Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment
Abstract
Simple Summary
Abstract
1. Introduction
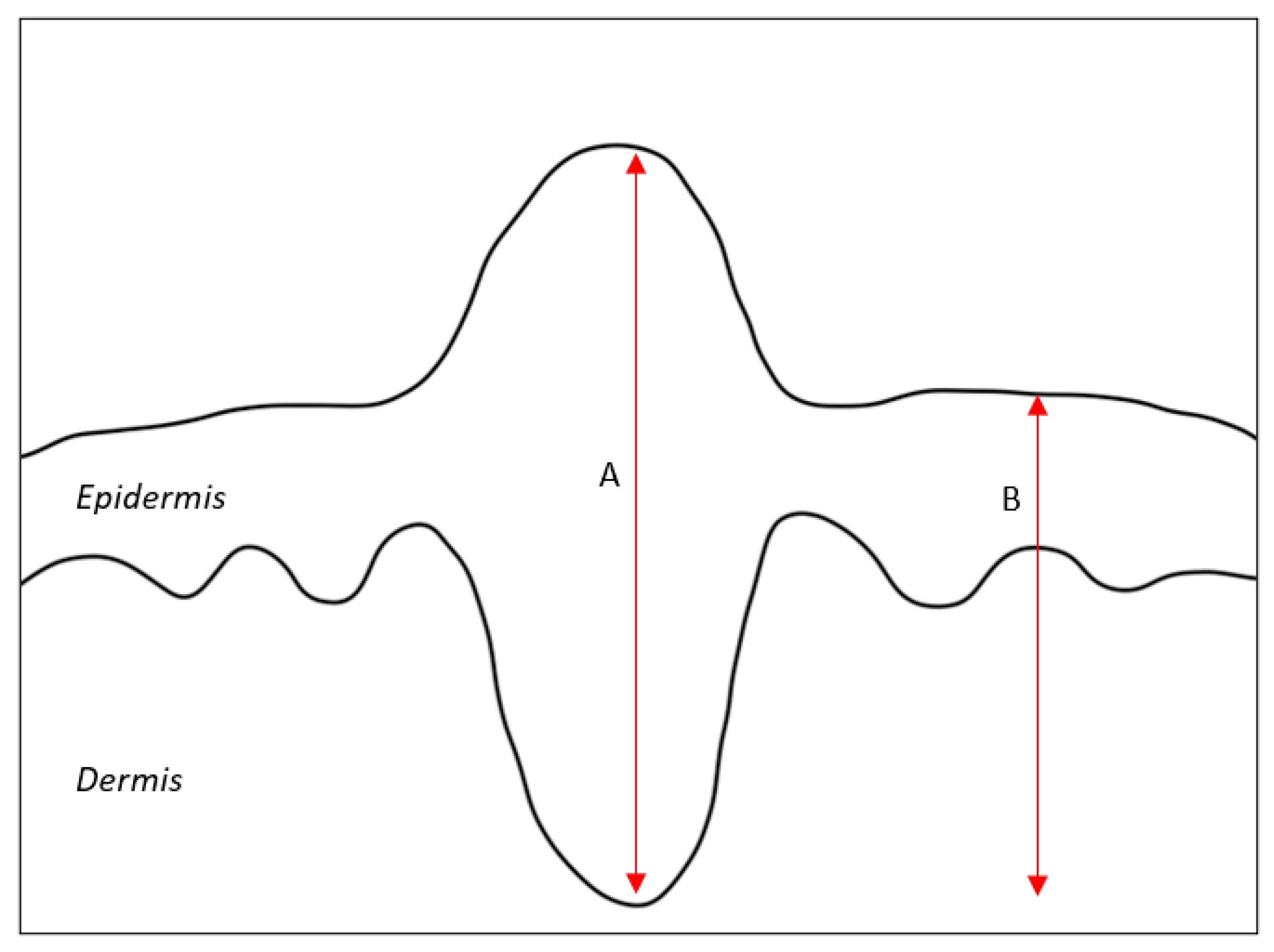
2. Depth of Invasion
3. Surgical Margins
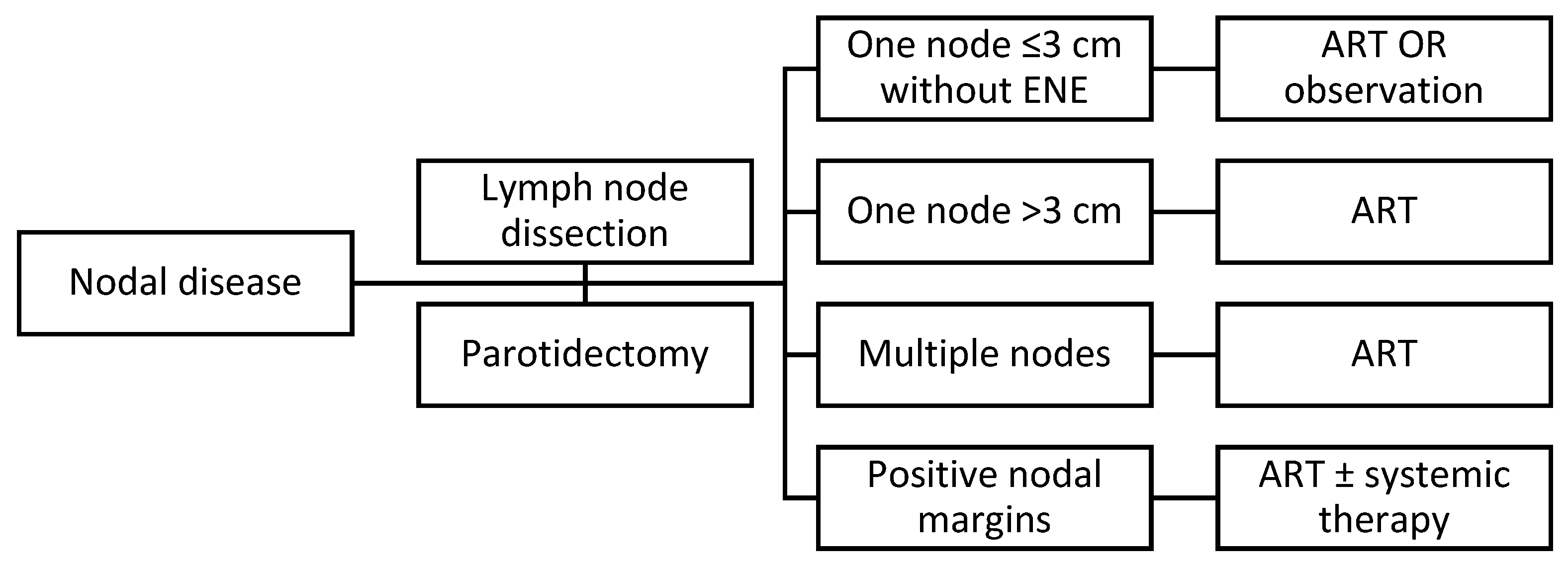
4. Positive Regional Node(s) and Extranodal Extension
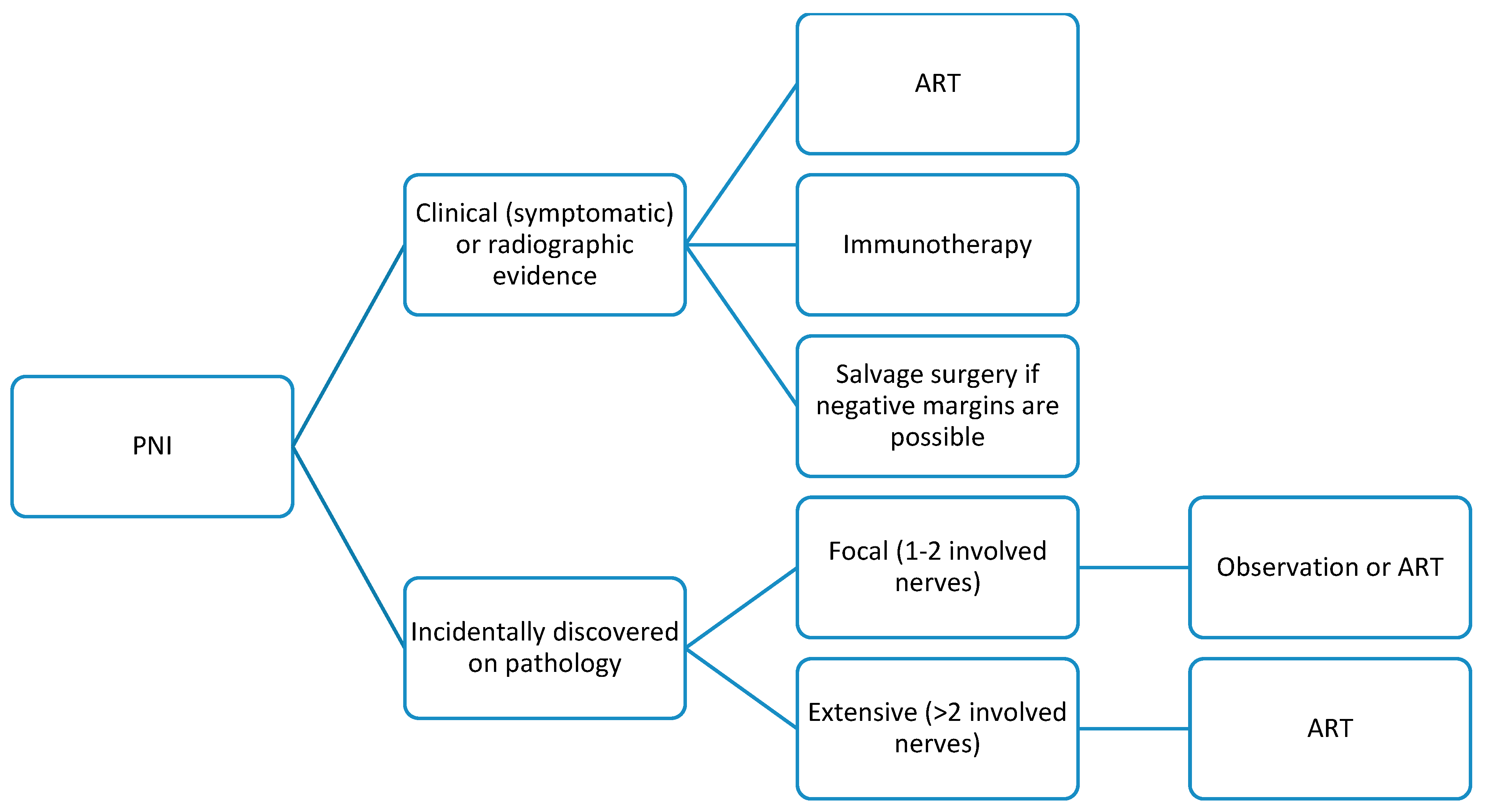
5. PNI
6. LVI
7. Tumor Grade
8. Histologic Subtype
9. Premalignant Lesions
10. Molecular Markers
11. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Que, S.K.T.; Zwald, F.O.; Schmults, C.D. Cutaneous squamous cell carcinoma: Incidence, risk factors, diagnosis, and staging. J. Am. Acad. Dermatol. 2018, 78, 237–247. [Google Scholar] [CrossRef]
- Lubov, J.; Labbe, M.; Sioufi, K.; Morand, G.B.; Hier, M.P.; Khanna, M.; Sultanem, K.; Mlynarek, A.M. Prognostic factors of head and neck cutaneous squamous cell carcinoma: A systematic review. J. Otolaryngol. Head. Neck Surg. 2021, 50, 54. [Google Scholar] [CrossRef]
- NCCN. Squamous Cell Skin Cancer (Version 1.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/squamous.pdf (accessed on 1 August 2024).
- Thompson, A.K.; Kelley, B.F.; Prokop, L.J.; Murad, M.H.; Baum, C.L. Risk Factors for Cutaneous Squamous Cell Carcinoma Recurrence, Metastasis, and Disease-Specific Death: A Systematic Review and Meta-analysis. JAMA Dermatol. 2016, 152, 419–428. [Google Scholar] [CrossRef]
- Edge, S.B.; Byrd, D.R.; Carducci, M.A.; Compton, C.C.; Fritz, A.; Greene, F. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2010; Volume 7. [Google Scholar]
- Amin, M.B.; Edge, S.B.; Greene, F.L.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; Volume 1024. [Google Scholar]
- Yildiz, P.; Aung, P.P.; Milton, D.R.; Hruska, C.; Ivan, D.; Nagarajan, P.; Tetzlaff, M.T.; Curry, J.L.; Torres-Cabala, C.; Prieto, V.G. Measurement of Tumor Thickness in Cutaneous Squamous Cell Carcinomas: Do the Different Methods Provide Better Prognostic Data? Am. J. Dermatopathol. 2020, 42, 337–342. [Google Scholar] [CrossRef]
- Marsidi, N.; Ottevanger, R.; Bouwes Bavinck, J.N.; Krekel-Taminiau, N.M.A.; Goeman, J.J.; Genders, R.E. Risk factors for incomplete excision of cutaneous squamous cell carcinoma: A large cohort study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1229–1234. [Google Scholar] [CrossRef]
- Ruiz, E.S.; Koyfman, S.A.; Que, S.K.T.; Kass, J.; Schmults, C.D. Evaluation of the utility of localized adjuvant radiation for node-negative primary cutaneous squamous cell carcinoma with clear histologic margins. J. Am. Acad. Dermatol. 2020, 82, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.Y.; Kim, S.K.; Ma, J.; Barker, C.A. Local recurrence and quality of life after adjuvant radiation therapy in high-risk squamous cell carcinoma. Br. J. Dermatol. 2019, 180, 417–418. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, C.B.; Andresen, N.S.; Kendell, N.; Al-Qurayshi, Z.; Pagedar, N.A. Survival Outcomes for Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck. Ann. Otol. Rhinol. Laryngol. 2019, 128, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Trosman, S.J.; Zhu, A.; Nicolli, E.A.; Leibowitz, J.M.; Sargi, Z.B. High-Risk Cutaneous Squamous Cell Cancer of the Head and Neck: Risk Factors for Recurrence and Impact of Adjuvant Treatment. Laryngoscope 2021, 131, E136–E143. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, G.A.; Pavlidis, L.; Trakatelli, M.; Foroglou, P.; Pagkalos, A.; Tsimponis, A.; Lampros, E.; Delimpaltas, A.; Demiri, E. Cutaneous squamous cell carcinoma with incomplete margins demonstrate higher tumour grade on re-excision. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1478–1481. [Google Scholar] [CrossRef] [PubMed]
- Genders, R.E.; Marsidi, N.; Michi, M.; Henny, E.P.; Goeman, J.J.; van Kester, M.S. Incomplete Excision of Cutaneous Squamous Cell Carcinoma; Systematic Review of the Literature. Acta Derm. Venereol. 2020, 100, adv00084. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.G.; Hall, M.A.; Kurley, S.J.; Cook, R.W.; Farberg, A.S.; Geiger, J.L.; Koyfman, S.A. Adjuvant therapy for high-risk cutaneous squamous cell carcinoma: 10-year review. Head. Neck 2021, 43, 2822–2843. [Google Scholar] [CrossRef] [PubMed]
- Maubec, E. Update of the Management of Cutaneous Squamous-cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00143. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Foncillas, J.; Tejera-Vaquerizo, A.; Sanmartin, O.; Rojo, F.; Mestre, J.; Martin, S.; Azinovic, I.; Mesia, R. Update on Management Recommendations for Advanced Cutaneous Squamous Cell Carcinoma. Cancers 2022, 14, 629. [Google Scholar] [CrossRef] [PubMed]
- Chambers, K.J.; Kraft, S.; Emerick, K. Evaluation of frozen section margins in high-risk cutaneous squamous cell carcinomas of the head and neck. Laryngoscope 2015, 125, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.A.; Weber, R.S.; Prieto, V.; El-Naggar, A.; Holsinger, F.C.; Zhou, X.; Lee, J.J.; Lippman, S.; Clayman, G.L. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope 2005, 115, 1561–1567. [Google Scholar] [CrossRef] [PubMed]
- Sahovaler, A.; Krishnan, R.J.; Yeh, D.H.; Zhou, Q.; Palma, D.; Fung, K.; Yoo, J.; Nichols, A.; MacNeil, S.D. Outcomes of Cutaneous Squamous Cell Carcinoma in the Head and Neck Region With Regional Lymph Node Metastasis: A Systematic Review and Meta-analysis. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Huis In’t Veld, E.A.; Boere, T.; Zuur, C.L.; Wouters, M.W.; van Akkooi, A.C.J.; Haanen, J.; Crijns, M.B.; Smith, M.J.; Mooyaart, A.; Wakkee, M.; et al. Oncological Outcome After Lymph Node Dissection for Cutaneous Squamous Cell Carcinoma. Ann. Surg. Oncol. 2023, 30, 5017–5026. [Google Scholar] [CrossRef]
- Knuutila, J.S.; Riihila, P.; Kurki, S.; Nissinen, L.; Kahari, V.M. Risk Factors and Prognosis for Metastatic Cutaneous Squamous Cell Carcinoma: A Cohort Study. Acta Derm. Venereol. 2020, 100, adv00266. [Google Scholar] [CrossRef]
- Varra, V.; Woody, N.M.; Reddy, C.; Joshi, N.P.; Geiger, J.; Adelstein, D.J.; Burkey, B.B.; Scharpf, J.; Prendes, B.; Lamarre, E.D.; et al. Suboptimal Outcomes in Cutaneous Squamous Cell Cancer of the Head and Neck with Nodal Metastases. Anticancer Res. 2018, 38, 5825–5830. [Google Scholar] [CrossRef]
- Amit, M.; Liu, C.; Gleber-Netto, F.O.; Kini, S.; Tam, S.; Benov, A.; Aashiq, M.; El-Naggar, A.K.; Moreno, A.C.; Rosenthal, D.I.; et al. Inclusion of extranodal extension in the lymph node classification of cutaneous squamous cell carcinoma of the head and neck. Cancer 2021, 127, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Kelder, W.; Ebrahimi, A.; Forest, V.I.; Gao, K.; Murali, R.; Clark, J.R. Cutaneous head and neck squamous cell carcinoma with regional metastases: The prognostic importance of soft tissue metastases and extranodal spread. Ann. Surg. Oncol. 2012, 19, 274–279. [Google Scholar] [CrossRef]
- Givi, B.; Andersen, P.E.; Diggs, B.S.; Wax, M.K.; Gross, N.D. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head. Neck 2011, 33, 999–1004. [Google Scholar] [CrossRef]
- Kampel, L.; Dorman, A.; Horowitz, G.; Fliss, D.M.; Gutfeld, O.; Muhanna, N. Surgically Treated Advanced Cutaneous Squamous Cell Carcinoma of the Head and Neck: Outcome Predictors and the Role of Adjuvant Radiation Therapy. Ann. Otol. Rhinol. Laryngol. 2021, 130, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Karia, P.S.; Morgan, F.C.; Ruiz, E.S.; Schmults, C.D. Clinical and Incidental Perineural Invasion of Cutaneous Squamous Cell Carcinoma: A Systematic Review and Pooled Analysis of Outcomes Data. JAMA Dermatol. 2017, 153, 781–788. [Google Scholar] [CrossRef]
- Sapir, E.; Tolpadi, A.; McHugh, J.; Samuels, S.E.; Elalfy, E.; Spector, M.; Shuman, A.G.; Malloy, K.M.; Prince, M.E.; Bradford, C.R.; et al. Skin cancer of the head and neck with gross or microscopic perineural involvement: Patterns of failure. Radiother. Oncol. 2016, 120, 81–86. [Google Scholar] [CrossRef]
- Wu, M.P.; Reinshagen, K.L.; Cunnane, M.B.; Shalhout, S.Z.; Kaufman, H.L.; Miller, D.; Emerick, K.S. Clinical Perineural Invasion and Immunotherapy for Head and Neck Cutaneous Squamous Cell Carcinoma. Laryngoscope 2022, 132, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Erkan, S.; Savundra, J.M.; Wood, B.; Acharya, A.N.; Rajan, G.P. Clinical perineural invasion of the trigeminal and facial nerves in cutaneous head and neck squamous cell carcinoma: Outcomes and prognostic implications of multimodality and salvage treatment. Head. Neck 2017, 39, 1280–1286. [Google Scholar] [CrossRef]
- Farah, M.; Milton, D.R.; Gross, N.D.; Nagarajan, P.; Gu, J.; Curry, J.L.; Ivan, D.; Torres-Cabala, C.A.; Myers, J.N.; Prieto, V.G.; et al. Histopathologic features predictive of metastasis and survival in 230 patients with cutaneous squamous cell carcinoma of the head and neck and non-head and neck locations: A single-center retrospective study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1246–1255. [Google Scholar] [CrossRef]
- Kus, K.J.B.; Murad, F.; Smile, T.D.; Chang, M.; Ashrafzadeh, S.; Zhou, G.; Ilori, E.O.; Koyfman, S.A.; Vidimos, A.T.; Schmults, C.D.; et al. Higher metastasis and death rates in cutaneous squamous cell carcinomas with lymphovascular invasion. J. Am. Acad. Dermatol. 2022, 86, 766–773. [Google Scholar] [CrossRef]
- Durham, A.B.; Lowe, L.; Malloy, K.M.; McHugh, J.B.; Bradford, C.R.; Chubb, H.; Johnson, T.M.; McLean, S.A. Sentinel Lymph Node Biopsy for Cutaneous Squamous Cell Carcinoma on the Head and Neck. JAMA Otolaryngol. Head. Neck Surg. 2016, 142, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Kadakia, S.; Ducic, Y.; Marra, D.; Saman, M. The role of elective superficial parotidectomy in the treatment of temporal region squamous cell carcinoma. Oral. Maxillofac. Surg. 2016, 20, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J.N.; Hajder, E.; van der Holt, B.; Den Bakker, M.A.; Hovius, S.E.; Mureau, M.A. The Effect of Differentiation Grade of Cutaneous Squamous Cell Carcinoma on Excision Margins, Local Recurrence, Metastasis, and Patient Survival: A Retrospective Follow-Up Study. Ann. Plast. Surg. 2015, 75, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Wako, B.D.; Dese, K.; Ulfata, R.E.; Nigatu, T.A.; Turunbedu, S.K.; Kwa, T. Squamous Cell Carcinoma of Skin Cancer Margin Classification From Digital Histopathology Images Using Deep Learning. Cancer Control 2022, 29, 10732748221132528. [Google Scholar] [CrossRef] [PubMed]
- Kiely, J.; Kostusiak, M.; Bloom, O.; Roshan, A. Poorly differentiated cutaneous squamous cell carcinomas have high incomplete excision rates with UK minimum recommended pre-determined surgical margins. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Edition, S.; Edge, S.; Byrd, D. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2017; Volume 19. [Google Scholar]
- Karia, P.S.; Morgan, F.C.; Califano, J.A.; Schmults, C.D. Comparison of Tumor Classifications for Cutaneous Squamous Cell Carcinoma of the Head and Neck in the 7th vs 8th Edition of the AJCC Cancer Staging Manual. JAMA Dermatol. 2018, 154, 175–181. [Google Scholar] [CrossRef]
- Kuo, T. Clear cell carcinoma of the skin. A variant of the squamous cell carcinoma that simulates sebaceous carcinoma. Am. J. Surg. Pathol. 1980, 4, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Adisa, A.O.; Olajide, M.A.; Olusanya, A.A. Clear cell variant of squamous cell carcinoma of skin: A report of a case. J. Oral. Maxillofac. Pathol. 2013, 17, 110–112. [Google Scholar] [CrossRef]
- Loesch, M.; Ganocy, S.J.; Jaworsky, C. Clear Cell Squamous Cell Carcinoma: Clinical and Histologic Parameters and a Review of the Literature. SKIN J. Cutan. Med. 2020, 4, 130–138. [Google Scholar] [CrossRef]
- Cassarino, D.S.; Derienzo, D.P.; Barr, R.J. Cutaneous squamous cell carcinoma: A comprehensive clinicopathologic classification. Part one. J. Cutan. Pathol. 2006, 33, 191–206. [Google Scholar] [CrossRef]
- Hollmig, S.T.; Sachdev, R.; Cockerell, C.J.; Posten, W.; Chiang, M.; Kim, J. Spindle cell neoplasms encountered in dermatologic surgery: A review. Dermatol. Surg. 2012, 38, 825–850. [Google Scholar] [CrossRef] [PubMed]
- Bennardo, L.; Passante, M.; Cameli, N.; Cristaudo, A.; Patruno, C.; Nistico, S.P.; Silvestri, M. Skin Manifestations after Ionizing Radiation Exposure: A Systematic Review. Bioengineering 2021, 8, 153. [Google Scholar] [CrossRef]
- Vandeweyer, E.; Sales, F.; Deraemaecker, R. Cutaneous verrucous carcinoma. Br. J. Plast. Surg. 2001, 54, 168–170. [Google Scholar] [CrossRef]
- Chong, S.; Huang, L.; Yu, H.; Huang, H.; Ming, W.K.; Ip, C.C.; Mu, H.H.; Li, K.; Zhang, X.; Lyu, J.; et al. Crafting a prognostic nomogram for the overall survival rate of cutaneous verrucous carcinoma using the surveillance, epidemiology, and end results database. Front. Endocrinol. 2023, 14, 1142014. [Google Scholar] [CrossRef]
- Ye, Q.; Hu, L.; Jia, M.; Deng, L.J.; Fang, S. Cutaneous verrucous carcinoma: A clinicopathological study of 21 cases with long-term clinical follow-up. Front. Oncol. 2022, 12, 953932. [Google Scholar] [CrossRef]
- Morteza Abedi, S.; Salama, S.; Alowami, S. Lymphoepithelioma-like carcinoma of the skin: Case report and approach to surgical pathology sign out. Rare Tumors 2013, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Lassen, C.B.; Lock-Andersen, J. Lymphoepithelioma-like Carcinoma of the Skin: A Case with Perineural Invasion. Plast. Reconstr. Surg. Glob. Open 2014, 2, e252. [Google Scholar] [CrossRef] [PubMed]
- Glaich, A.S.; Behroozan, D.S.; Cohen, J.L.; Goldberg, L.H. Lymphoepithelioma-like carcinoma of the skin: A report of two cases treated with complete microscopic margin control and review of the literature. Dermatol. Surg. 2006, 32, 316–319. [Google Scholar] [CrossRef]
- Breuninger, H.; Schaumburg-Lever, G.; Holzschuh, J.; Horny, H.P. Desmoplastic squamous cell carcinoma of skin and vermilion surface: A highly malignant subtype of skin cancer. Cancer 1997, 79, 915–919. [Google Scholar] [CrossRef]
- Brantsch, K.D.; Meisner, C.; Schonfisch, B.; Trilling, B.; Wehner-Caroli, J.; Rocken, M.; Breuninger, H. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008, 9, 713–720. [Google Scholar] [CrossRef]
- Eigentler, T.K.; Leiter, U.; Hafner, H.M.; Garbe, C.; Rocken, M.; Breuninger, H. Survival of Patients with Cutaneous Squamous Cell Carcinoma: Results of a Prospective Cohort Study. J. Investig. Dermatol. 2017, 137, 2309–2315. [Google Scholar] [CrossRef]
- Neugebauer, R.; Su, K.A.; Zhu, Z.; Sokil, M.; Chren, M.M.; Friedman, G.D.; Asgari, M.M. Comparative effectiveness of treatment of actinic keratosis with topical fluorouracil and imiquimod in the prevention of keratinocyte carcinoma: A cohort study. J. Am. Acad. Dermatol. 2019, 80, 998–1005. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, C.P.H.; Bakos, R.M. Actinic keratoses: Review of clinical, dermoscopic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 637–657. [Google Scholar] [CrossRef]
- Balcere, A.; Konrade-Jilmaza, L.; Paulina, L.A.; Cema, I.; Krumina, A. Clinical Characteristics of Actinic Keratosis Associated with the Risk of Progression to Invasive Squamous Cell Carcinoma: A Systematic Review. J. Clin. Med. 2022, 11, 5899. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, V.R.; Mercer, S.E.; Phelps, R.G. Histopathological variants of cutaneous squamous cell carcinoma: A review. J. Skin. Cancer 2011, 2011, 210813. [Google Scholar] [CrossRef] [PubMed]
- Gutzmer, R.; Wiegand, S.; Kolbl, O.; Wermker, K.; Heppt, M.; Berking, C. Actinic Keratosis and Cutaneous Squamous Cell Carcinoma. Dtsch. Arztebl. Int. 2019, 116, 616–626. [Google Scholar] [CrossRef]
- Worley, B.; Harikumar, V.; Reynolds, K.; Dirr, M.A.; Christensen, R.E.; Anvery, N.; Yi, M.D.; Poon, E.; Alam, M. Treatment of actinic keratosis: A systematic review. Arch. Dermatol. Res. 2023, 315, 1099–1108. [Google Scholar] [CrossRef]
- Ferrandiz, C.; Malvehy, J.; Guillen, C.; Ferrandiz-Pulido, C.; Fernandez-Figueras, M. Precancerous Skin Lesions. Actas Dermosifiliogr. 2017, 108, 31–41. [Google Scholar] [CrossRef]
- Eimpunth, S.; Goldenberg, A.; Hamman, M.S.; Oganesyan, G.; Lee, R.A.; Hunnangkul, S.; Song, S.S.; Greywal, T.; Jiang, S.I.B. Squamous Cell Carcinoma In Situ Upstaged to Invasive Squamous Cell Carcinoma: A 5-Year, Single Institution Retrospective Review. Dermatol. Surg. 2017, 43, 698–703. [Google Scholar] [CrossRef]
- Palaniappan, V.; Karthikeyan, K. Bowen’s Disease. Indian Dermatol. Online J. 2022, 13, 177–189. [Google Scholar] [CrossRef]
- Morton, C.A.; Birnie, A.J.; Eedy, D.J. British Association of Dermatologists’ guidelines for the management of squamous cell carcinoma in situ (Bowen’s disease) 2014. Br. J. Dermatol. 2014, 170, 245–260. [Google Scholar] [CrossRef]
- Lukas VanderSpek, L.A.; Pond, G.R.; Wells, W.; Tsang, R.W. Radiation therapy for Bowen’s disease of the skin. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 505–510. [Google Scholar] [CrossRef]
- Jansen, M.H.E.; Kessels, J.; Nelemans, P.J.; Kouloubis, N.; Arits, A.; van Pelt, H.P.A.; Quaedvlieg, P.J.F.; Essers, B.A.B.; Steijlen, P.M.; Kelleners-Smeets, N.W.J.; et al. Randomized Trial of Four Treatment Approaches for Actinic Keratosis. N. Engl. J. Med. 2019, 380, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Hesse, K.; Satzger, I.; Schacht, V.; Kother, B.; Hillen, U.; Klode, J.; Schaper, K.; Gutzmer, R. Characterisation of Prognosis and Invasion of Cutaneous Squamous Cell Carcinoma by Podoplanin and E-Cadherin Expression. Dermatology 2016, 232, 558–565. [Google Scholar] [CrossRef]
- Mulvaney, P.M.; Massey, P.R.; Yu, K.K.; Drinan, J.E.; Schmults, C.D. Differential Molecular Expression Patterns Associated with Metastasis in Cutaneous Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. J. Investig. Dermatol. 2021, 141, 2161–2169. [Google Scholar] [CrossRef]
- Kreppel, M.; Krakowezki, A.; Kreppel, B.; Drebber, U.; Wedemeyer, I.; Mauch, C.; Zoller, J.E.; Scheer, M. Podoplanin expression in cutaneous head and neck squamous cell carcinoma--prognostic value and clinicopathologic implications. J. Surg. Oncol. 2013, 107, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Mulvaney, P.M.; Schmults, C.D. Molecular prediction of metastasis in cutaneous squamous cell carcinoma. Curr. Opin. Oncol. 2020, 32, 129–136. [Google Scholar] [CrossRef]
- Kamiya, S.; Kato, J.; Kamiya, T.; Yamashita, T.; Sumikawa, Y.; Hida, T.; Horimoto, K.; Sato, S.; Takahashi, H.; Sawada, M.; et al. Association between PD-L1 expression and lymph node metastasis in cutaneous squamous cell carcinoma. Asia Pac. J. Clin. Oncol. 2020, 16, e108–e112. [Google Scholar] [CrossRef] [PubMed]
- Canueto, J.; Cardenoso, E.; Garcia, J.L.; Santos-Briz, A.; Castellanos-Martin, A.; Fernandez-Lopez, E.; Blanco Gomez, A.; Perez-Losada, J.; Roman-Curto, C. Epidermal growth factor receptor expression is associated with poor outcome in cutaneous squamous cell carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Dean, N.R.; Magnuson, J.S.; Carroll, W.R.; Helman, E.E.; Hyde, S.O.; Desmond, R.L.; Rosenthal, E.L. EGFR expression in advanced head and neck cutaneous squamous cell carcinoma. Head. Neck 2012, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Balasescu, E.; Gheorghe, A.C.; Moroianu, A.; Turcu, G.; Brinzea, A.; Antohe, M.; Hodorogea, A.; Manea, L.; Balaban, M.; Andrei, R.; et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamous-cell carcinomas (Review). Exp. Ther. Med. 2022, 23, 383. [Google Scholar] [CrossRef] [PubMed]
| Feature | Threshold | Recommendations |
|---|---|---|
| DOI | 6 mm from adjacent uninvolved granular layer | No specific therapy recommendations |
| Invasion beyond subcutaneous fat | ||
| Margins | Positive | Re-excision if feasible, ART ± systemic therapy if not |
| LVI | Present | Evaluate for nodal disease and treat appropriately |
| ENE | Present | ART ± systemic therapy |
| PNI | Clinical or radiographic | ART |
| Microscopic | ART vs. observation | |
| Grade | Poor differentiation | Evaluate margins and treat appropriately |
| Subtype | Desmoplastic | ART vs. neoadjuvant cemiplimab |
| Molecular markers | PD-L1, Podoplanin, EGFR | Evaluate for nodal/distant metastasis and treat appropriately |
| Treatment Options | Indicated Feature(s) |
|---|---|
| ART | PNI |
| Positive margins with unfavorable resection | |
| Multiple lymph nodes or one node >3 cm | |
| ENE | |
| LVI | |
| Poorly differentiated primary tumor | |
| Desmoplastic subtype | |
| Re-operation | Positive margins |
| PNI if adequate margins are feasible | |
| Immunotherapy | PNI |
| Desmoplastic subtype | |
| Other systemic therapy | Only as ART adjunct or if ART is infeasible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, U.; Chiang, E.; Stafford, H.; Buell, J.; Materia, F.; Amit, M.; Yaniv, D. Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment. Cancers 2024, 16, 2866. https://doi.org/10.3390/cancers16162866
Ramesh U, Chiang E, Stafford H, Buell J, Materia F, Amit M, Yaniv D. Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment. Cancers. 2024; 16(16):2866. https://doi.org/10.3390/cancers16162866
Chicago/Turabian StyleRamesh, Uma, Elizabeth Chiang, Haleigh Stafford, Jane Buell, Frank Materia, Moran Amit, and Dan Yaniv. 2024. "Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment" Cancers 16, no. 16: 2866. https://doi.org/10.3390/cancers16162866
APA StyleRamesh, U., Chiang, E., Stafford, H., Buell, J., Materia, F., Amit, M., & Yaniv, D. (2024). Cutaneous Squamous Cell Carcinoma of the Head and Neck: Pathological Features and What They Mean for Prognosis and Treatment. Cancers, 16(16), 2866. https://doi.org/10.3390/cancers16162866







