
| Grade 2 | 29 (13.9) | 6 (12.5) | 22 (14.8) | 1 (8.3) | ||
| Grade 3 | 4 (1.9) | 2 (4.2) | 1 (0.7) | 1 (8.3) | ||
| GI | Grade 1 | 42 (20.1) | 9 (18.8) | 32 (21.5) | 1 (8.3) | |
| Grade 2 | 19 (9.1) | 3 (6.3) | 15 (10.1) | 1 (8.3) | ||
| Grade 3 | 4 (1.9) | 2 (4.2) | 2 (1.3) | 0 (0.0) | ||
| Sacral insufficiency fracture | 1 (0.5) | 0 (0.0) | 0 (0.0) | 1 (8.3) | ||
| RT Coverage | |||
|---|---|---|---|
| Prostate/SV (N = 48) | Prostate/SV + Pelvis (N = 145) | p | |
| Positive core/total core ratio; median (IQR) | 0.42 (0.17, 0.58) | 0.50 (0.25, 0.83) | 0.015 |
| ISUP grade group 4–5 core/total core ratio; median (IQR) | 0.00 (0.00, 0.00) | 0.00 (0.00, 0.20) | 0.019 |
| PNI; N (%) | 0.457 | ||
| Negative | 12 (25.0) | 28 (19.3) | |
| Positive | 31 (64.6) | 92 (63.4) | |
| Not available | 5 (10.4) | 25 (17.2) | |
| ISUP grade group; N (%) | 0.063 | ||
| 1–3 | 35 (72.9) | 78 (53.8) | |
| 4 | 8 (16.7) | 46 (31.7) | |
| 5 | 5 (10.4) | 21 (14.5) | |
| T stage; N (%) | 0.089 | ||
| 1–2 | 16 (33.3) | 67 (46.2) | |
| 3a | 23 (47.9) | 44 (30.3) | |
| 3b-4 | 9 (18.8) | 34 (23.4) | |
| Baseline PSA, ng/mL; median (IQR) | 13.5 (8.2, 22.0) | 25.2 (15.4, 43.1) | 0.051 |
| Roach estimated nodal risk, %; median (IQR) | 15.7 (13.8, 33.3) | 33.4 (24.5, 43.9) | 0.004 |
| NCCN risk group; N (%) | 0.134 | ||
| High-risk cN0 | 29 (60.4) | 68 (46.9) | |
| Very high-risk cN0 | 19 (39.6) | 77 (53.1) | |
| ADT; N (%) | 0.865 | ||
| 2 years | 3 (6.3) | 7 (4.8) | |
| 3 years | 44 (91.7) | 134 (92.4) | |
| >3 years ^ | 1 (2.1) | 2 (1.4) | |
| Others | 0 (0.0) | 2 (1.4) | |
| BRFS | RRFS | ||||
|---|---|---|---|---|---|
| p * | HR (95% CI) * | p * | HR (95% CI) * | ||
| Clinicopathological characteristics | |||||
| Positive core/total core ratio | Continuous variable | 0.465 | 0.080 | 18.335 (0.705–476.549) | |
| ISUP grade group 4–5 core/total core ratio | Continuous variable | 0.603 | 0.277 | ||
| PNI | Negative | 0.672 | 0.209 | ||
| Positive | |||||
| ISUP grade group | 1–3 | 0.386 | 0.078 | Reference | |
| 4 | 0.975 | ||||
| 5 | 0.024 | 7.939 (1.313–48.001) | |||
| T stage | 1–2 | 0.903 | 0.616 | ||
| 3a | |||||
| 3b-4 | |||||
| N stage | 0 | 0.186 | 0.000 | Reference | |
| 1 | 42.508 (6.495–278.201) | ||||
| Baseline PSA, ng/mL | Continuous variable | 0.837 | 0.585 | ||
| Roach estimated nodal risk, % | Continuous variable | 0.424 | 0.157 | ||
| Investigations | |||||
| Prostate biopsy method | Systematic biopsy | 0.951 | 0.087 | Reference | |
| Systematic + targeted biopsy | 4.815 (0.795–29.173) | ||||
| Staging imaging | Conventional imaging | 0.083 | Reference | 0.632 | |
| PSMA PET-CT | 0.428 (0.159–1.150) | ||||
| BRFS | ||
|---|---|---|
| p * | ||
| Positive core/total core ratio | Continuous variable | 0.325 |
| ISUP grade group 4–5 core/total core ratio | Continuous variable | 0.096 |
| PNI | Categorial variable | 0.908 |
| Negative | ||
| Positive | ||
| Not available | ||
| ISUP grade group | Categorial variable | 0.039 |
| 1–3 | Reference | |
| 4 | HR * 0.130 95% CI * 0.006–3.001 | |
| 5 | HR * 0.010 95% CI * 0.000–0.367 | |
| T stage | Categorial variable | 0.630 |
| 1–2 | ||
| 3a | ||
| 3b-4 | ||
| Baseline PSA, ng/mL | Continuous variable | 0.098 |
| NHT + WPRT Arm in RTOG 9413 [16,17,18] | WPRT Arm in GETUG-01 [27,48] | WPRT Arm in POP-RT [19] | Elective Pelvic RT in the Current Study | |
|---|---|---|---|---|
| Median age | Not specified | 69.8 years | 66 years | 71 years |
| Roach estimated nodal risk | ≥15% | 48.7% patients ≥ 15% | ≥20% | ≥15% |
| Baseline PSA, ng/mL | 33% patients with PSA > 30 | Median 12 | Median 29.9 | Median 25.5 |
| GS | 74% patients with GS 7–10 | 12.5% patients with GS 8–10 | 48.1% patients with GS 8–10 | 45.8% patients with GS 8–10 |
| Clinical T stage | 67% patients with T2c-T4 | 24.2% patients with cT3 | 74.6% patients with cT3–4 | 53.5% patients with cT3–4 |
| Staging modality | Conventional | Conventional | Conventional and/or PSMA PET-CT (80%) | Conventional and/or PSMA PET-CT (42%) |
| ADT duration | 4 months | 6 months | ≥2 years (14.5% orchidectomy) | 3 years |
| RT technique | Conventional 2-D box | Conventional 2-D box and 3-D conformal | IMRT or tomotherapy | VMAT |
| Dose and fractionation to prostate (BED) | 70.2 Gy in 1.8-Gy fractions (112.32 Gy) | 66–70 Gy in 1.8 to 2-Gy fractions (110–122 Gy) | 68 Gy in 2.72-Gy fractions (129.6 Gy) | 76 Gy in 2-Gy fractions (126.7 Gy) |
| Dose and fractionation to whole pelvis | 50.4 Gy in 1.8-Gy fractions | 46 Gy in 2-Gy fractions | 50 Gy in 2-Gy fractions | 46 Gy in 2-Gy fractions |
| Definition of biochemical failure | Phoenix criteria | ASTRO definition | Phoenix criteria | ASTRO definition |
| Median follow-up | 8.8 years | 11.4 years | 5.7 years | 4 years |
| Primary outcome | 10-year PFS 28.4% | 5-year EFS 69.2% | 5-year BFFS 95% | 4-year BRFS 87% |
| Other clinical outcome estimates | Not specified | Not specified | 5-year | 4-year |
| DFS 89.5% | BRFS 87% | |||
| MFS 95.9% | RRFS 99% | |||
| MFS 99% | ||||
| PCSS 100% | ||||
| Grade ≥ 3 late toxicities | ||||
| GU toxicity | 6% | 15.3% | 1.8% | 0.7% |
| GI toxicity | 7% | 10.7% | 1.8% | 1.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Q.; Chan, K.; Kam, M.T.-Y.; Zheng, K.Y.-C.; Hung, R.H.-M.; Wu, P.Y. Volumetric Modulated Arc Therapy for High-Risk and Very High-Risk Locoregional Prostate Cancer in the Modern Era: Real-World Experience from an Asian Cohort. Cancers 2024, 16, 2964. https://doi.org/10.3390/cancers16172964
Du Q, Chan K, Kam MT-Y, Zheng KY-C, Hung RH-M, Wu PY. Volumetric Modulated Arc Therapy for High-Risk and Very High-Risk Locoregional Prostate Cancer in the Modern Era: Real-World Experience from an Asian Cohort. Cancers. 2024; 16(17):2964. https://doi.org/10.3390/cancers16172964
Chicago/Turabian StyleDu, Qijun, Kuen Chan, Michael Tsz-Yeung Kam, Kelvin Yu-Chen Zheng, Rico Hing-Ming Hung, and Philip Yuguang Wu. 2024. "Volumetric Modulated Arc Therapy for High-Risk and Very High-Risk Locoregional Prostate Cancer in the Modern Era: Real-World Experience from an Asian Cohort" Cancers 16, no. 17: 2964. https://doi.org/10.3390/cancers16172964
APA StyleDu, Q., Chan, K., Kam, M. T.-Y., Zheng, K. Y.-C., Hung, R. H.-M., & Wu, P. Y. (2024). Volumetric Modulated Arc Therapy for High-Risk and Very High-Risk Locoregional Prostate Cancer in the Modern Era: Real-World Experience from an Asian Cohort. Cancers, 16(17), 2964. https://doi.org/10.3390/cancers16172964






