The Pivotal Role of the Key Angiogenic Factors in the Development of Endometrioid Pathologies of the Uterus and Ovary
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Cultivation Protocols
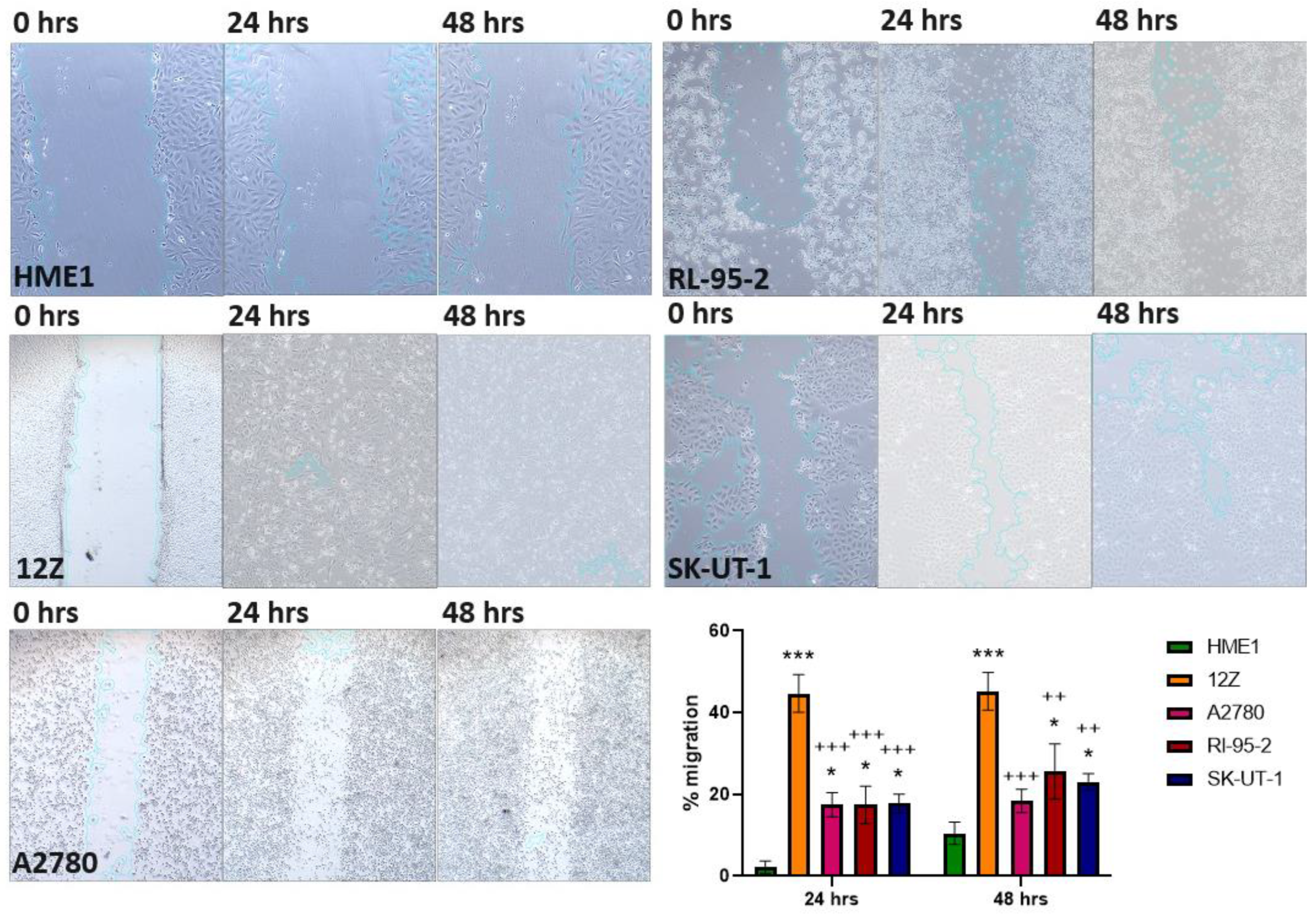
2.2. Wound Healing Assay
2.3. mRNA Isolation and PCR
2.4. Statistical Analysis
3. Results
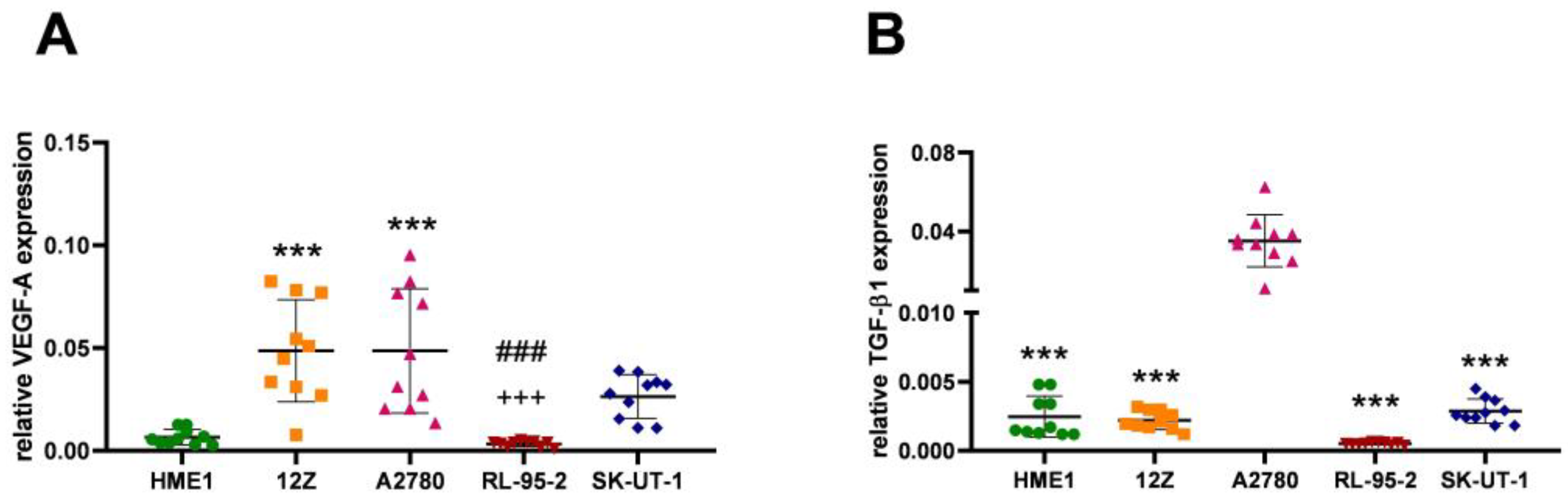
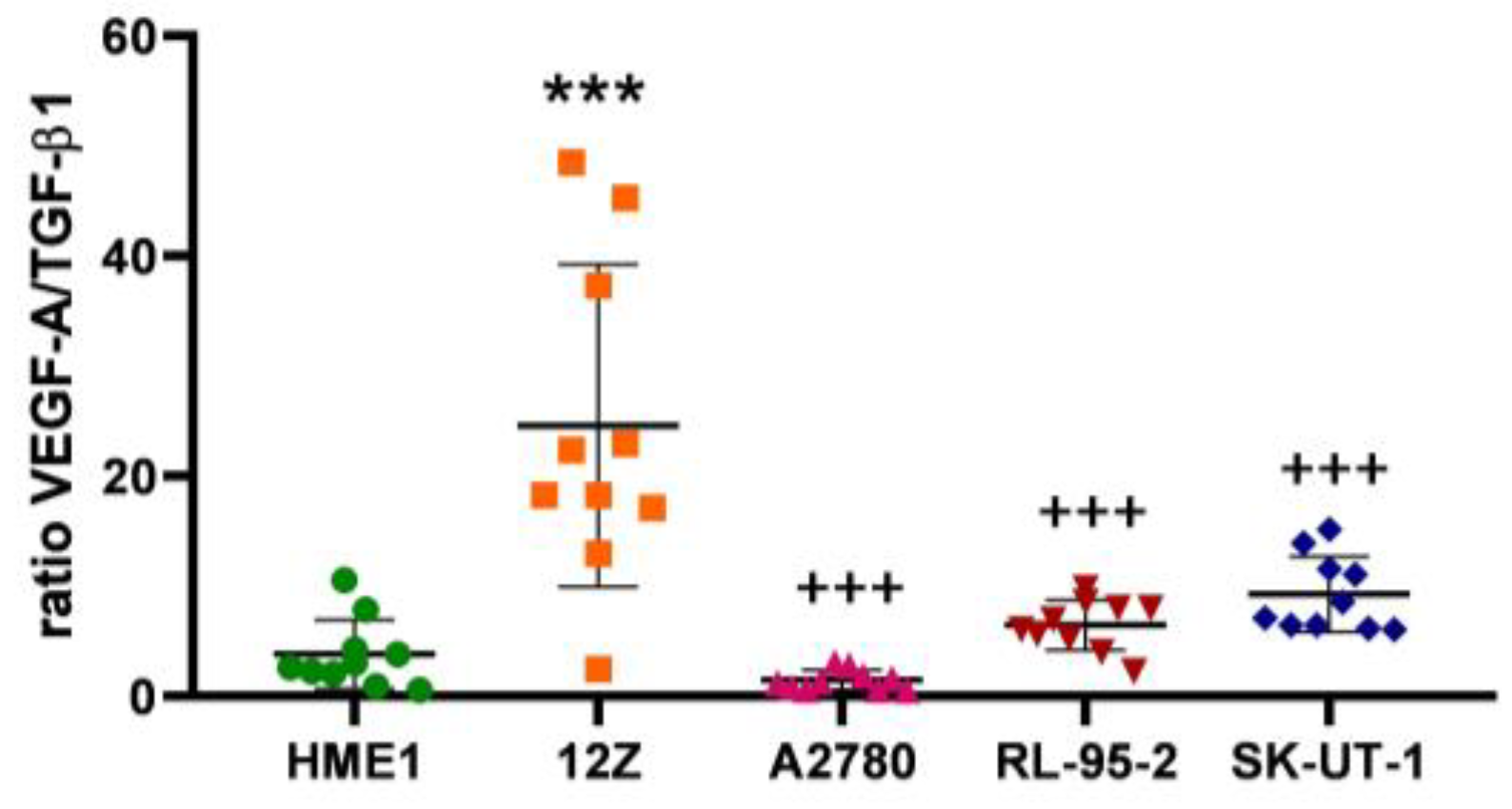
3.1. Angiogenesis Regulated via the VEGF-A/TGF-β1 Axis
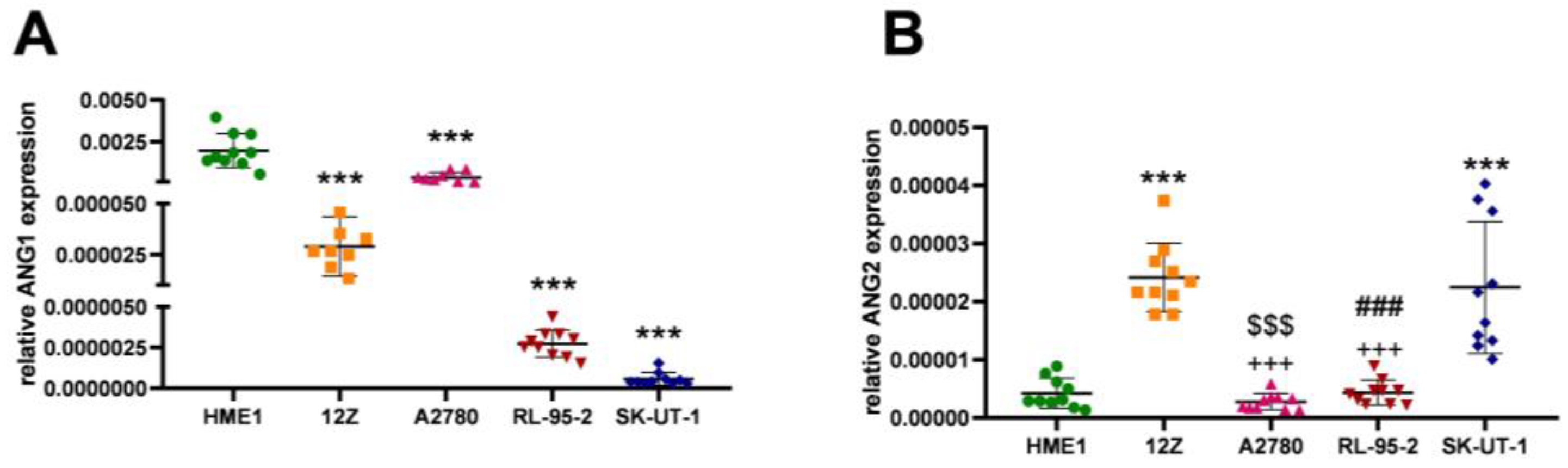
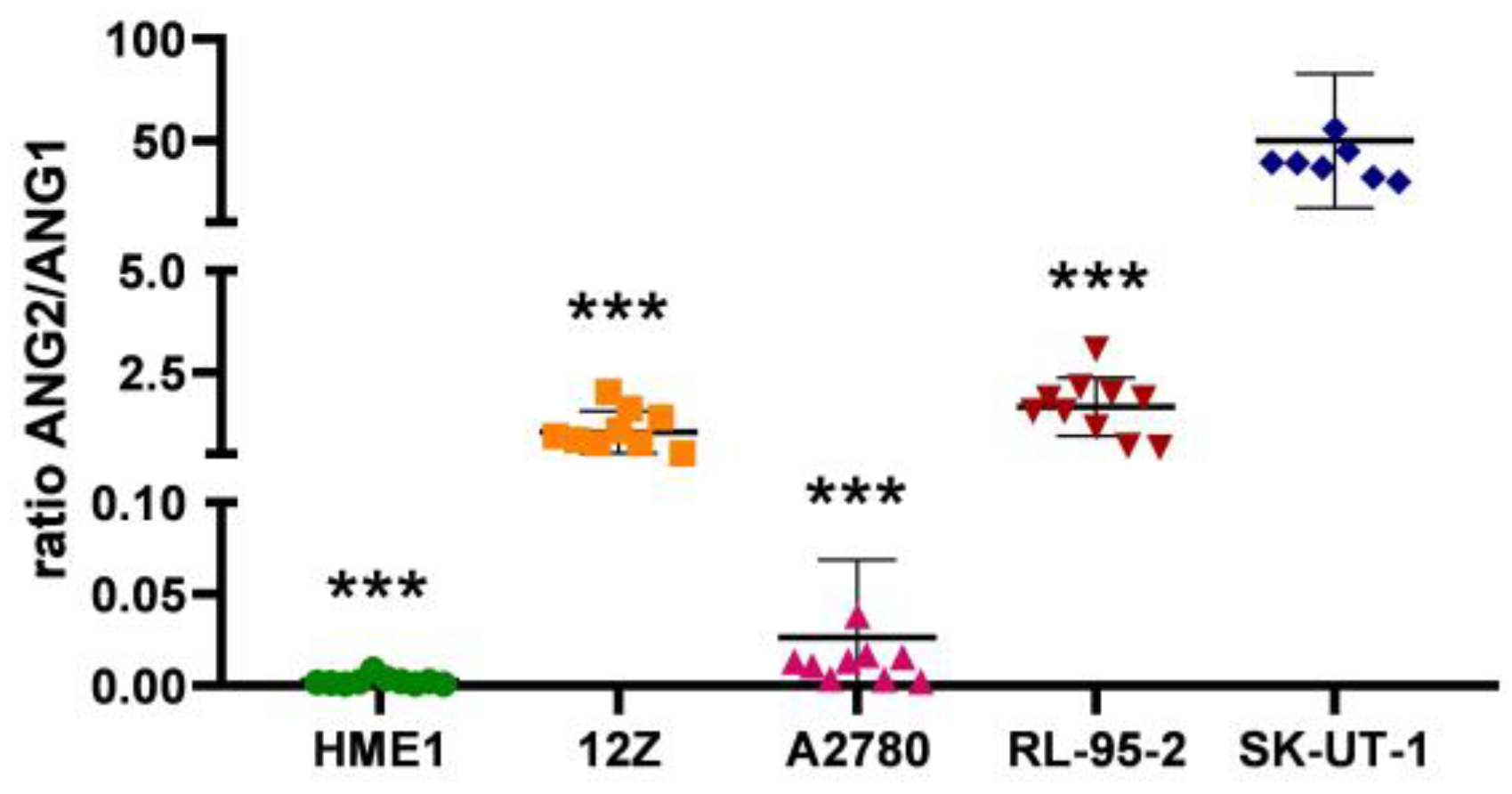
3.2. Angiogenesis Regulated via the ANG2/ANG1 Axis
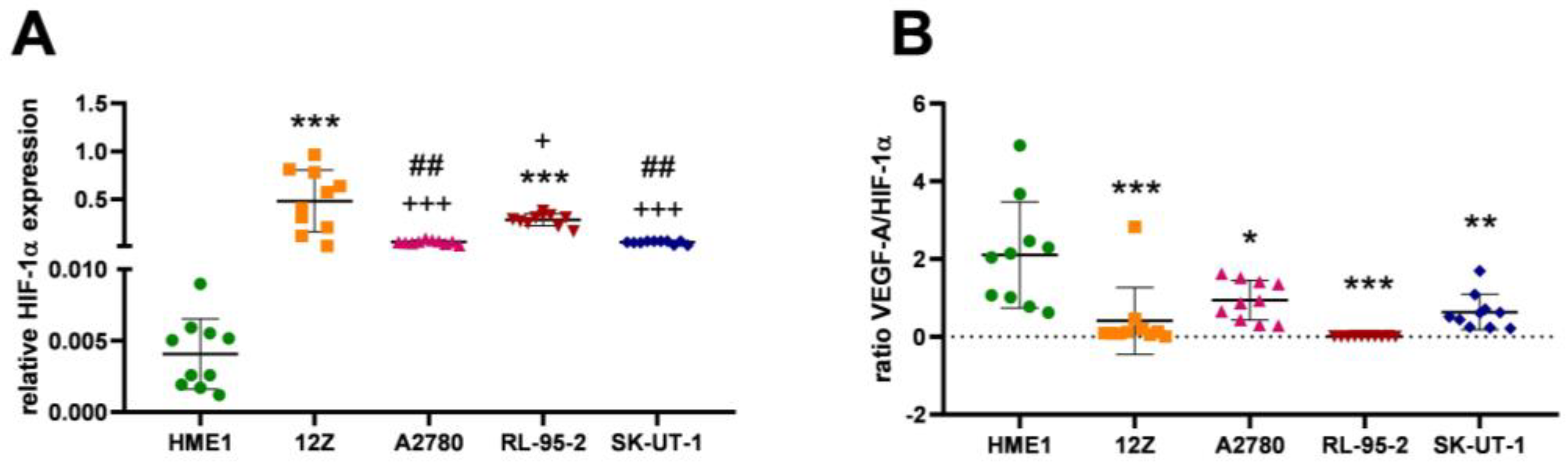
3.3. Angiogenesis Regulated via Hypoxia
3.4. Wound Healing Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, R.N.; Lebovic, D.I.; Mueller, M.D. Angiogenic Factors in Endometriosis. Ann. N. Y. Acad. Sci. USA 2002, 955, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.S.; Han, S.J. Endometriosis-Associated Angiogenesis and Anti-Angiogenic Therapy for Endometriosis. Front. Glob. Womens Health 2022, 3, 856316. [Google Scholar] [CrossRef]
- Shibuya, M. Differential Roles of Vascular Endothelial Growth Factor Receptor-1 and Receptor-2 in Angiogenesis. BMB Rep. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, J.; Zhu, J.; Yin, X.; You, H.; Lin, Y.; Zhu, H. MicroRNA-125 Inhibits RKO Colorectal Cancer Cell Growth by Targeting VEGF. Int. J. Mol. Med. 2018, 42, 665–673. [Google Scholar] [CrossRef]
- Mathonnet, M. Hallmarks in Colorectal Cancer: Angiogenesis and Cancer Stem-like Cells. World J. Gastroenterol. 2014, 20, 4189. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF Blockade Inhibits Angiogenesis and Reepithelialization of Endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.K.; Dey, S.K. Differential Expression of Vascular Endothelial Growth Factor and Its Receptor mRNAs in the Mouse Uterus around the Time of Implantation. J. Endocrinol. 1995, 147, 339–352. [Google Scholar] [CrossRef]
- Wittko-Schneider, I.M.; Schneider, F.T.; Plate, K.H. Brain Homeostasis: VEGF Receptor 1 and 2—Two Unequal Brothers in Mind. Cell. Mol. Life Sci. 2013, 70, 1705–1725. [Google Scholar] [CrossRef]
- Lan, J.; Li, H.; Luo, X.; Hu, J.; Wang, G. BRG1 Promotes VEGF-A Expression and Angiogenesis in Human Colorectal Cancer Cells. Exp. Cell Res. 2017, 360, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Geindreau, M.; Bruchard, M.; Vegran, F. Role of Cytokines and Chemokines in Angiogenesis in a Tumor Context. Cancers 2022, 14, 2446. [Google Scholar] [CrossRef]
- Choudhry, H.; Harris, A.L. Advances in Hypoxia-Inducible Factor Biology. Cell Metab. 2018, 27, 281–298. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1α. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Moradi, M.; Mousavi, A.; Emamgholipour, Z.; Giovannini, J.; Moghimi, S.; Peytam, F.; Honarmand, A.; Bach, S.; Foroumadi, A. Quinazoline-Based VEGFR-2 Inhibitors as Potential Anti-Angiogenic Agents: A Contemporary Perspective of SAR and Molecular Docking Studies. Eur. J. Med. Chem. 2023, 259, 115626. [Google Scholar] [CrossRef]
- Nishigaki, A.; Okada, H.; Tsuzuki, T.; Cho, H.; Yasuda, K.; Kanzaki, H. Angiopoietin 1 and Angiopoietin 2 in Follicular Fluid of Women Undergoing a Long Protocol. Fertil. Steril. 2011, 96, 1378–1383. [Google Scholar] [CrossRef]
- Michinaga, S.; Koyama, Y. Angiopoietin-1/Tie-2 Signaling in Traumatic Brain Injury. In Cellular, Molecular, Physiological, and Behavioral Aspects of Traumatic Brain Injury; Elsevier: Amsterdam, The Netherlands, 2022; pp. 219–230. ISBN 978-0-12-823036-7. [Google Scholar]
- Davis, S.; Aldrich, T.H.; Jones, P.F.; Acheson, A.; Compton, D.L.; Jain, V.; Ryan, T.E.; Bruno, J.; Radziejewski, C.; Maisonpierre, P.C.; et al. Isolation of Angiopoietin-1, a Ligand for the TIE2 Receptor, by Secretion-Trap Expression Cloning. Cell 1996, 87, 1161–1169. [Google Scholar] [CrossRef]
- Bate, N.; Lodge, J.; Brindle, N.P.J. Intrinsic Differences in the Mechanisms of Tie2 Binding to Angiopoietins Exploited by Directed Evolution to Create an Ang2-Selective Ligand Trap. J. Biol. Chem. 2021, 297, 100888. [Google Scholar] [CrossRef]
- Khan, A.A.; Sandhya, V.K.; Singh, P.; Parthasarathy, D.; Kumar, A.; Advani, J.; Gattu, R.; Ranjit, D.V.; Vaidyanathan, R.; Mathur, P.P.; et al. Signaling Network Map of Endothelial TEK Tyrosine Kinase. J. Signal Transduct. 2014, 2014, 173026. [Google Scholar] [CrossRef]
- Thurston, G.; Rudge, J.S.; Ioffe, E.; Zhou, H.; Ross, L.; Croll, S.D.; Glazer, N.; Holash, J.; McDonald, D.M.; Yancopoulos, G.D. Angiopoietin-1 Protects the Adult Vasculature against Plasma Leakage. Nat. Med. 2000, 6, 460–463. [Google Scholar] [CrossRef]
- Hashizume, H.; Falcón, B.L.; Kuroda, T.; Baluk, P.; Coxon, A.; Yu, D.; Bready, J.V.; Oliner, J.D.; McDonald, D.M. Complementary Actions of Inhibitors of Angiopoietin-2 and VEGF on Tumor Angiogenesis and Growth. Cancer Res. 2010, 70, 2213–2223. [Google Scholar] [CrossRef]
- Goede, V.; Schmidt, T.; Kimmina, S.; Kozian, D.; Augustin, H.G. Analysis of Blood Vessel Maturation Processes during Cyclic Ovarian Angiogenesis. Lab. Investig. 1998, 78, 1385–1394. [Google Scholar]
- Tsuzuki, T.; Okada, H.; Cho, H.; Shimoi, K.; Miyashiro, H.; Yasuda, K.; Kanzaki, H. Divergent Regulation of Angiopoietin-1, Angiopoietin-2, and Vascular Endothelial Growth Factor by Hypoxia and Female Sex Steroids in Human Endometrial Stromal Cells. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 95–101. [Google Scholar] [CrossRef]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef]
- Ferrari, G.; Cook, B.D.; Terushkin, V.; Pintucci, G.; Mignatti, P. Transforming Growth Factor-beta 1 (TGF-β1) Induces Angiogenesis through Vascular Endothelial Growth Factor (VEGF)-mediated Apoptosis. J. Cell. Physiol. 2009, 219, 449–458. [Google Scholar] [CrossRef]
- Pollman, M.J.; Naumovski, L.; Gibbons, G.H. Endothelial Cell Apoptosis in Capillary Network Remodeling. J. Cell. Physiol. 1999, 178, 359–370. [Google Scholar] [CrossRef]
- Larrivée, B.; Prahst, C.; Gordon, E.; del Toro, R.; Mathivet, T.; Duarte, A.; Simons, M.; Eichmann, A. ALK1 Signaling Inhibits Angiogenesis by Cooperating with the Notch Pathway. Dev. Cell 2012, 22, 489–500. [Google Scholar] [CrossRef]
- Roman, B.L.; Hinck, A.P. ALK1 Signaling in Development and Disease: New Paradigms. Cell. Mol. Life Sci. 2017, 74, 4539–4560. [Google Scholar] [CrossRef]
- Yu, X.; Ye, F. Role of Angiopoietins in Development of Cancer and Neoplasia Associated with Viral Infection. Cells 2020, 9, 457. [Google Scholar] [CrossRef]
- Yang, P.; Chen, N.; Yang, D.; Crane, J.; Yang, S.; Wang, H.; Dong, R.; Yi, X.; Xie, L.; Jing, G.; et al. The Ratio of Serum Angiopoietin-1 to Angiopoietin-2 in Patients with Cervical Cancer Is a Valuable Diagnostic and Prognostic Biomarker. PeerJ 2017, 5, e3387. [Google Scholar] [CrossRef]
- Sfiligoi, C.; De Luca, A.; Cascone, I.; Sorbello, V.; Fuso, L.; Ponzone, R.; Biglia, N.; Audero, E.; Arisio, R.; Bussolino, F.; et al. Angiopoietin-2 Expression in Breast Cancer Correlates with Lymph Node Invasion and Short Survival. Int. J. Cancer 2003, 103, 466–474. [Google Scholar] [CrossRef]
- Hong, S. Ratio of Angiopoietin-2 to Angiopoietin-1 Predicts Mortality in Acute Lung Injury Induced by Paraquat. Med. Sci. Monit. 2013, 19, 28–33. [Google Scholar] [CrossRef][Green Version]
- Fang, Y.; Li, C.; Shao, R.; Yu, H.; Zhang, Q.; Zhao, L. Prognostic Significance of the Angiopoietin-2/Angiopoietin-1 and Angiopoietin-1/Tie-2 Ratios for Early Sepsis in an Emergency Department. Crit. Care 2015, 19, 367. [Google Scholar] [CrossRef] [PubMed]
- Palazon, A.; Tyrakis, P.A.; Macias, D.; Veliça, P.; Rundqvist, H.; Fitzpatrick, S.; Vojnovic, N.; Phan, A.T.; Loman, N.; Hedenfalk, I.; et al. An HIF-1α/VEGF-A Axis in Cytotoxic T Cells Regulates Tumor Progression. Cancer Cell 2017, 32, 669–683. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Bhat, G.R.; Besina, S.; Thakur, N.; Zahoor, S.; Rather, R.A.; Mushtaq, I.; Dar, S.; Rah, B.; Bhat, A.A.; et al. Expression of HIF-1α and Markers of Angiogenesis and Metabolic Adaptation in Molecular Subtypes of Breast Cancer. Transl. Med. Commun. 2023, 8, 2. [Google Scholar] [CrossRef]
- Rmali, K.A.; Watkins, G.; Douglas-Jones, A.; Mansel, R.E.; Jiang, W.G. Angiopoietins Lack of Prognostic Significance in Ductal Mammary Carcinoma. Int. Semin. Surg. Oncol. 2007, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhang, M.; Mao, X.-Y.; Chang, H.; Perez-Losada, J.; Mao, J.-H. Distinct Clinical Impact and Biological Function of Angiopoietin and Angiopoietin-like Proteins in Human Breast Cancer. Cells 2021, 10, 2590. [Google Scholar] [CrossRef]
- Young, V.J.; Ahmad, S.F.; Brown, J.K.; Duncan, W.C.; Horne, A.W. Peritoneal VEGF-A Expression Is Regulated by TGF-Β1 through an ID1 Pathway in Women with Endometriosis. Sci. Rep. 2015, 5, 16859. [Google Scholar] [CrossRef]
- Delbandi, A.-A.; Mahmoudi, M.; Shervin, A.; Heidari, S.; Kolahdouz-Mohammadi, R.; Zarnani, A.-H. Evaluation of Apoptosis and Angiogenesis in Ectopic and Eutopic Stromal Cells of Patients with Endometriosis Compared to Non-Endometriotic Controls. BMC Women’s Health 2020, 20, 3. [Google Scholar] [CrossRef]
- Dziobek, K.; Opławski, M.; Grabarek, B.O.; Zmarzły, N.; Tomala, B.; Halski, T.; Leśniak, E.; Januszyk, K.; Brus, R.; Kiełbasiński, R.; et al. Changes in the Expression Profile of VEGF-A, VEGF-B, VEGFR-1, VEGFR-2 in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 955–963. [Google Scholar] [CrossRef]
- Prager, G.W.; Poettler, M.; Unseld, M.; Zielinski, C.C. Angiogenesis in Cancer: Anti-VEGF Escape Mechanisms. Transl. Lung Cancer Res. 2012, 1, 14–25. [Google Scholar] [CrossRef]
- Arablou, T.; Aryaeian, N.; Khodaverdi, S.; Kolahdouz-Mohammadi, R.; Moradi, Z.; Rashidi, N.; Delbandi, A.-A. The Effects of Resveratrol on the Expression of VEGF, TGF-β, and MMP-9 in Endometrial Stromal Cells of Women with Endometriosis. Sci. Rep. 2021, 11, 6054. [Google Scholar] [CrossRef]
- Li, X.; Hu, Z.; Shi, H.; Wang, C.; Lei, J.; Cheng, Y. Inhibition of VEGFA Increases the Sensitivity of Ovarian Cancer Cells to Chemotherapy by Suppressing VEGFA-Mediated Autophagy. Onco Targets Ther. 2020, 13, 8161–8171. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Watanabe, Y.; Nakai, H.; Hata, T.; Hoshiai, H. Expression of the Vascular Endothelial Growth Factor (VEGF) Gene in Epithelial Ovarian Cancer: An Approach to Anti-VEGF Therapy. Anticancer. Res. 2011, 31, 731–737. [Google Scholar] [PubMed]
- Fujimoto, J.; Ichigo, S.; Hirose, R.; Sakaguchi, H.; Tamaya, T. Expressions of Vascular Endothelial Growth Factor (VEGF) and Its mRNA in Uterine Endometrial Cancers. Cancer Lett. 1998, 134, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Emoto, M.; Charnock-Jones, D.S.; Licence, D.R.; Ishiguro, M.; Kawai, M.; Yanaihara, A.; Saito, T.; Hachisuga, T.; Iwasaki, H.; Kawarabayashi, T.; et al. Localization of the VEGF and Angiopoietin Genes in Uterine Carcinosarcoma. Gynecol. Oncol. 2004, 95, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Näyhä, V.; Stenbäck, F. Angiogenesis and Expression of Angiogenic Agents in Uterine and Ovarian Carcinosarcomas. APMIS 2008, 116, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.C.; Haisley, C.; Hurteau, J.; Moser, T.L.; Whitaker, R.; Bast, R.C.; Stack, M.S. Regulation of Invasion of Epithelial Ovarian Cancer by Transforming Growth Factor-β. Gynecol. Oncol. 2001, 80, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Shi, Y.; Dong, M. Measurements of interleukin-6, interleukin-8 and transforming growth factor-beta 1 levels in peritoneal fluid of patients with endometriosis. Zhonghua Fu Chan Ke Za Zhi 2000, 35, 329–331. [Google Scholar] [PubMed]
- Young, V.J.; Ahmad, S.F.; Duncan, W.C.; Horne, A.W. The Role of TGF-β in the Pathophysiology of Peritoneal Endometriosis. Hum. Reprod. Update 2017, 23, 548–559. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, A.A.; Lee, L.R.; Raboteau, D.; Hamilton, C.A.; Maxwell, G.L.; Rodriguez, G.C.; Syed, V. Progesterone Inhibits Endometrial Cancer Invasiveness by Inhibiting the TGFβ Pathway. Cancer Prev. Res. 2014, 7, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Klar, M.; Matsuzaki, S.; Roman, L.D.; Sood, A.K.; Matsuo, K. Uterine Carcinosarcoma: Contemporary Clinical Summary, Molecular Updates, and Future Research Opportunity. Gynecol. Oncol. 2021, 160, 586–601. [Google Scholar] [CrossRef]
- Dwivedi, S.K.D.; Rao, G.; Dey, A.; Buechel, M.; Zhang, Y.; Zhang, M.; Yang, D.; Mukherjee, P.; Bhattacharya, R. Targeting the TGFβ Pathway in Uterine Carcinosarcoma. Cell Stress 2020, 4, 252–260. [Google Scholar] [CrossRef]
- Shi, X.; Yang, J.; Deng, S.; Xu, H.; Wu, D.; Zeng, Q.; Wang, S.; Hu, T.; Wu, F.; Zhou, H. TGF-β Signaling in the Tumor Metabolic Microenvironment and Targeted Therapies. J. Hematol. Oncol. 2022, 15, 135. [Google Scholar] [CrossRef]
- Guerrero, P.A.; McCarty, J.H. TGF-β Activation and Signaling in Angiogenesis. In Physiologic and Pathologic Angiogenesis—Signaling Mechanisms and Targeted Therapy; Simionescu, D., Simionescu, A., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3023-9. [Google Scholar]
- Mallikarjuna, P.; Zhou, Y.; Landström, M. The Synergistic Cooperation between TGF-β and Hypoxia in Cancer and Fibrosis. Biomolecules 2022, 12, 635. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, Q.; Lian, X.; Jiang, P.; Cui, J. Hypoxia-Inducible Factor-1α (HIF-1α) Promotes Hypoxia-Induced Invasion and Metastasis in Ovarian Cancer by Targeting Matrix Metallopeptidase 13 (MMP13). Med. Sci. Monit. 2019, 25, 7202–7208. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Qiu, H.; Song, H.; Feng, D.; Jiang, Y.; Deng, S.; Meng, H.; Geng, J. AEG-1 Contributes to Metastasis in Hypoxia-Related Ovarian Cancer by Modulating the HIF-1alpha/NF-kappaB/VEGF Pathway. BioMed Res. Int. 2018, 2018, 3145689. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Y.; Feng, Y.; Gao, S. SNAIL Gene Inhibited by Hypoxia-Inducible Factor 1α (HIF-1α) in Epithelial Ovarian Cancer. Int. J. Immunopathol. Pharmacol. 2016, 29, 364–375. [Google Scholar] [CrossRef]
- Filippi, I.; Carrarelli, P.; Luisi, S.; Batteux, F.; Chapron, C.; Naldini, A.; Petraglia, F. Different Expression of Hypoxic and Angiogenic Factors in Human Endometriotic Lesions. Reprod. Sci. 2016, 23, 492–497. [Google Scholar] [CrossRef]
- Varga, J.; Reviczká, A.; Háková, H.; Švajdler, P.; Rabajdová, M.; Ostró, A. Predictive Factors of Endometriosis Progression into Ovarian Cancer. J. Ovarian Res. 2022, 15, 5. [Google Scholar] [CrossRef]
- Drenkhahn, M.; Gescher, D.M.; Wolber, E.-M.; Meyhoefer-Malik, A.; Malik, E. Expression of Angiopoietin 1 and 2 in Ectopic Endometrium on the Chicken Chorioallantoic Membrane. Fertil. Steril. 2004, 81, 869–875. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Wakikawa, A.; Ueno, A.; Nagai, R.; Matsumoto, M.; Komatsu, J.; Kinoshita, H.; Minami, S.; Hayashi, K. Comparison of Endometriotic Cysts and Ovarian Cancer in Association with Endometriotic Cysts. Cancer Treat. Res. Commun. 2018, 14, 26–29. [Google Scholar] [CrossRef]
- Hu, B.; Cheng, S.-Y. Angiopoietin-2: Development of Inhibitors for Cancer Therapy. Curr. Oncol. Rep. 2009, 11, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Aspriţoiu, V.M.; Stoica, I.; Bleotu, C.; Diaconu, C.C. Epigenetic Regulation of Angiogenesis in Development and Tumors Progression: Potential Implications for Cancer Treatment. Front. Cell Dev. Biol. 2021, 9, 689962. [Google Scholar] [CrossRef] [PubMed]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial Cell Migration During Angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef] [PubMed]
- Kuzmic, N.; Moore, T.; Devadas, D.; Young, E.W.K. Modelling of Endothelial Cell Migration and Angiogenesis in Microfluidic Cell Culture Systems. Biomech. Model. Mechanobiol. 2019, 18, 717–731. [Google Scholar] [CrossRef]
- Motizuki, M.; Koinuma, D.; Yokoyama, T.; Itoh, Y.; Omata, C.; Miyazono, K.; Saitoh, M.; Miyazawa, K. TGF-β-Induced Cell Motility Requires Downregulation of ARHGAPs to Sustain Rac1 Activity. J. Biol. Chem. 2021, 296, 100545. [Google Scholar] [CrossRef] [PubMed]
| Target Gene | Forward | Reverse |
|---|---|---|
| Β-Actin | AGAGCCCAGTCTTCATTGCT | TGTCCTGTTGCATACCGTCT |
| VEGF-A | ATAAGTCCTGGAGCGTTCCCT | GTTTAACTCAAGCTGCCTCGC |
| TGF-β1 | AAGATGGAGAGAGGACTGCG | AGAGGGAGAGAGAGGGAGTG |
| ANG1 | CGTGGAACCGGATTTCTCTTC | TGGGCCATCTCCGACTTCAT |
| ANG2 | AACCAAACAGCGGAGCAAAC | AGGGAGTGTTCCAAGAGCTG |
| HIF-1α | GACCGATTCACCATGGAGGG | GTGGCAACTGATGAGCAAGC |
| Cell Line | VEGF-A | TGF-β1 |
|---|---|---|
| HME1 | 0.006709 ± 0.003730 | 0.002470 ± 0.001492 |
| 12Z | 0.048710 ± 0.024825 | 0.002206 ± 0.000678 |
| A2780 | 0.048700 ± 0.030207 | 0.035170 ± 0.013240 |
| RL-95-2 | 0.003395 ± 0.001363 | 0.000519 ± 0.000094 |
| SK-UT-1 | 0.026450 ± 0.010603 | 0.002874 ± 0.000883 |
| 95.00% CI VEGF-A | 95.00% CI TGF-β1 | |
| HME1 vs. 12Z | −0.06514 to −0.01887 | −0.007337 to 0.007860 |
| HME1 vs. A2780 | −0.06512 to −0.01886 | −0.04030 to −0.02511 |
| HME1 vs. RL-95-2 | −0.01982 to 0.02645 | −0.005648 to 0.009549 |
| HME1 vs. SK-UT-1 | −0.04288 to 0.003388 | −0.008005 to 0.007192 |
| 12Z vs. A2780 | −0.02312 to 0.02315 | −0.04057 to −0.02537 |
| 12Z vs. RL-95-2 | 0.02219 to 0.06845 | −0.005909 to 0.009288 |
| 12Z vs. SK-UT-1 | −0.0008711 to 0.04539 | −0.008267 to 0.006930 |
| A2780 vs. RL-95-2 | 0.02217 to 0.06844 | 0.02706 to 0.04225 |
| A2780 vs. SK-UT-1 | −0.0008844 to 0.04538 | 0.02470 to 0.03990 |
| RL-95-2 vs. SK-UT-1 | −0.04619 to 7.441 × 10−5 | −0.009956 to 0.005241 |
| Cell Line | ANG1 | ANG2 |
|---|---|---|
| HME1 | 0.001974 ± 0.001028 | 0.000004 ± 0.000003 |
| 12Z | 0.000029 ± 0.000015 | 0.000024 ± 0.000006 |
| A2780 | 0.000035 ± 0.000297 | 0.000003 ± 0.000001 |
| RL-95-2 | 0.000003 ± 0.000001 | 0.000004 ± 0.000002 |
| SK-UT-1 | 0.000001 ± 0.000004 | 0.000022 ± 0.000011 |
| 95.00% CI ANG1 | 95.00% CI ANG2 | |
| HME1 vs. 12Z | 0.001337 to 0.002553 | −2.750 × 10−5 to −1.238 × 10−5 |
| HME1 vs. A2780 | 0.001014 to 0.002231 | −6.098 × 10−6 to 9.020 × 10−6 |
| HME1 vs. RL-95-2 | 0.001363 to 0.002580 | −7.684 × 10−6 to 7.434 × 10−6 |
| HME1 vs. SK-UT-1 | 0.001365 to 0.002582 | −2.577 × 10−5 to −1.065 × 10−5 |
| 12Z vs. A2780 | −0.0009312 to 0.0002856 | 1.384 × 10−5 to 2.896 × 10−5 |
| 12Z vs. RL-95-2 | −0.0005822 to 0.0006346 | 1.226 × 10−5 to 2.738 × 10−5 |
| 12Z vs. SK-UT-1 | −0.0005801 to 0.0006367 | −5.829 × 10−6 to 9.289 × 10−6 |
| A2780 vs. RL-95-2 | −0.0002594 to 0.0009574 | −9.145 × 10−6 to 5.973 × 10−6 |
| A2780 vs. SK-UT-1 | −0.0002573 to 0.0009595 | −2.723 × 10−5 to −1.211 × 10−5 |
| RL-95-2 vs. SK-UT-1 | −0.0006062 to 0.0006106 | −2.565 × 10−5 to −1.053 × 10−5 |
| Cell Line | HIF-1α |
|---|---|
| HME1 | 0.004057 ± 0.002464 |
| 12Z | 0.481800 ± 0.320725 |
| A2780 | 0.053650 ± 0.018822 |
| RL-95-2 | 0.284200 ± 0.061784 |
| SK-UT-1 | 0.049860 ± 0.016882 |
| 95.00% CI HIF-1α | |
| HME1 vs. 12Z | −0.6639 to −0.2915 |
| HME1 vs. A2780 | −0.2358 to 0.1366 |
| HME1 vs. RL-95-2 | −0.4663 to −0.09396 |
| HME1 vs. SK-UT-1 | −0.2320 to 0.1404 |
| 12Z vs. A2780 | 0.2419 to 0.6143 |
| 12Z vs. RL-95-2 | 0.01139 to 0.3837 |
| 12Z vs. SK-UT-1 | 0.2457 to 0.6181 |
| A2780 vs. RL-95-2 | −0.4167 to −0.04437 |
| A2780 vs. SK-UT-1 | −0.1824 to 0.1900 |
| RL-95-2 vs. SK-UT-1 | 0.04816 to 0.4205 |
| Cell Line | 24 h | 48 h |
|---|---|---|
| HME1 | 2.226 ± 1.237 | 10.468 ± 2.254 |
| 12Z | 44.682 ± 23.244 | 45.237 ± 17.074 |
| A2780 | 17.464 ± 3.772 | 18.427 ± 3.746 |
| RL-95-2 | 17.468 ± 16.385 | 25.578 ± 16.192 |
| SK-UT-1 | 17.712 ± 2.431 | 23.083 ± 2.319 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabolová, G.; Špaková, I.; Artimovič, P.; Bohuš, P.; Rabajdová, M.; Mareková, M. The Pivotal Role of the Key Angiogenic Factors in the Development of Endometrioid Pathologies of the Uterus and Ovary. Cancers 2024, 16, 2772. https://doi.org/10.3390/cancers16162772
Sabolová G, Špaková I, Artimovič P, Bohuš P, Rabajdová M, Mareková M. The Pivotal Role of the Key Angiogenic Factors in the Development of Endometrioid Pathologies of the Uterus and Ovary. Cancers. 2024; 16(16):2772. https://doi.org/10.3390/cancers16162772
Chicago/Turabian StyleSabolová, Gabriela, Ivana Špaková, Peter Artimovič, Peter Bohuš, Miroslava Rabajdová, and Mária Mareková. 2024. "The Pivotal Role of the Key Angiogenic Factors in the Development of Endometrioid Pathologies of the Uterus and Ovary" Cancers 16, no. 16: 2772. https://doi.org/10.3390/cancers16162772
APA StyleSabolová, G., Špaková, I., Artimovič, P., Bohuš, P., Rabajdová, M., & Mareková, M. (2024). The Pivotal Role of the Key Angiogenic Factors in the Development of Endometrioid Pathologies of the Uterus and Ovary. Cancers, 16(16), 2772. https://doi.org/10.3390/cancers16162772






