Prediction of PSA Response after Dexamethasone Switch during Abiraterone Acetate + Prednisolone Treatment of Metastatic Castration-Resistant Prostate Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Treatment and Follow-Up
2.2. Laboratory Measurements
2.3. Statistics
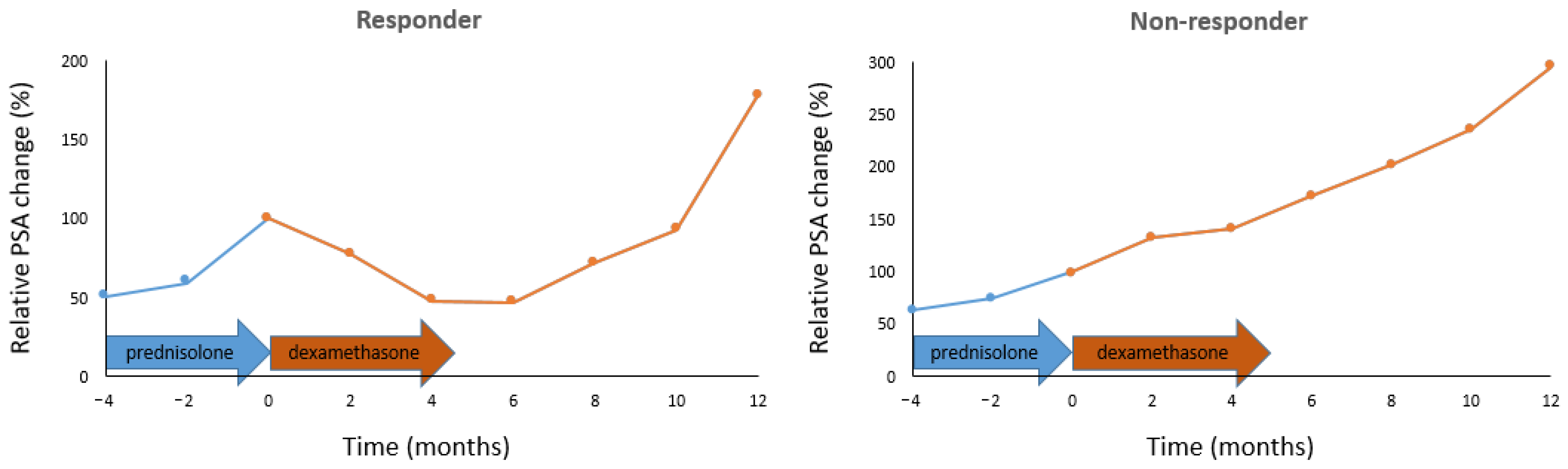
3. Results
4. Discussion
- -
- The presence of melanocortin receptors (MCR) in prostate cancer [41,42] raises the importance of ACTH inhibition, since ACTH is a ligand for MCR [43] and exclusively for melanocortin 2 receptor and promotes prostate cancer cell progression in a concentration-dependent manner [41]. ACTH production decreases to 33% after 1.5 mg of dexamethasone given for 3 weeks (p = 10−6) [35], while 10 mg of prednisolone given for 8 months decreases ACTH to only 58% (p > 0.05) [44].
- -
- -
- IL-6 is another mediator of prostate cancer progression to the castration-resistant state and promotion of tumor metastasis and resistance [24,48]. IL-6 is inhibited by dexamethasone at an order of magnitude lower concentration than prednisolone (IC50 prednisolone = 0.7 × 10−7 vs. IC50 dexamethasone = 0.5 × 10−8 [49].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Szarvas, T.; Csizmarik, A.; Nagy, N.; Keresztes, D.; Váradi, M.; Küronya, Z.; Riesz, P.; Nyirády, P. Molecular underpinnings of systemic treatment resistance in metastatic castration-resistant prostate cancer. Orv. Hetil. 2020, 161, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Fenioux, C.; Louvet, C.; Charton, E.; Rozet, F.; Ropert, S.; Prapotnich, D.; Barret, E.; Sanchez-Salas, R.; Mombet, A.; Cathala, N.; et al. Switch from abiraterone plus prednisone to abiraterone plus dexamethasone at asymptomatic PSA progression in patients with metastatic castration-resistant prostate cancer. BJU Int. 2019, 123, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Qiu, S.; Yi, X.; Xu, H.; Liao, D.; Lei, H.; Bai, S.; Peng, G.; Ai, J.; Yang, L. Steroid switch after progression on abiraterone plus prednisone in patients with metastatic castration-resistant prostate cancer: A systematic review. Urol. Oncol. 2021, 39, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ni, Y.; Zhao, D.; Zhang, Y.; Wang, J.; Jiang, L.; Chen, D.; Wu, Z.; Wang, Y.; He, L.; et al. Corticosteroid switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients with biochemical progression on abiraterone acetate plus prednisone. BMC Cancer 2021, 21, 919. [Google Scholar] [CrossRef] [PubMed]
- Walter, B.; Rogenhofer, S.; Vogelhuber, M.; Berand, A.; Wieland, W.F.; Andreesen, R.; Reichle, A. Modular therapy approach in metastatic castration-refractory prostate cancer. World J. Urol. 2010, 28, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Lorente, D.; Omlin, A.; Ferraldeschi, R.; Pezaro, C.; Perez, R.; Mateo, J.; Altavilla, A.; Zafeirou, Z.; Tunariu, N.; Parker, C.; et al. Tumour responses following a steroid switch from prednisone to dexamethasone in castration-resistant prostate cancer patients progressing on abiraterone. Br. J. Cancer 2014, 111, 2248–2253. [Google Scholar] [CrossRef] [PubMed]
- Arciero, V.S.; Lien, K.; McDonald, E.; Lee, E.K.; Blanchette, P.S.; Emmenegger, U. Identifying candidates for prednisone to dexamethasone switch amongst patients with castrationresistant prostate cancer undergoing abiraterone therapy. J. Clin. Oncol. 2016, 34 (Suppl. S15), e16568. [Google Scholar] [CrossRef]
- Roviello, G.; Petrioli, R.; Bonetta, A.; Conca, R.; Rodriquenz, M.G.; Aieta, M. Corticosteroid switch in heavily pre-treated castration-resistant prostate cancer patients progressed on abiraterone acetate plus prednisone. Investig. New Drugs 2018, 36, 1110–1115. [Google Scholar] [CrossRef]
- Romero-Laorden, N.; Lozano, R.; Jayaram, A.; López-Campos, F.; Saez, M.I.; Montesa, A.; Gutierrez-Pecharoman, A.; Villatoro, R.; Herrera, B.; Correa, R.; et al. Phase II pilot study of the prednisone to dexamethasone switch in metastatic castration-resistant prostate cancer (mCRPC) patients with limited progression on abiraterone plus prednisone (SWITCH study). Br. J. Cancer 2018, 119, 1052–1059. [Google Scholar] [CrossRef]
- Zanardi, E.; Soldato, D.; Latocca, M.M.; Cattrini, C.; Boccardo, F. To switch or not to switch? A real-life experience using dexamethasone in combination with abiraterone. Ther. Adv. Urol. 2019, 11, 1756287219854908. [Google Scholar] [CrossRef]
- Belenchón, I.R.; Calahorro, C.M.; García, I.O.; Villar, P.B.; Ruíz, C.C.; Jiménez, B.S.J.R.; Bortolo, C.J.; Ibarguren, L.R.; Cameno, E.J.; López, M.R.A.; et al. Switch from prednisone to dexamethasone in patients with metastatic castration resistant prostate cancer (mCRPC) treated with abiraterone acetate (AA) in a multicenter study. Eur. Urol. Suppl. 2019, 18, e3464. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, Y.; Li, Z.; Li, Y.; Jiang, L.; Chen, D.; Wu, Z.; Wang, Y.; He, L.; Shi, Y.; et al. Switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients progressing on abiraterone plus prednisone. Chin. J. Urol. 2020, 41, 597–602. [Google Scholar] [CrossRef]
- Barua, R.; Phillips, C.; Arciero, V.S.; Zhang, L.; Rahmadian, A.; Santos, S.D.; Parshad, S.; Emmenegger, U. Development of a predictive model of PSA response to a switch from prednisone to dexamethasone in metastatic castrate-resistant prostate cancer patients biochemically progressing on abiraterone. J. Clin. Oncol. 2020, 38 (Suppl. S6), 88. [Google Scholar] [CrossRef]
- Jamelot, M.; Pressat-Laffouilhere, T.; Baciarello, G.; Dumont, C.; Bonnet, C.; Fizazi, K.; Culine, S. Abiraterone and dexamethasone in castration-resistant prostate cancer: Biological response after switch or rechallenge. Ann. Oncol. 2020, 31 (Suppl. S4), S529. [Google Scholar] [CrossRef]
- Belenchón, I.R.; Calahorro, C.M.; García, I.O.; Villar, P.B.; Ruíz, C.C.; Jiménez, B.S.J.R.; Bartolo, C.J.; Ibarguren, L.R.; Cameno, E.J.; López, M.R.A. Switching from prednisone to dexamethasone in metastatic castration resistant prostate cancer treated with abiraterone acetate in a multicenter study. Eur. Urol. Open Sci. 2020, 19, e897–e898. [Google Scholar] [CrossRef]
- Ni, Y.; Zhao, J.; Chen, J.; Sun, G.; Zhu, S.; Zhang, X.; Dai, J.; Wang, Z.; Zhang, H.; Zhu, X.; et al. The effect of AKR1C3 on the switch from prednisone to dexamethasone in metastatic castration-resistant prostate cancer patients receiving abiraterone. J. Clin. Oncol. 2020, 38 (Suppl. S6), 133. [Google Scholar] [CrossRef]
- Ni, Y.C.; Zhao, J.G.; Zhang, M.N.; Zhang, Y.J.; Yang, Z.Y.; Chen, N.; Chen, J.R.; Shen, P.F.; Sun, G.X.; Zhang, X.M.; et al. Predictors of efficacy of corticosteroid switching from abiraterone plus prednisone to dexamethasone in patients with metastatic castration-resistant prostate cancer. Asian J. Androl. 2022, 24, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.; Leaper, A.; Yadollahi, S.; Teasdale, K.; Irfan, S.; Theivendrampillai, S.; Elbayyar, O.; Wheater, M. AbiDex: Retrospective U.K. analysis of steroid switching in patients with progression of mCRPC treated with abiraterone acetate. J. Clin. Oncol. 2022, 40 (Suppl. S6), 114. [Google Scholar] [CrossRef]
- Venkitaraman, R.; Lorente, D.; Murthy, V.; Thomas, K.; Parker, L.; Ahiabor, R.; Dearnaley, D.; Huddart, R.; de Bono, J.; Parker, C. A randomised phase 2 trial of dexamethasone versus prednisolone in castration-resistant prostate cancer. Eur. Urol. 2015, 67, 673–679. [Google Scholar] [CrossRef]
- Venkitaraman, R.; Thomas, K.; Huddart, R.A.; Horwich, A.; Dearnaley, D.P.; Parker, C.C. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 2008, 101, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Reid, A.H.; A’Hern, R.; Parker, C.; Oommen, N.B.; Folkerd, E.; Messiou, C.; Molife, L.R.; Maier, G.; Thompson, E.; et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 2009, 27, 3742–3748. [Google Scholar] [CrossRef] [PubMed]
- Vanky, E.; Salvesen, K.A.; Carlsen, S.M. Six-month treatment with low-dose dexamethasone further reduces androgen levels in PCOS women treated with diet and lifestyle advice, and metformin. Hum. Reprod. 2004, 19, 529–533. [Google Scholar] [CrossRef][Green Version]
- Akakura, K.; Suzuki, H.; Ueda, T.; Komiya, A.; Ichikawa, T.; Igarashi, T.; Ito, H. Possible mechanism of dexamethasone therapy for prostate cancer: Suppression of circulating level of interleukin-6. Prostate 2003, 56, 106–109. [Google Scholar] [CrossRef]
- Suzuki, K.; Sakamoto, M.; Terakawa, T.; Furukawa, J.; Harada, K.; Hinata, N.; Nakano, Y.; Fujisawa, M. Serum DHEA-S Is a Predictive Parameter of Abiraterone Acetate in Patients with Castration-resistant Prostate Cancer. Anticancer Res. 2018, 38, 5929–5935. [Google Scholar] [CrossRef] [PubMed]
- Lea-Currie, Y.R.; Wen, P.; McIntosh, M.K. Dehydroepiandrosterone reduces proliferation and differentiation of 3T3-L1 preadipocytes. Biochem. Biophys. Res. Commun. 1998, 248, 497–504. [Google Scholar] [CrossRef]
- McIntosh, M.K.; Berdanier, C.D. Antiobesity effects of dehydroepiandrosterone are mediated by futile substrate cycling in hepatocytes of BHE/cdb rats. J. Nutr. 1991, 121, 2037–2043. [Google Scholar] [CrossRef]
- Ibáñez, L.; Jaramillo, A.M.; Ferrer, A.; de Zegher, F. High neutrophil count in girls and women with hyperinsulinaemic hyperandrogenism: Normalization with metformin and flutamide overcomes the aggravation by oral contraception. Hum. Reprod. 2005, 20, 2457–2462. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N.A.; Upton, J.; Phillips, A.C.; Hampson, P.; Lord, J.M. Depressive symptoms are associated with reduced neutrophil function in hip fracture patients. Brain Behav. Immun. 2013, 33, 173–182. [Google Scholar] [CrossRef]
- Özay, A.C.; Özay, Ö.E. The importance of inflammation markers in polycystic ovary syndrome. Rev. Assoc. Med. Bras. 2021, 67, 411–417. [Google Scholar] [CrossRef]
- Suh, E.; Cho, A.R.; Haam, J.H.; Gil, M.; Lee, Y.K.; Kim, Y.S. Relationship between Serum Cortisol, Dehydroepiandrosterone Sulfate (DHEAS) Levels, and Natural Killer Cell Activity: A Cross-Sectional Study. J. Clin. Med. 2023, 12, 4027. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Foppiani, L.; Prete, C.; Ballarino, P.; Sulli, A.; Villaggio, B.; Seriolo, B.; Giusti, M.; Accardo, S. Hypothalamic-pituitary-adrenocortical axis function in premenopausal women with rheumatoid arthritis not treated with glucocorticoids. J. Rheumatol. 1999, 26, 282–288. [Google Scholar] [PubMed]
- Safwat, S.M.; Hussein, A.M.; Eid, E.A.; Serria, M.S.; Elesawy, B.H.; Sakr, H.F. Dehydroepiandrosterone (DHEA) Improves the Metabolic and Haemostatic Disturbances in Rats with Male Hypogonadism. Sci. Pharm. 2022, 90, 6. [Google Scholar] [CrossRef]
- Karolczak, K.; Konieczna, L.; Kostka, T.; Witas, P.J.; Soltysik, B.; Baczek, T.; Watala, C. Testosterone and dihydrotestosterone reduce platelet activation and reactivity in older men and women. Aging 2018, 10, 902–929. [Google Scholar] [CrossRef] [PubMed]
- Demirci, I.; Haymana, C.; Orhan, D.; Akin, O.; Meric, C.; Aydoğan, A.; Alper, S. Hematological indices in congenital male hypogonadism and the effects of testosterone replacement therapy: A retrospective study. Gulhane Med. J. 2021, 63, 205–211. [Google Scholar] [CrossRef]
- Sakr, H.F.; Hussein, A.M.; Eid, E.A.; Boudaka, A.; Lashin, L.S. Impact of Dehydroepiandrosterone (DHEA) on Bone Mineral Density and Bone Mineral Content in a Rat Model of Male Hypogonadism. Vet. Sci. 2020, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Hanayama, Y.; Obika, M.; Itoshima, K.; Okada, K.; Otsuka, F. Involvement of serum dehydroepiandrosterone sulfate in erythropoietic activity. Aging Male 2020, 23, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Lac, G.; Marquet, P.; Chassain, A.P.; Galen, F.X. Dexamethasone in resting and exercising men. II. Effects on adrenocortical hormones. J. Appl. Physiol. 1999, 87, 183–188. [Google Scholar] [CrossRef][Green Version]
- Sulli, A.; Montecucco, C.M.; Caporali, R.; Cavagna, L.; Montagna, P.; Capellino, S.; Fazzuoli, L.; Seriolo, B.; Alessandro, C.; Secchi, M.E.; et al. Glucocorticoid effects on adrenal steroids and cytokine responsiveness in polymyalgia rheumatica and elderly onset rheumatoid arthritis. Ann. N. Y. Acad. Sci. 2006, 1069, 307–314. [Google Scholar] [CrossRef]
- Han, T.S.; Stimson, R.H.; Rees, D.A.; Krone, N.; Willis, D.S.; Conway, G.S.; Arlt, W.; Walker, B.R.; Ross, R.J.; United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin. Endocrinol. 2013, 78, 197–203. [Google Scholar] [CrossRef]
- Hafiz, S.; Dennis, J.C.; Schwartz, D.; Judd, R.; Tao, Y.X.; Khazal, K.; Akingbemi, B.; Mo, X.L.; Abdel-Mageed, A.B.; Morrison, E.; et al. Expression of melanocortin receptors in human prostate cancer cell lines: MC2R activation by ACTH increases prostate cancer cell proliferation. Int. J. Oncol. 2012, 41, 1373–1380. [Google Scholar] [CrossRef]
- Li, S.; Cao, L. Demethyltransferase FTO alpha-ketoglutarate dependent dioxygenase (FTO) regulates the proliferation, migration, invasion and tumor growth of prostate cancer by modulating the expression of melanocortin 4 receptor (MC4R). Bioengineered 2022, 13, 5598–5612. [Google Scholar] [CrossRef]
- Yuan, X.C.; Tao, Y.X. Ligands for Melanocortin Receptors: Beyond Melanocyte-Stimulating Hormones and Adrenocorticotropin. Biomolecules 2022, 12, 1407. [Google Scholar] [CrossRef]
- Yukioka, M.; Komatsubara, Y.; Yukioka, K.; Toyosaki-Maeda, T.; Yonenobu, K.; Ochi, T. Adrenocorticotropic hormone and dehydroepiandrosterone sulfate levels of rheumatoid arthritis patients treated with glucocorticoids. Mod. Rheumatol. 2006, 16, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kang, M.; Kim, S.; An, H.T.; Gettemans, J.; Ko, J. α-Actinin-4 Promotes the Progression of Prostate Cancer Through the Akt/GSK-3β/β-Catenin Signaling Pathway. Front. Cell Dev. Biol. 2020, 8, 588544. [Google Scholar] [CrossRef]
- Zhao, X.; Khurana, S.; Charkraborty, S.; Tian, Y.; Sedor, J.R.; Bruggman, L.A.; Kao, H.Y. α Actinin 4 (ACTN4) Regulates Glucocorticoid Receptor-mediated Transactivation and Transrepression in Podocytes. J. Biol. Chem. 2017, 292, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Ding, J.; Fan, Q.; Guan, N. Diversities of podocyte molecular changes induced by different antiproteinuria drugs. Exp. Biol. Med. 2006, 231, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.P.; Li, J.; Tewari, A.K. Inflammation and prostate cancer: The role of interleukin 6 (IL-6). BJU Int. 2014, 113, 986–992. [Google Scholar] [CrossRef]
- Carvalho, L.A.; Urbanova, L.; Hamer, M.; Hackett, R.A.; Lazzarino, A.I.; Steptoe, A. Blunted glucocorticoid and mineralocorticoid sensitivity to stress in people with diabetes. Psychoneuroendocrinology 2015, 51, 209–218. [Google Scholar] [CrossRef]
- Attard, G.; Murphy, L.; Clarke, N.W.; Sachdeva, A.; Jones, C.; Hoyle, A.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: Final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023, 24, 443–456. [Google Scholar] [CrossRef]
| Parameters | Non-Responders | Responders | p |
|---|---|---|---|
| (Dichotomized Value) | N = 60 | N = 49 | |
| Age, mean + SD, years | 69.5 + 8.2 | 71.2 + 7.3 | 0.253 |
| <69.2 (0) | 32 | 15 | 0.017 |
| ≥69.2 (1) | 28 | 34 | |
| Gleason score (Gl) | |||
| <8 (0) | 27 | 16 | 0.19 |
| ≥8 (1) | 33 | 33 | |
| CRFS, mean + SD, months | 51.3 + 45.3 | 68.6 + 58.9 | 0.121 |
| <115.6 (0) | 55 | 37 | 0.021 |
| ≥115.6 (1) | 5 | 12 | |
| ECOG at start of AA | |||
| 0 | 52 | 39 | 0.322 |
| 1 | 8 | 10 | |
| Local recurrence present at start of AA | 13 | 8 | 0.482 |
| Site of distant metastasis at start of AA | |||
| Bone (1) | 51 | 40 | 0.638 |
| Lymph node (1) | 35 | 25 | 0.445 |
| Visceral (visc) (1) | 5 | 5 | 0.736 |
| Chemotherapy before AA (pre) | |||
| no (0) | 27 | 33 | 0.019 |
| yes (1) | 33 | 16 | |
| PSA at start of AA (PSAs), mean + SD, ng/mL | 78.6 + 84.7 | 131.6 + 301 | 0.328 |
| <4.64 (0) | 1 | 10 | 0.002 |
| ≥4.64 (1) | 59 | 39 | |
| ALP at start of AA, mean + SD, U/L | 493 + 925 | 451 + 1065 | 0.423 |
| <237 (0) | 31 | 25 | 0.59 |
| ≥237 (1) | 23 | 23 | |
| ND | 6 | 1 | |
| LDH at start of AA (LDHs), mean + SD, U/L | 469 + 761 | 343 + 162 | 0.295 |
| <416 (0) | 38 | 37 | 0.172 |
| ≥416 (1) | 22 | 12 | |
| Hemoglobin at start of AA, mean + SD, g/dL | 12.5 + 1.4 | 12.6 + 1.3 | 0.671 |
| <11.8 (0) | 22 | 9 | 0.022 |
| ≥11.8 (1) | 33 | 38 | |
| ND | 5 | 2 | |
| WBC at start of AA (WBCs), mean + SD, 109/L | 6.67 + 1.7 | 7.26 + 2.4 | 0.074 |
| <6.43 (0) | 33 | 14 | 0.006 |
| ≥6.43 (1) | 27 | 35 | |
| Neutrophils at start of AA, mean + SD, 109/L | 4.36 + 1.4 | 4.76 + 1.8 | 0.146 |
| <4.27 (0) | 28 | 18 | 0.063 |
| ≥4.27 (1) | 32 | 31 | |
| Lymphocytes at start of AA (lys), mean + SD, 109/L | 1.66 + 0.7 | 1.86 + 1 | 0.427 |
| <1.58 (0) | 28 | 18 | 0.296 |
| ≥1.58 (1) | 32 | 31 | |
| Platelets at start of AA (pls), mean + SD, 109/L | 241 + 68.5 | 229 + 69.8 | 0.372 |
| <220 (0) | 21 | 27 | 0.035 |
| ≥220 (1) | 39 | 22 | |
| PPFS, mean + SD, months | 16.1 + 13.1 | 12.5 + 8.1 | 0.445 |
| <22.7 (0) | 44 | 46 | 0.005 |
| ≥22.7 (1) | 16 | 3 | |
| ECOG at switch | |||
| 0 | 53 | 41 | 0.482 |
| 1 | 7 | 8 | |
| PSA at switch (PSAw), mean + SD, ng/mL | 69.8 + 137 | 99.7 + 331 | 0.264 |
| <5.62 (0) | 18 | 23 | 0.069 |
| ≥5.62 (1) | 42 | 26 | |
| ALP at switch (ALPw), mean + SD, U/L | 264 + 601 | 316 + 790 | 0.949 |
| <199 (0) | 35 | 31 | 0.6 |
| ≥199 (1) | 25 | 18 | |
| LDH at switch (LDHw), mean + SD, U/L | 413 + 955 | 321 + 196 | 0.522 |
| <177 (0) | 13 | 4 | 0.053 |
| ≥177 (1) | 47 | 45 | |
| Hemoglobin at switch (hgbw), mean + SD, g/dL | 12.8 + 1.3 | 12.9 + 1.5 | 0.809 |
| <13.3 (0) | 44 | 27 | 0.047 |
| ≥13.3 (1) | 16 | 22 | |
| WBC at switch (WBCw), mean + SD, 109/L | 7.78 + 2.1 | 7.83 + 1.8 | 0.899 |
| <7.16 (0) | 27 | 17 | 0.275 |
| ≥7.16 (1) | 33 | 32 | |
| Neutrophils at switch (neuw), mean + SD, 109/L | 5.48 + 1.8 | 5.4 + 1.5 | 0.813 |
| <4.32 (0) | 23 | 11 | 0.075 |
| ≥4.32 (1) | 37 | 38 | |
| Lymphocytes at switch (lyw), mean + SD, 109/L | 1.64 + 0.8 | 1.78 + 0.9 | 0.288 |
| <2.02 (0) | 46 | 30 | 0.081 |
| ≥2.02 (1) | 14 | 19 | |
| Platelets at switch (plw), mean + SD, 109/L | 235 + 70.7 | 227 + 51.1 | 0.497 |
| <221 (0) | 23 | 26 | 0.124 |
| ≥221 (1) | 37 | 23 | |
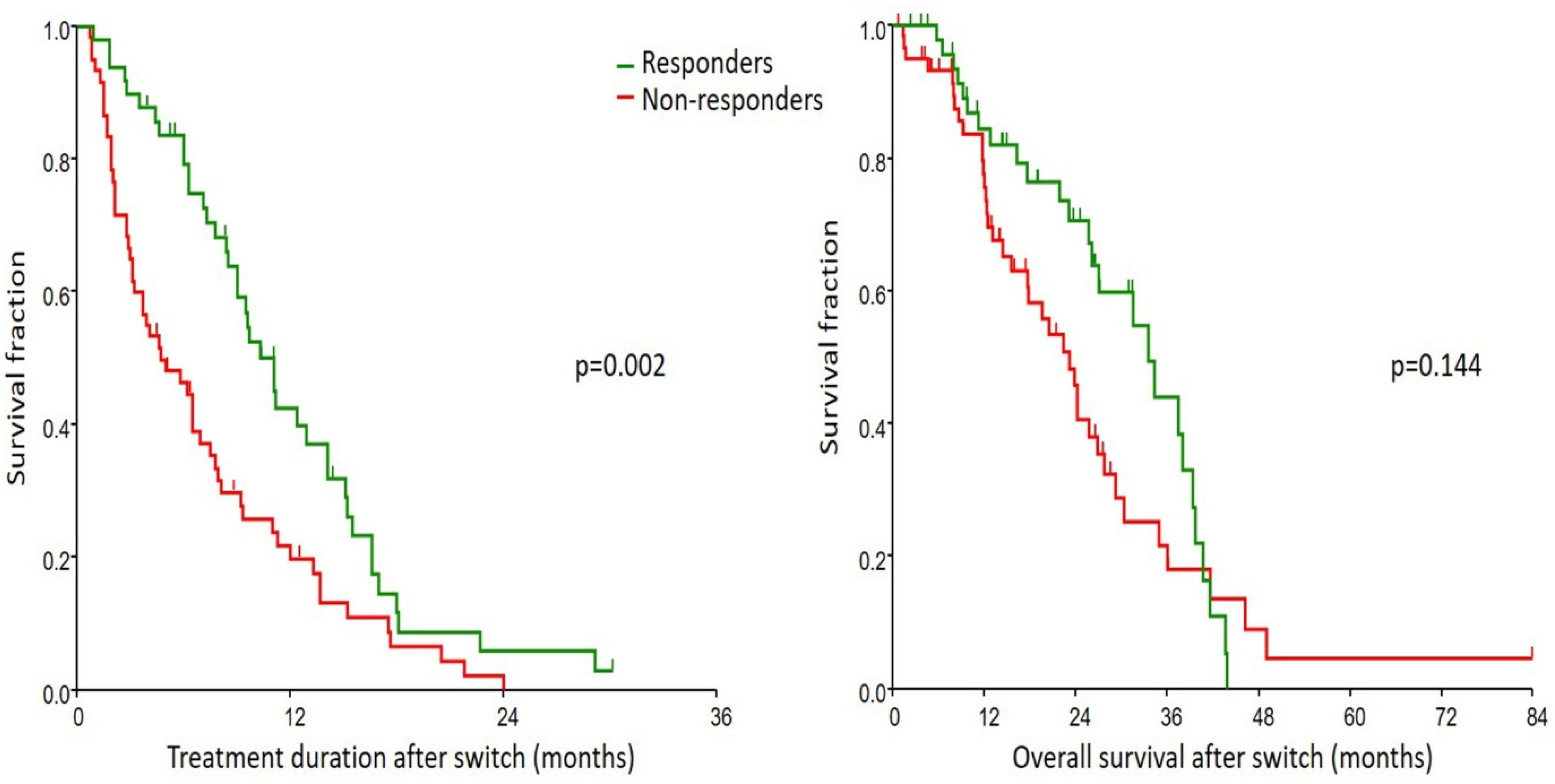
| TD after switch, median (95% CI), months | 4.7 (3.1–6.5) | 11.1 (8.5–12.9) | 0.002 |
| Treatment lines after AA | |||
| 0 | 22 | 20 | 0.888 |
| 1 | 14 | 11 | |
| 2 | 17 | 11 | |
| >2 | 7 | 7 | |
| OS after switch, median (95% CI), months | 23.2 (15.6–25.8) | 33.5 (26.1–38) | 0.144 |
| Parameter and Condition | Yes * | No * |
|---|---|---|
| Age ≥ 69.2 years | 251 | 0 |
| Gleason score ≥ 8 | 65 | 0 |
| CRFS ≥ 115.6 months | 260 | 0 |
| AA as pre-chemotherapy | 158 | 0 |
| PPFS ≥ 22.7 months | −410 | 0 |
| Visceral metastasis at start of AA | 95 | 0 |
| PSA at start of AA ≥ 4.64 ng/mL | −717 | 0 |
| LDH at start of AA ≥ 416 U/L | −150 | 0 |
| WBC at start of AA ≥ 6.43 × 109/L | 37 | 0 |
| Lymphocytes at start of AA ≥ 1.58 × 109/L | −58 | 0 |
| Platelets at start of AA ≥ 220 × 109/L | −282 | 0 |
| PSA at switch ≥ 5.62 ng/mL | 117 | 0 |
| LDH at switch ≥ 177 U/L | 502 | 0 |
| ALP at switch ≥ 199 U/L | −74 | 0 |
| Hemoglobin at switch ≥ 13.3 g/dL | 248 | 0 |
| WBC at switch ≥ 7.16 × 109/L | −84 | 0 |
| Neutrophils at switch ≥ 4.32 × 109/L | 248 | 0 |
| Lymphocytes at switch ≥ 2.02 × 109/L | 178 | 0 |
| Platelets at switch ≥ 221 × 109/L | 115 | 0 |
| Total | …… | |
| Responder if total−169 > 0 | ||
| Parameters | TD | OS | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Responder | ||||
| No | 1 (reference) | 0.006 | 1 (reference) | 0.045 |
| Yes | 0.54 | 0.58 (0.34–0.99) | ||
| ALP at switch, U/L | ||||
| <199 | 1 (reference) | 0.019 | 1 (reference) | 2.3 × 10−4 |
| ≥199 | 1.63 (1.08–2.47) | 2.74 (1.6–4.7) | ||
| WBC at switch, 109/L | ||||
| <7.16 | 1 (reference) | 0.372 | - | |
| ≥7.16 | 0.82 (0.53–1.27) | - | ||
| Number of systemic treatment lines after AA | ||||
| 0 | - | 1 (reference) | ||
| 1 | - | 0.43 (0.21–0.85) | 0.016 | |
| 2 | - | 0.17 (0.08–0.36) | 7.4 × 10−6 | |
| >2 | - | 0.11 (0.04–0.27) | 1.9 × 10−6 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fekete, B.; Biró, K.; Gyergyay, F.; Polk, N.; Horváth, O.; Géczi, L.; Patócs, A.; Budai, B. Prediction of PSA Response after Dexamethasone Switch during Abiraterone Acetate + Prednisolone Treatment of Metastatic Castration-Resistant Prostate Cancer Patients. Cancers 2024, 16, 2760. https://doi.org/10.3390/cancers16152760
Fekete B, Biró K, Gyergyay F, Polk N, Horváth O, Géczi L, Patócs A, Budai B. Prediction of PSA Response after Dexamethasone Switch during Abiraterone Acetate + Prednisolone Treatment of Metastatic Castration-Resistant Prostate Cancer Patients. Cancers. 2024; 16(15):2760. https://doi.org/10.3390/cancers16152760
Chicago/Turabian StyleFekete, Bertalan, Krisztina Biró, Fruzsina Gyergyay, Nándor Polk, Orsolya Horváth, Lajos Géczi, Attila Patócs, and Barna Budai. 2024. "Prediction of PSA Response after Dexamethasone Switch during Abiraterone Acetate + Prednisolone Treatment of Metastatic Castration-Resistant Prostate Cancer Patients" Cancers 16, no. 15: 2760. https://doi.org/10.3390/cancers16152760
APA StyleFekete, B., Biró, K., Gyergyay, F., Polk, N., Horváth, O., Géczi, L., Patócs, A., & Budai, B. (2024). Prediction of PSA Response after Dexamethasone Switch during Abiraterone Acetate + Prednisolone Treatment of Metastatic Castration-Resistant Prostate Cancer Patients. Cancers, 16(15), 2760. https://doi.org/10.3390/cancers16152760







