How to Tackle Discordance in Adjuvant Chemotherapy Recommendations by Using Oncotype DX Results, in Early-Stage Breast Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.J.; Carey, L.A.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Xie, S.J.; Wu, S.G.; He, Z.Y. Impact of the 21-gene expression assay on treatment decisions and clinical outcomes in breast cancer with one to three positive lymph nodes. Front. Endocrinol. 2023, 14, 1103949. [Google Scholar] [CrossRef]
- Licata, L.; Viale, G.; Giuliano, M.; Curigliano, G.; Chavez-MacGregor, M.; Foldi, J.; Oke, O.; Collins, J.; Del Mastro, L.; Puglisi, F.; et al. Oncotype DX results increase concordance in adjuvant chemotherapy recommendations for early-stage breast cancer. NPJ Breast Cancer 2023, 9, 51. [Google Scholar] [CrossRef]
- Swain, S.M.; Tang, G.; Puhalla, S.L.; Ganz, P.A.; Henry, N.L.; Cecchini, R.S.; Reid, S.A.; Rastogi, P.; Geyer, C.E.; White, J.R.; et al. A phase III trial evaluating addition of adjuvant chemotherapy to ovarian function suppression + endocrine therapy in premenopausal women with pN0-1, HR+/HER2- breast cancer (BC) and oncotype recurrence score (RS) ≤25 (OFSET): NRG-BR009. J. Clin. Oncol. 2024, 42 (Suppl. 16), TPS612. [Google Scholar] [CrossRef]
- Sparano, J.A.; Gray, R.J.; Makower, D.F.; Pritchard, K.I.; Albain, K.S.; Hayes, D.F.; Geyer, C.E., Jr.; Dees, E.C.; Goetz, M.P.; Olson, J.A., Jr.; et al. Adjuvant Chemotherapy Guided by a 21-Gene Expression Assay in Breast Cancer. N. Engl. J. Med. 2018, 379, 111–121. [Google Scholar] [CrossRef]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.L.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N. Engl. J. Med. 2021, 385, 2336–2347. [Google Scholar] [CrossRef]
- Fleiss, J.L. Measuring nominal scale agreement among many raters. Psychol. Bull. 1971, 76, 378–382. [Google Scholar] [CrossRef]
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Medica 2012, 22, 276–282. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Breast Cancer (Version 1.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 1 February 2024).
- Horváth, Z.; Boér, K.; Dank, M.; Kahán, Z.; Kocsis, J.; Kövér, E.; Máhr, K.; Pikó, B.; Rubovszky, G. Az emlőrák szisztémás kezelése: Szakmai irányelvek [Systemic treatment of breast cancer: Professional guideline]. Magy. Onkológia 2020, 64, 348–368. [Google Scholar]
- R. Core Team. A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R. Core Team: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 2 January 2024).
- Wu, P.; Wu, S.G.; He, Z.Y. Nomogram Update to Predict the High Genomic Risk Breast Cancer by Different Races. Clin. Breast Cancer 2024, 24, e61–e70. [Google Scholar] [CrossRef] [PubMed]
- Orucevic, A.; Bell, J.L.; King, M.; McNabb, A.P.; Heidel, R.E. Nomogram update based on TAILORx clinical trial results—Oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data. Breast 2019, 46, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Humphris, K.; Stephenson, J.; Kumaraswamy, V. Predicting oncotype DX recurrence scores using locally available immunohistochemical markers: Experience in a district general hospital. J. Clin. Pathol. 2021, 76, 252–255. [Google Scholar] [CrossRef]
- Baltres, A.; Al Masry, Z.; Zemouri, R.; Valmary-Degano, S.; Arnould, L.; Zerhouni, N.; Devalland, C. Prediction of Oncotype DX recurrence score using deep multi-layer perceptrons in estrogen receptor-positive, HER2-negative breast cancer. Breast Cancer. 2020, 27, 1007–1016. [Google Scholar] [CrossRef]
- Thibodeau, S.; Voutsadakis, I.A. Prediction of Oncotype Dx recurrence score using clinical parameters: A comparison of available tools and a simple predictor based on grade and progesterone receptor. Hematol. Oncol. Stem Cell Ther. 2019, 12, 89–96. [Google Scholar] [CrossRef]
- Levine, M.N.; Julian, J.A.; Bedard, P.L.; Eisen, A.; Trudeau, M.E.; Higgins, B.; Bordeleau, L.; Pritchard, K.I. Prospective Evaluation of the 21-Gene Recurrence Score Assay for Breast Cancer Decision-Making in Ontario. J. Clin. Oncol. 2016, 34, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, S.; Gudiwada, S.P.; Kaji, A.H.; Chlebowski, R.T.; Venegas, R.; Ozao-Choy, J.; Dauphine, C. Can Oncotype DX testing be omitted in invasive breast cancer patients with clinicopathologic factors predicting very high pretest probability of a concordant result? Breast J. 2020, 26, 2199–2202. [Google Scholar] [CrossRef]
- Crager, M.; Wijayawardana, S.R.; Gruver, A.M.; Blacklock, A.; Russell, C.; Baehner, F.L.; Sapunar, F. Population-based estimate for the correlation of the Oncotype Dx Breast Recurrence Score® result and Ki-67 IHC MIB-1 pharmDx in HR+, HER2-, node-positive early breast cancer. Breast Cancer Res. 2022, 24, 74. [Google Scholar] [CrossRef]
- Saigosoom, N.; Sa-Nguanraksa, D.; O-Charoenrat, E.; Thumrongtaradol, T.; O-Charoenrat, P. The Evaluation of Magee Equation 2 in Predicting Response and Outcome in Hormone Receptor-Positive and HER2-Negative Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Manag. Res. 2020, 12, 2491–2499. [Google Scholar] [CrossRef]
- Glasgow, A.; Sechrist, H.; Bomeisl, P.; Gilmore, H.; Harbhajanka, A. Correlation between modified Magee equation-2 and Oncotype-Dx recurrence scores using both traditional and TAILORx cutoffs and the clinical application of the Magee Decision Algorithm: A single institutional review. Breast Cancer 2021, 28, 321–328. [Google Scholar] [CrossRef]
- Slembrouck, L.; Vanden Bempt, I.; Wildiers, H.; Smeets, A.; Van Rompuy, A.S.; Van Ongeval, C.; Jongen, L.; Weltens, C.; Punie, K.; Hoste, G.; et al. Concordance between results of inexpensive statistical models and multigene signatures in patients with ER+/HER2- early breast cancer. Mod. Pathol. 2021, 34, 1297–1309. [Google Scholar] [CrossRef]
- Alkushi, A.; Omair, A.; Masuadi, E.; Alamri, G.; Abusanad, A.; Abdelhafiez, N.; Mohamed, A.E.; Abulkhair, O. The Level of Agreement Among Medical Oncologists on Adjuvant Chemotherapy Decision for Breast Cancer in Pre and Post-Oncotype DX Settings. Cureus 2021, 13, e13298. [Google Scholar]
- Albanell, J.; Svedman, C.; Gligorov, J.; Holt, S.D.; Bertelli, G.; Blohmer, J.U.; Rouzier, R.; Lluch, A.; Eiermann, W. Pooled analysis of prospective European studies assessing the impact of using the 21-gene Recurrence Score assay on clinical decision making in women with oestrogen receptor-positive, human epidermal growth factor receptor 2-negative early-stage breast cancer. Eur. J. Cancer 2016, 66, 104–113. [Google Scholar]
- Soliman, H.; Shah, V.; Srkalovic, G.; Mahtani, R.; Levine, E.; Mavromatis, B.; Srinivasiah, J.; Kassar, M.; Gabordi, R.; Qamar, R.; et al. MammaPrint guides treatment decisions in breast Cancer: Results of the IMPACt trial. BMC Cancer 2020, 20, 81. [Google Scholar] [CrossRef]
- Martín, M.; González-Rivera, M.; Morales, S.; de la Haba-Rodriguez, J.; González-Cortijo, L.; Manso, L.; Albanell, J.; González-Martín, A.; González, S.; Arcusa, A.; et al. Prospective study of the impact of the Prosigna assay on adjuvant clinical decision-making in unselected patients with estrogen receptor positive, human epidermal growth factor receptor negative, node negative earlystage breast cancer. Curr. Med. Res. Opin. 2015, 31, 1129–1137. [Google Scholar] [CrossRef]
- Lo, S.S.; Mumby, P.B.; Norton, J.; Rychlik, K.; Smerage, J.; Kash, J.; Chew, H.K.; Gaynor, E.R.; Hayes, D.F.; Epstein, A.; et al. Prospective multicenter study of the impact of the 21-gene recurrence score assay on medical oncologist and patient adjuvant breast cancer treatment selection. J. Clin. Oncol. 2010, 28, 1671–1676. [Google Scholar] [CrossRef]
- Oratz, R.; Kim, B.; Chao, C.; Skrzypczak, S.; Ory, C.; Bugarini, R.; Broder, M. Physician survey of the effect of the 21-gene recurrence score assay results on treatment recommendations for patients with lymph node-positive, estrogen receptor-positive breast cancer. J. Oncol. Pract. 2011, 7, 94–99. [Google Scholar] [CrossRef]
- Dieci, M.V.; Guarneri, V.; Zustovich, F.; Mion, M.; Morandi, P.; Bria, E.; Merlini, L.; Bullian, P.; Oliani, C.; Gori, S.; et al. Impact of 21-Gene Breast Cancer Assay on Treatment Decision for Patients with T1-T3, N0-N1, Estrogen Receptor-Positive/Human Epidermal Growth Receptor 2-Negative Breast Cancer: Final Results of the Prospective Multicenter ROXANE Study. Oncologist 2019, 24, 1424–1431. [Google Scholar] [CrossRef]
- Kuchel, A.; Robinson, T.; Comins, C.; Shere, M.; Varughese, M.; Sparrow, G.; Sahu, A.; Saunders, L.; Bahl, A.; Cawthorn, S.J.; et al. The impact of the 21-gene assay on adjuvant treatment decisions in oestrogen receptor-positive early breast cancer: A prospective study. Br. J. Cancer 2016, 114, 731–736. [Google Scholar] [CrossRef][Green Version]
- Wuerstlein, R.; Kates, R.; Gluz, O.; Grischke, E.M.; Schem, C.; Thill, M.; Hasmueller, S.; Köhler, A.; Otremba, B.; Griesinger, F.; et al. WSG-PRIMe investigators in Germany, Austria, Switzerland. Strong impact of MammaPrint and BluePrint on treatment decisions in luminal early breast cancer: Results of the WSG-PRIMe study. Breast Cancer Res. Treat. 2019, 175, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Penault-Llorca, F.; Kwiatkowski, F.; Arnaud, A.; Levy, C.; Leheurteur, M.; Uwer, L.; Derbel, O.; Le Rol, A.; Jacquin, J.P.; Jouannaud, C.; et al. Decision of adjuvant chemotherapy in intermediate risk luminal breast cancer patients: A prospective multicenter trial assessing the clinical and psychological impact of EndoPredict® (EpClin) use (UCBG 2-14). Breast 2020, 49, 132–140. [Google Scholar] [CrossRef]
- Cheng, R.; Kong, X.; Wang, X.; Fang, Y.; Wang, J. Oncotype DX Breast Recurrence Score Distribution and Chemotherapy Benefit Among Women of Different Age Groups With HR-Positive, HER2-Negative, Node-Negative Breast Cancer in the SEER Database. Front. Oncol. 2020, 10, 1583. [Google Scholar] [CrossRef]
- Crolley, V.E.; Marashi, H.; Rawther, S.; Sirohi, B.; Parton, M.; Graham, J.; Vinayan, A.; Sutherland, S.; Rigg, A.; Wadhawan, A.; et al. The impact of Oncotype DX breast cancer assay results on clinical practice: A UK experience. Breast Cancer Res. Treat. 2020, 180, 809–817. [Google Scholar] [CrossRef]
- Iles, K.; Roberson, M.L.; Spanheimer, P.; Gallagher, K.; Ollila, D.W.; Strassle, P.D.; Downs-Canner, S. The impact of age and nodal status on variations in oncotype DX testing and adjuvant treatment. NPJ Breast Cancer 2022, 8, 27. [Google Scholar] [CrossRef]
- McSorley, L.M.; Tharmabala, M.; Al Rahbi, F.; Keane, F.; Evoy, D.; Geraghty, J.G.; Rothwell, J.; McCartan, D.P.; Greally, M.; O’Connor, M.; et al. Real-World Analysis of the Clinical and Economic Impact of the 21-Gene Recurrence Score (RS) in Invasive Lobular Early-Stage Breast Carcinoma in Ireland. Curr. Oncol. 2024, 31, 1302–1310. [Google Scholar] [CrossRef]
- de Jongh, F.E.; Efe, R.; Herrmann, K.H.; Spoorendonk, J.A. Cost and Clinical Benefits Associated with Oncotype DX® Test in Patients with Early-Stage HR+/HER2- Node-Negative Breast Cancer in the Netherlands. Int. J. Breast Cancer 2022, 2022, 5909724. [Google Scholar] [CrossRef]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef]
- Pece, S.; Sestak, I.; Montani, F.; Tillhon, M.; Maisonneuve, P.; Freddi, S.; Chu, K.; Colleoni, M.; Veronesi, P.; Disalvatore, D.; et al. Comparison of StemPrintER with Oncotype DX Recurrence Score for predicting risk of breast cancer distant recurrence after endocrine therapy. Eur. J. Cancer 2022, 164, 52–61. [Google Scholar] [CrossRef]
| Characteristic | Mean (min.-max. Value) | Categories (%) |
|---|---|---|
| age (year) | 52.9 (25–75) | |
| <50 year | 86 (43%) | |
| ≥50 year | 115 (57%) | |
| grade I | 10 (5%) | |
| grade II | 122 (60.1%) | |
| grade III | 69 (34.3%) | |
| pT (mm) | 26 (2.8–85) | |
| 1a | 1 (0.5%) | |
| 1b | 2 (1%) | |
| 1c | 79 (39.3%) | |
| 2 | 106 (52.7%) | |
| 3 | 13 (6.5%) | |
| pN | ||
| 0 | 102 (50.7%) | |
| 1 | 98 (48.8%) | |
| 2 | 1 (0.5%) | |
| ER (%) | 94 (1–100) | |
| PR (%) | 59 (0–100) | missing: 1 |
| Ki67 (%) | 20 (<1–60) | |
| MAI | 12.5 (1–65) | missing: 6 |
| No positive lymph node | 0.73 (0–5) | |
| No excised lymph nodes | 4.16 (1–31) | |
| perinodal infiltration | ||
| yes | 62 (30.8%) | |
| no | 25 (12.4%) | |
| missing | 12 (6%) | |
| TILs (%) | 5.8 (0–70) | missing: 24 (11.9%) |
| vascular invasion | ||
| yes | 90 (44.8%) | |
| no | 102 (50.7%) | |
| missing | 10 (5%) | |
| NPI | 4.31 (2.5–5.7) | |
| RS | 19.85 (0–66) | |
| 0–10 | 33 (16.4%) | |
| 11–15 | 31 (15.4%) | |
| 16–20 | 48 (23.9%) | |
| 21–25 | 48 (23.9%) | |
| 26–30 | 14 (7%) | |
| >30 | 27 (13.4%) | |
| adjuvant chemotherapy | ||
| yes | 46 (22.9%) | |
| no | 155 (77%) |
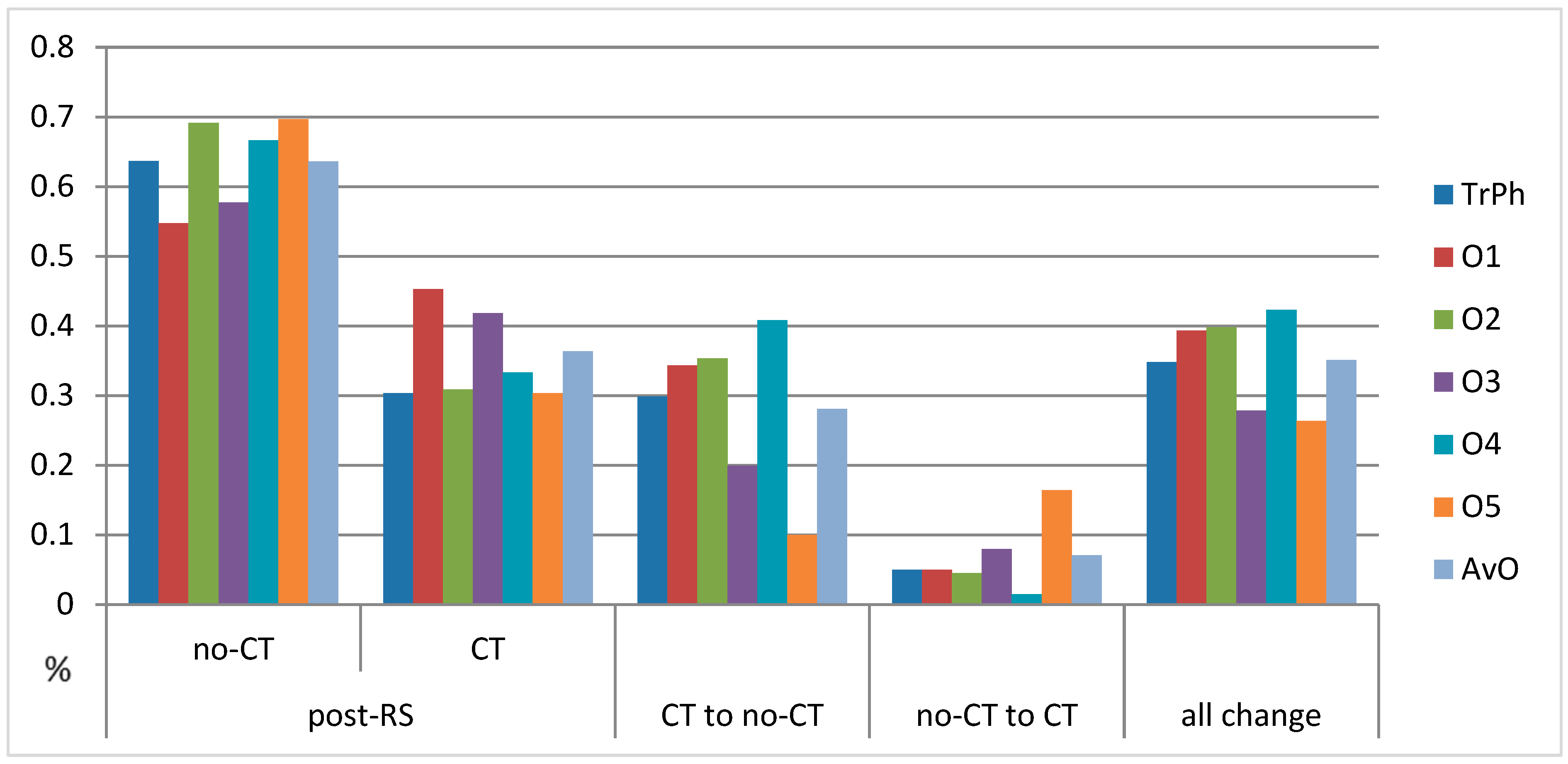
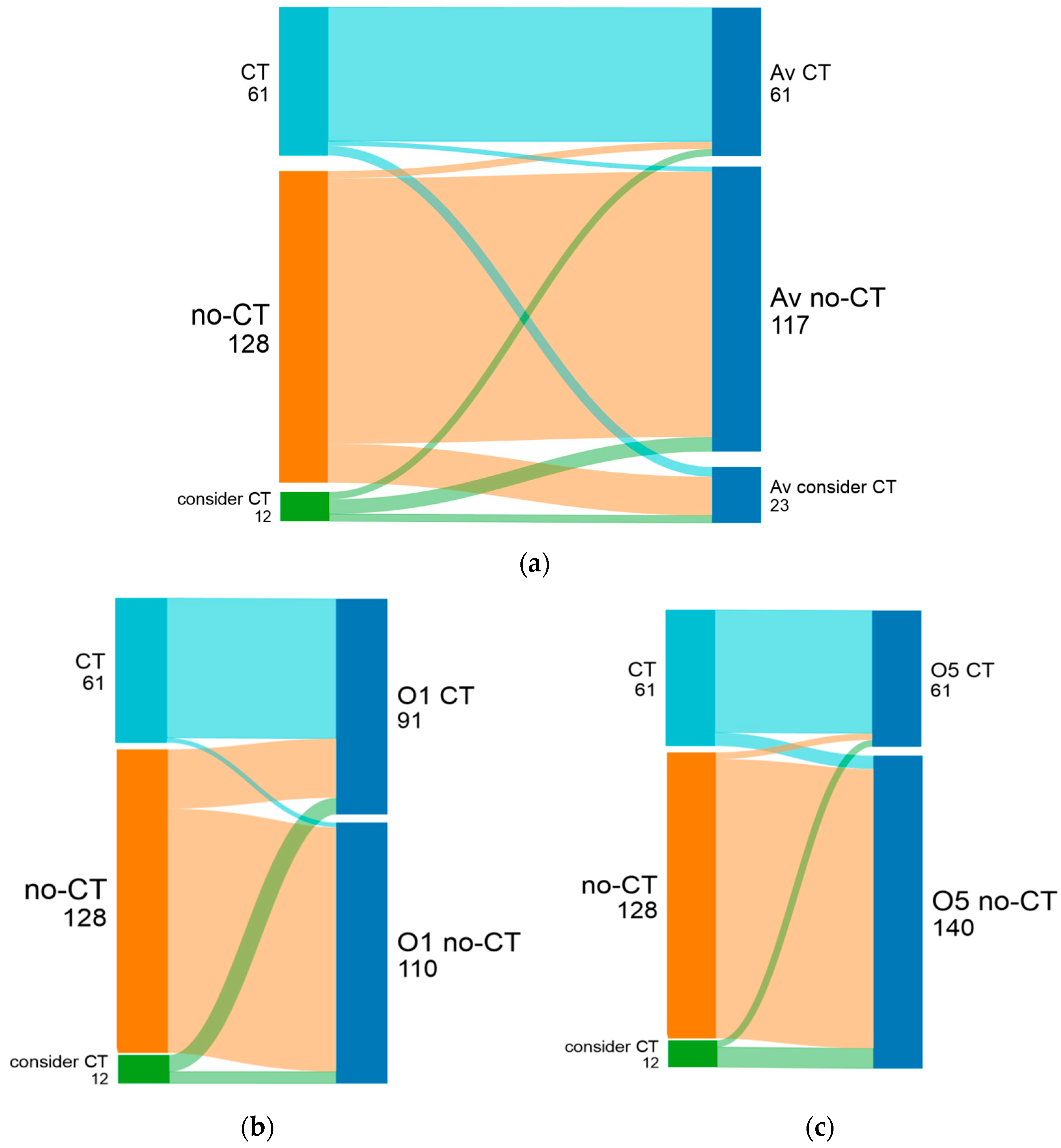
| Oncologist | Pre-RS | Post-RS | CT Recommendation Decrease (Percentage of All Patients) | Change from CT to No-CT | Change from No-CT to CT | All Changes | ||
|---|---|---|---|---|---|---|---|---|
| No-CT | CT | No-CT | CT | |||||
| treating physician | 82 (41%) | 119 (59%) | 128 (63.7%) | 61 (30.3%) | 48.7% (28.9%) uncertain after RS:12 (6%) | 60 (29.9%) | 10 (5%) | 70 (34.9%) |
| oncologist 1 | 51 (25.4%) | 150 (74.6%) | 110 (54.7%) | 91 (45.3%) | 35% (26.4%) | 69 (34.3%) | 10 (5%) | 79 (39.3) |
| oncologist 2 | 77 (38.3%) | 124 (61.7%) | 139 (69.2%) | 62 (62%) | 50% (30.8%) | 71 (35.3%) | 9 (4.5%) | 80 (39.8%) |
| oncologist 3 | 92 (45.8%) | 109 (54.2%) | 116 (57.7%) | 84 (41.8%) | 22.9% (12.4%) | 40 (19.9%) | 16 (8%) | 56 (27.9%) |
| oncologist 4 | 55 (27.4%) | 146 (72.6%) | 134 (66.7%) | 67 (33.3%) | 54.1% (39.3%) | 82 (40.8%) | 3 (1.5%) | 85 (42.3%) |
| oncologist 5 | 127 (63.2%) | 74 (36.8%) | 140 (69.7%) | 61 (30.3%) | 17.6% (6.5%) | 33 (16.4%) | 20 (10%) | 53 (26.4%) |
| average of oncologists 1–5 | 80.4 (40%) | 120.6 (60%) | 127.8 (63.6%) | 73 (36.3%) | 39.5% (23.7%) | 56.4 (28.1%) | 14.2 (7%) | 70.6 (35%) |
| Agreement Level | Pre-RS | Post-RS | ||
|---|---|---|---|---|
| complete concordance | 74 (36.8%) | no-CT: 23 (11.4%) | 153 (76.1%) | no-CT 103 (51.2%) |
| CT: 51 (25.4%) | CT 50 (24.9%) | |||
| concordant | 69 (34.3%) | no-CT: 28 (13.9%) | 25 (12.4%) | no-CT 14 (7%) |
| CT: 41 (20.4%) | CT 11 (5.4%) | |||
| discordance | 58 (28.9%) | 23 (11.4%) | ||
| NCCN Category (Number of Patients) | Concord 0 | Concord 1 | Concord 2 | Concord 3 | Concord 4 | Concord 5 |
|---|---|---|---|---|---|---|
| no-CT (n = 104) | 93 | 6 | 1 | 4 | 0 | 0 |
| CT (n = 41) | 0 | 0 | 0 | 0 | 1 | 40 |
| consider CT (n = 56) | 10 | 8 | 11 | 7 | 10 | 10 |
| Pre-RS Test Opinion for Chemotherapy by Experts | Number | RS Result | Final Decision of Experts | Chemotherapy Was Given |
|---|---|---|---|---|
| CCA | 23 | all RS ≤ 24 | CCA: 23 CA: 1 ambiguous: 1 | 1 |
| CA | 28 | RS ≤ 25: 27 RS ≥ 26: 1 | CCA: 24 CA: 3 ambiguous: 1 CCF: 1 | 0 |
| CF | 41 | RS ≤ 25: 34 RS ≥ 26: 7 | CCA: 16 CA: 4 ambiguous: 7 CF: 3 CCF: 11 | 9 |
| CCF | 51 | RS ≤ 25: 26 RS ≥ 26: 25 | CCA: 9 CA: 2 ambiguous: 5 CF: 4 CCF: 30 | 26 |
| Age | Grade (1–2 vs. 3) | T (1 vs. 2–3) | N (0 vs. 1) | ER (≥30% vs. <30%) | PR (≥30% vs. <30%) | Ki67 (≥20% vs. <20%) | MAI (≥20 vs. <20) | Vascular Invasion (Yes vs. No) | |
|---|---|---|---|---|---|---|---|---|---|
| O1 | ** | *** | |||||||
| O2 | *** | * | *** | *** | * | *** | |||
| O3 | *** | *** | * | ** | *** | ||||
| O4 | *** | * | *** | ** | |||||
| O5 | * | *** | *** | * | * | *** | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boér, K.; Kaposi, A.; Kocsis, J.; Horváth, Z.; Madaras, B.; Sávolt, Á.; Klément, G.B.; Rubovszky, G. How to Tackle Discordance in Adjuvant Chemotherapy Recommendations by Using Oncotype DX Results, in Early-Stage Breast Cancer. Cancers 2024, 16, 2928. https://doi.org/10.3390/cancers16172928
Boér K, Kaposi A, Kocsis J, Horváth Z, Madaras B, Sávolt Á, Klément GB, Rubovszky G. How to Tackle Discordance in Adjuvant Chemotherapy Recommendations by Using Oncotype DX Results, in Early-Stage Breast Cancer. Cancers. 2024; 16(17):2928. https://doi.org/10.3390/cancers16172928
Chicago/Turabian StyleBoér, Katalin, Ambrus Kaposi, Judit Kocsis, Zsolt Horváth, Balázs Madaras, Ákos Sávolt, Gyorgy Benjamin Klément, and Gábor Rubovszky. 2024. "How to Tackle Discordance in Adjuvant Chemotherapy Recommendations by Using Oncotype DX Results, in Early-Stage Breast Cancer" Cancers 16, no. 17: 2928. https://doi.org/10.3390/cancers16172928
APA StyleBoér, K., Kaposi, A., Kocsis, J., Horváth, Z., Madaras, B., Sávolt, Á., Klément, G. B., & Rubovszky, G. (2024). How to Tackle Discordance in Adjuvant Chemotherapy Recommendations by Using Oncotype DX Results, in Early-Stage Breast Cancer. Cancers, 16(17), 2928. https://doi.org/10.3390/cancers16172928






