Perinatal Famine Exposure and Young-Onset Cancer—Lessons from China Health and Nutrition Survey
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
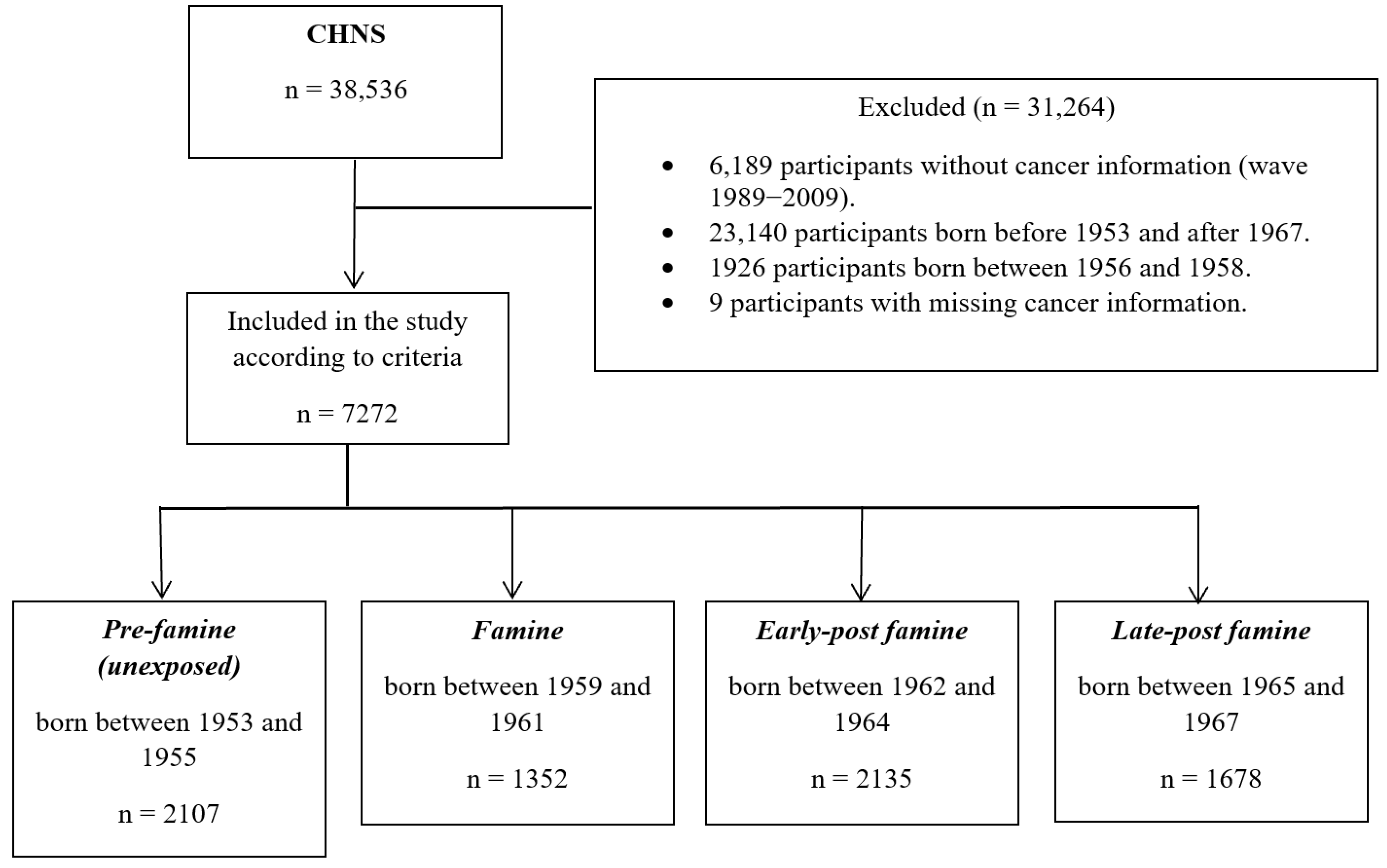
2.1. Study Population
2.2. Assessment of Perinatal Famine Exposure
2.3. Assessment of Outcome
2.4. Assessment of Covariates
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics (Table 1)
3.2. Outcomes (Table 2)
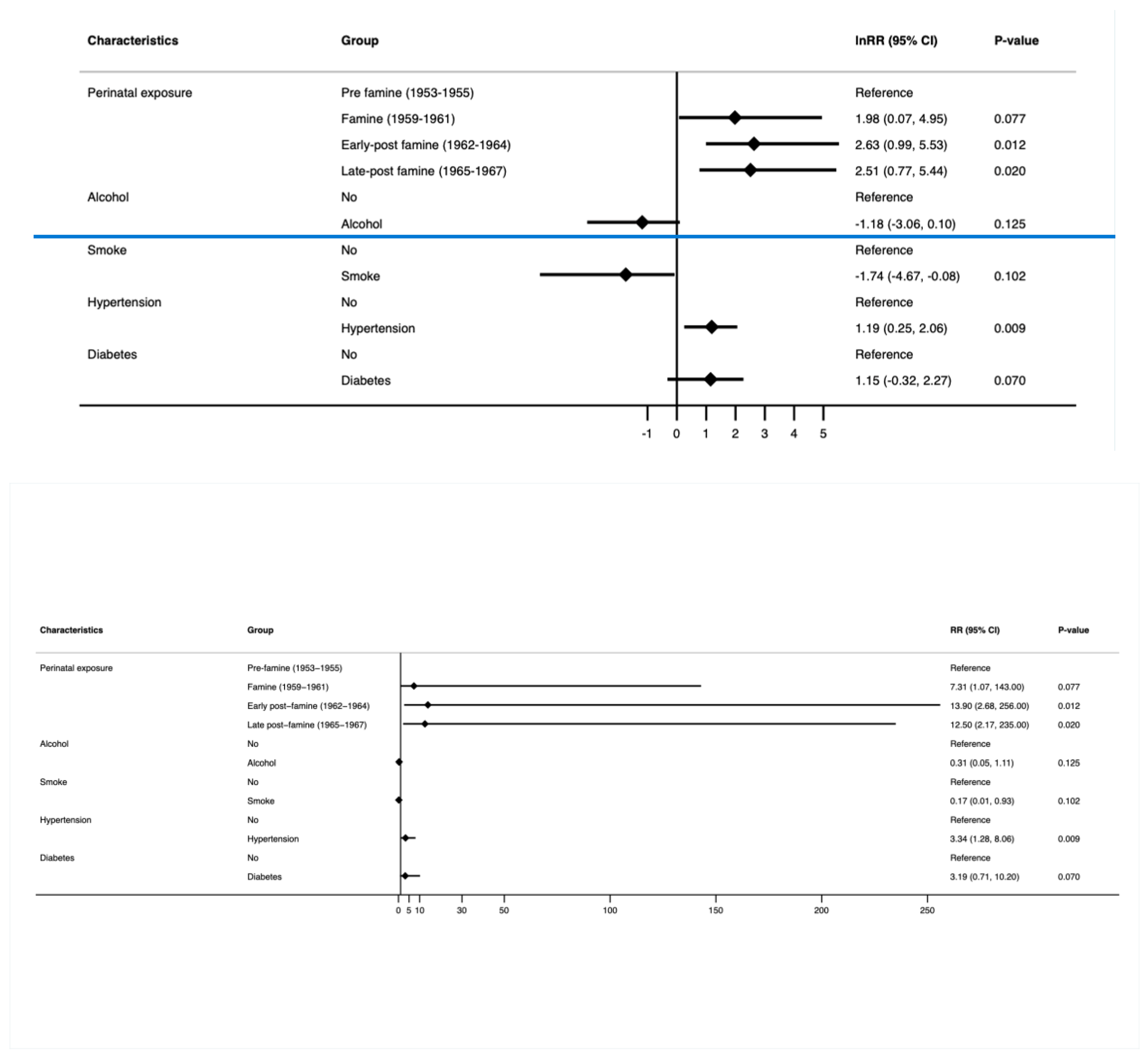
3.2.1. Young-Onset Cancer (Figure 2 and Supplementary Table S1)
3.2.2. Young-Onset Genitourinary Cancer (Figure 3 and Supplementary Table S2)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schell, D.; Ullah, S.; Brooke-Smith, M.E.; Hollington, P.; Yeow, M.; Karapetis, C.S.; Watson, D.I.; Pandol, S.J.; Roberts, C.T.; Barreto, S.G. Gastrointestinal Adenocarcinoma Incidence and Survival Trends in South Australia, 1990–2017. Cancers 2022, 14, 275. [Google Scholar] [CrossRef] [PubMed]
- Fanny, E.R.V.; Stella, A.V.N.; Bardou, M.; Lansdorp-Vogelaar, I.; Dinis-Ribeiro, M.; Bento, M.J.; Zadnik, V.; Pellisé, M.; Esteban, L.; Kaminski, M.F.; et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut 2019, 68, 1820. [Google Scholar] [CrossRef]
- Murphy, C.C.; Singal, A.G.; Baron, J.A.; Sandler, R.S. Decrease in Incidence of Young-Onset Colorectal Cancer Before Recent Increase. Gastroenterology 2018, 155, 1716–1719.e14. [Google Scholar] [CrossRef] [PubMed]
- McWilliams, R.R.; Maisonneuve, P.; Bamlet, W.R.; Petersen, G.M.; Li, D.; Risch, H.A.; Yu, H.; Fontham, E.T.H.; Luckett, B.; Bosetti, C.; et al. Risk Factors for Early-Onset and Very-Early-Onset Pancreatic Adenocarcinoma: A Pancreatic Cancer Case-Control Consortium (PanC4) Analysis. Pancreas 2016, 45, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Bernards, S.S.; Norquist, B.M.; Harrell, M.I.; Agnew, K.J.; Lee, M.K.; Walsh, T.; Swisher, E.M. Genetic characterization of early onset ovarian carcinoma. Gynecol. Oncol. 2016, 140, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y. Rectal Cancer in Asian vs. Western Countries: Why the Variation in Incidence? Curr. Treat. Options Oncol. 2017, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, K.M.; Desai, D.C. Epidemiology of digestive tract cancers in India. V. Large and small bowel. Indian J. Gastroenterol. 1999, 18, 118–121. [Google Scholar] [PubMed]
- American Cancer Society. Special Section: Cancer in Adolescents and Young Adults. In Cancer Facts & Figures; American Cancer Society: New York, NY, USA, 2020; p. 29. [Google Scholar]
- Shepherdson, M.; Leemaqz, S.; Singh, G.; Ryder, C.; Ullah, S.; Canuto, K.; Young, J.P.; Price, T.J.; McKinnon, R.A.; Pandol, S.J.; et al. Young-Onset Gastrointestinal Adenocarcinoma Incidence and Survival Trends in the Northern Territory, Australia, with Emphasis on Indigenous Peoples. Cancers 2022, 14, 2870. [Google Scholar] [CrossRef] [PubMed]
- Chelmow, D.; Pearlman, M.D.; Young, A.; Bozzuto, L.; Dayaratna, S.; Jeudy, M.; Kremer, M.E.; Scott, D.M.; O’Hara, J.S. Executive Summary of the Early-Onset Breast Cancer Evidence Review Conference. Obstet. Gynecol. 2020, 135, 1457–1478. [Google Scholar] [CrossRef]
- van Nistelrooij, A.M.J.; van Marion, R.; Biermann, K.; Spaander, M.C.W.; van Lanschot, J.J.B.; Wijnhoven, B.P.L.; Dinjens, W.N.M. Early onset esophageal adenocarcinoma: A distinct molecular entity? Oncoscience 2016, 3, 42–48. [Google Scholar] [CrossRef]
- Ben-Aharon, I.; van Laarhoven, H.W.M.; Fontana, E.; Obermannova, R.; Nilsson, M.; Lordick, F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise-Evidence and Implications. Cancer Discov. 2023, 13, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Barreto, S.G.; Pandol, S.J. Young-Onset Carcinogenesis—The Potential Impact of Perinatal and Early Life Metabolic Influences on the Epigenome. Front. Oncol. 2021, 11, 653289. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Knudson, A.G., Jr. Mutation and cancer: Statistical study of retinoblastoma. Proc. Natl. Acad. Sci. USA 1971, 68, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Lahouel, K.; Younes, L.; Danilova, L.; Giardiello, F.M.; Hruban, R.H.; Groopman, J.; Kinzler, K.W.; Vogelstein, B.; Geman, D.; Tomasetti, C. Revisiting the tumorigenesis timeline with a data-driven generative model. Proc. Natl. Acad. Sci. USA 2020, 117, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nembhard, W.N.; Stockwell, H.G. Sex-Specific Effects of Fetal Exposure to the 1959–1961 Chinese Famine on Risk of Adult Hypertension. Matern. Child Health J. 2014, 18, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gooch, E. Estimating the Long-Term Impact of the Great Chinese Famine (1959–61) on Modern China. World Dev. 2017, 89, 140–151. [Google Scholar] [CrossRef]
- Liu, D.; Yu, D.-M.; Zhao, L.-Y.; Fang, H.-Y.; Zhang, J.; Wang, J.-Z.; Yang, Z.-Y.; Zhao, W.-H. Exposure to Famine During Early Life and Abdominal Obesity in Adulthood: Findings from the Great Chinese Famine During 1959–1961. Nutrients 2019, 11, 903. [Google Scholar] [CrossRef] [PubMed]
- Song, S. Assessing the impact of in utero exposure to famine on fecundity: Evidence from the 1959–61 famine in China. Popul. Stud. 2013, 67, 293–308. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, X.; Liu, B.; Hu, H.; Wang, F.; Li, X.; Yang, K.; Yuan, J.; Yao, P.; et al. Exposure to the Chinese Famine in Childhood Increases Type 2 Diabetes Risk in Adults. J. Nutr. 2016, 146, 2289–2295. [Google Scholar] [CrossRef]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef]
- Prevention, China Center for Disease Control. China Health and Nutrition Survey. Available online: https://www.cpc.unc.edu/projects/china (accessed on 14 March 2023).
- Zhang, X.; Wang, G.; Forman, M.R.; Fu, Q.; Rogers, C.J.; Wu, S.; Gao, X. In utero and childhood exposure to the Great Chinese Famine and risk of cancer in adulthood: The Kailuan Study. Am. J. Clin. Nutr. 2021, 114, 2017–2024. [Google Scholar] [CrossRef]
- Shein-Chung, C.; Chow, S.-C.; Shao, J.; Wang, H.; Lokhnygina, Y. Sample Size Calculations in Clinical Research, 3rd. ed.; Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Schoenfeld, D.A. Sample-Size Formula for the Proportional-Hazards Regression Model. Biometrics 1983, 39, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Lin, J.Y.; Yang, D.T. Food Availability, Entitlements and the Chinese Famine of 1959-61. Econ. J. 2000, 110, 136–158. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 14 June 2023).
- Xie, S.H.; Lagergren, J. A possible link between famine exposure in early life and future risk of gastrointestinal cancers: Implications from age-period-cohort analysis. Int. J. Cancer 2017, 140, 636–645. [Google Scholar] [CrossRef]
- Kim, H.-I.; Lim, H.; Moon, A. Sex Differences in Cancer: Epidemiology, Genetics and Therapy. Biomol. Ther. 2018, 26, 335–342. [Google Scholar] [CrossRef]
- Eriksen, K.G.; Radford, E.J.; Silver, M.J.; Fulford, A.J.C.; Wegmüller, R.; Prentice, A.M. Influence of intergenerational in utero parental energy and nutrient restriction on offspring growth in rural Gambia. FASEB J. 2017, 31, 4928–4934. [Google Scholar] [CrossRef] [PubMed]
- Painter, R.C.; De Rooij, S.R.; Bossuyt, P.M.M.; Osmond, C.; Barker, D.J.P.; Bleker, O.P.; Roseboom, T.J. A possible link between prenatal exposure to famine and breast cancer: A preliminary study. Am. J. Hum. Biol. 2006, 18, 853–856. [Google Scholar] [CrossRef]
- Saulnier, D.D.; Brolin, K. A systematic review of the health effects of prenatal exposure to disaster. Int. J. Public Health 2015, 60, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, L.; Xuan, P.; Fan, Y.; Yang, L.; Hu, C.; Bo, Q.; Wang, G.; Sheng, J.; Wang, S. The relationship between famine exposure during early life and body mass index in adulthood: A systematic review and meta-analysis. PLoS ONE 2018, 13, e0192212. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Ren, J.; Song, X.; Zhang, D.; Liu, L.; Zhang, L.; Sun, J.; Zhang, D.; Pang, Z.; Qiao, Q.; et al. Famine Exposure in Early Life and Risk of Metabolic Syndrome in Adulthood: Comparisons of Different Metabolic Syndrome Definitions. J. Diabetes Res. 2019, 2019, 7954856. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, L.; Ning, Z.; Li, Q.; Han, B.; Cheng, J.; Chen, Y.; Nie, X.; Xia, F.; Wang, N.; et al. Famine exposure in early life is associated with visceral adipose dysfunction in adult females. Eur. J. Nutr. 2019, 58, 1625–1633. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, C.; Yang, Z.; Ma, J.; Zou, Z. Fetal and infant exposure to severe Chinese famine increases the risk of adult dyslipidemia: Results from the China health and retirement longitudinal study. BMC Public Health 2017, 17, 488. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Schatten, H.; Sun, Q.-Y. Environmental epigenetic inheritance through gametes and implications for human reproduction. Hum. Reprod. Update 2015, 21, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Radford, E.J.; Ito, M.; Shi, H.; Corish, J.A.; Yamazawa, K.; Isganaitis, E.; Seisenberger, S.; Hore, T.A.; Reik, W.; Erkek, S.; et al. In utero undernourishment perturbs the adult sperm methylome and intergenerational metabolism. Science 2014, 345, 785. [Google Scholar] [CrossRef] [PubMed]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally Induced Transgenerational Environmental Reprogramming of Metabolic Gene Expression in Mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Chillaron, J.C.; Isganaitis, E.; Patti, M.-E.; Charalambous, M.; Gesta, S.; Pentinat-Pelegrin, T.; Faucette, R.R.; Otis, J.P.; Chow, A.; Diaz, R.; et al. Intergenerational Transmission of Glucose Intolerance and Obesity by In Utero Undernutrition in Mice. Diabetes 2009, 58, 460–468. [Google Scholar] [CrossRef]
- Barreto, S.G. We Asked the Experts: Providing the Road Map to Uncovering the Pathophysiology of Young-Onset Cancer to Guide Treatment and Preventive Strategies. World J. Surg. 2020, 44, 3212–3213. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Spiegelman, D.; Garland, M.; Hertzmark, E.; Hunter, D.J.; Colditz, G.A.; Willett, W.C.; Wand, H.; Manson, J.E. Physical Activity, Body Mass Index, and Ovulatory Disorder Infertility. Epidemiology 2002, 13, 184–190. [Google Scholar] [CrossRef]
- Roba, K.T.; Hassen, T.A.; Wilfong, T.; Legese Alemu, N.; Darsene, H.; Zewdu, G.; Negese, T.; Yifru, B.; Mohammed, E.; Raru, T.B. Association of undernutrition and female infertility in East Africa: Finding from multi-country demographic and health surveys. Front. Glob. Women’s Health 2022, 3, 1049404. [Google Scholar] [CrossRef] [PubMed]
- Best, D.; Avenell, A.; Bhattacharya, S. How effective are weight-loss interventions for improving fertility in women and men who are overweight or obese? A systematic review and meta-analysis of the evidence. Hum. Reprod. Update 2017, 23, 681–705. [Google Scholar] [CrossRef] [PubMed]
- Farias, P.M.; Marcelino, G.; Santana, L.F.; de Almeida, E.B.; Guimaraes, R.C.A.; Pott, A.; Hiane, P.A.; Freitas, K.C. Minerals in Pregnancy and Their Impact on Child Growth and Development. Molecules 2020, 25, 5630. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, Y.; Li, L.; Xu, W. Exposure to the Great Famine in Early Life and the Risk of Obesity in Adulthood: A Report Based on the China Health and Nutrition Survey. Nutrients 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Ren, W.; Luo, R.; Zhang, S.; Zhang, J.H.; Zeng, Q. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur. J. Clin. Nutr. 2012, 66, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Marcelino, H.; Veyrat-Durebex, C.; Rohner-Jeanrenaud, F.; Dulloo, A.G.; Summermatter, S.; Sarafian, D.; Miles-Chan, J.; Arsenijevic, D.; Zani, F.; Montani, J.-P.; et al. A Role for Adipose Tissue De Novo Lipogenesis in Glucose Homeostasis During Catch-up Growth: A Randle Cycle Favoring Fat Storage. Diabetes 2013, 62, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Zong, X.; Li, Z.; Li, N.; Hur, J.; Fritz, C.D.L.; Chapman Jr, W.; Nickel, K.B.; Tipping, A.; et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut 2021, 70, 1147–1154. [Google Scholar] [CrossRef]
- Li, H.; Boakye, D.; Chen, X.; Hoffmeister, M.; Brenner, H. Association of Body Mass Index With Risk of Early-Onset Colorectal Cancer: Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2021, 116, 2173–2183. [Google Scholar] [CrossRef]
- Lutz, W.K. Carcinogens in the diet vs. overnutrition. Individual dietary habits, malnutrition, and genetic susceptibility modify carcinogenic potency and cancer risk. Mutat. Res. 1999, 443, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, L.-A. The long-term health and economic consequences of the 1959–1961 famine in China. J. Health Econ. 2007, 26, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Smil, V. China’s great famine: 40 years later. BMJ 1999, 319, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Guelinckx, I.; Devlieger, R.; Vansant, G. Reproductive outcome after bariatric surgery: A critical review. Hum. Reprod. Update 2009, 15, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Pavli, P.; Triantafyllidou, O.; Kapantais, E.; Vlahos, N.F.; Valsamakis, G. Infertility Improvement after Medical Weight Loss in Women and Men: A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 1909. [Google Scholar] [CrossRef]
- Mechanick, J.I.; Youdim, A.; Jones, D.B.; Garvey, W.T.; Hurley, D.L.; McMahon, M.M.; Heinberg, L.J.; Kushner, R.; Adams, T.D.; Shikora, S.; et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient—2013 update: Cosponsored by american association of clinical endocrinologists, The obesity society, and american society for metabolic & bariatric surgery. Obesity 2013, 21, S1–S27. [Google Scholar] [CrossRef]

| Characteristics | Overall (n 7272) | Perinatal Exposure Group | p-Value * | |||
|---|---|---|---|---|---|---|
| Pre-Famine (n 2107) | Famine (n 1352) | Early Post-Famine (n 2135) | Late Post-Famine (n 1678) | |||
| Age y, median (IQR) † | 52.0 (48.0–56.0) | 58.0 (57.0,61.0) | 53.0 (51.0,55.0) | 50.0 (48.0–52.0) | 47.0 (45.0–49.0) | <0.001 |
| Age | <0.001 | |||||
| ≤50 years | 3018 (41.5%) | 0 (0.0%) | 236 (17.5%) | 1104 (51.7%) | 1678 (100.0%) | |
| >50 years | 4254 (58.5%) | 2107 (100.0%) | 1116 (82.5%) | 1031 (48.3%) | 0 (0.0%) | |
| Sex | 0.267 | |||||
| Male | 3434 (47.2%) | 967 (45.9%) | 645 (47.7%) | 1041 (48.8%) | 781 (46.5%) | |
| Female | 3838 (52.8%) | 1140 (54.1%) | 707 (52.3%) | 1094 (51.2%) | 897 (53.5%) | |
| Area | <0.001 | |||||
| Urban | 2843 (39.1%) | 865 (41.1%) | 574 (42.5%) | 802 (37.6%) | 602 (35.9%) | |
| Rural | 4429 (60.9%) | 1242 (58.9%) | 778 (57.5%) | 1333 (62.4%) | 1076 (64.1%) | |
| Alcohol | <0.001 | |||||
| Yes | 2422 (33.3%) | 620 (29.4%) | 471 (34.8%) | 775 (36.3%) | 556 (33.1%) | |
| Do not know | 135 (1.9%) | 29 (1.4%) | 23 (1.7%) | 47 (2.2%) | 36 (2.1%) | |
| Smoke | 0.217 | |||||
| Yes | 2190 (30.1%) | 643 (30.5%) | 428 (31.7%) | 647 (30.3%) | 472 (28.1%) | |
| Do not know | 136 (1.9%) | 30 (1.4%) | 23 (1.7%) | 46 (2.2%) | 37 (2.2%) | |
| BMI ‡ | <0.001 | |||||
| Underweight | 168 (2.3%) | 66 (3.1%) | 27 (2.0%) | 36 (1.7%) | 39 (2.3%) | |
| Normal | 3923 (53.9%) | 1149 (54.5%) | 740 (54.7%) | 1148 (53.8%) | 886 (52.8%) | |
| Overweight or obese | 2840 (39.1%) | 823 (39.1%) | 525 (38.8%) | 848 (39.7%) | 644 (38.4%) | |
| Missing | 341 (4.7%) | 69 (3.3%) | 60 (4.4%) | 103 (4.8%) | 109 (6.5%) | |
| Hypertension | <0.001 | |||||
| Yes | 1111 (15.3%) | 458 (21.7%) | 220 (16.3%) | 286 (13.4%) | 147 (8.8%) | |
| Do not know | 17 (0.2%) | 6 (0.3%) | 1 (0.1%) | 9 (0.4%) | 1 (0.1%) | |
| Diabetes | <0.001 | |||||
| Yes | 295 (4.1%) | 119 (5.6%) | 65 (4.8%) | 73 (3.4%) | 38 (2.3%) | |
| Do not know | 19 (0.3%) | 5 (0.2%) | 3 (0.2%) | 8 (0.4%) | 3 (0.2%) | |
| Specific Site-Specific Young-Onset Cancer | Overall (n 7272) | Perinatal Exposure | |||
|---|---|---|---|---|---|
| Pre-Famine (n 2107) | Famine (n 1352) | Early Post-Famine (n 2135) | Late Post-Famine (n 1678) | ||
| Young-onset cancer | 53 (0.73%) | 12 (0.57%) | 8 (0.59%) | 23 (1.08%) | 10 (0.60%) |
| Genitourinary cancer | |||||
| Uterine cancer | 22 (0.30%) | 1 (0.05%) | 4 (0.30%) | 11 (0.52%) | 6 (0.36%) |
| Cervical cancer | 2 (0.02%) | 0 (0.00%) | 0 (0.00%) | 1 (0.05%) | 1 (0.06%) |
| Breast cancer | 8 (0.11%) | 1 (0.05%) | 2 (0.15%) | 3 (0.14%) | 2 (0.12%) |
| Gastrointestinal cancer Colon cancer | 9 (0.12%) | 6 (0.28%) | 2 (0.15%) | 1 (0.05%) | 0 (0.00%) |
| Hepatic cancer | 1 (0.01%) | 1 (0.05%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) |
| Brain cancer | 4 (0.06%) | 1 (0.05%) | 0 (0.00%) | 2 (0.09%) | 1 (0.06%) |
| Lung cancer | 2 (0.03%) | 1 (0.05%) | 0 (0.00%) | 1 (0.05%) | 0 (0.00%) |
| Other cancer | 9 (0.12%) | 4 (0.19%) | 0 (0.00%) | 5 (0.23%) | 0 (0.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, A.; Ullah, S.; Yu, Y.; Pandol, S.J.; Barreto, S.G. Perinatal Famine Exposure and Young-Onset Cancer—Lessons from China Health and Nutrition Survey. Cancers 2024, 16, 2537. https://doi.org/10.3390/cancers16142537
Shuai A, Ullah S, Yu Y, Pandol SJ, Barreto SG. Perinatal Famine Exposure and Young-Onset Cancer—Lessons from China Health and Nutrition Survey. Cancers. 2024; 16(14):2537. https://doi.org/10.3390/cancers16142537
Chicago/Turabian StyleShuai, Aidi, Shahid Ullah, Yongfu Yu, Stephen J. Pandol, and Savio George Barreto. 2024. "Perinatal Famine Exposure and Young-Onset Cancer—Lessons from China Health and Nutrition Survey" Cancers 16, no. 14: 2537. https://doi.org/10.3390/cancers16142537
APA StyleShuai, A., Ullah, S., Yu, Y., Pandol, S. J., & Barreto, S. G. (2024). Perinatal Famine Exposure and Young-Onset Cancer—Lessons from China Health and Nutrition Survey. Cancers, 16(14), 2537. https://doi.org/10.3390/cancers16142537







