Safety and Feasibility of Neoadjuvant-Modified FOLFIRINOX in Elderly Patients with Pancreatic Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. NAC
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. NAC
3.3. AEs
3.4. Perioperative and Postoperative Outcomes
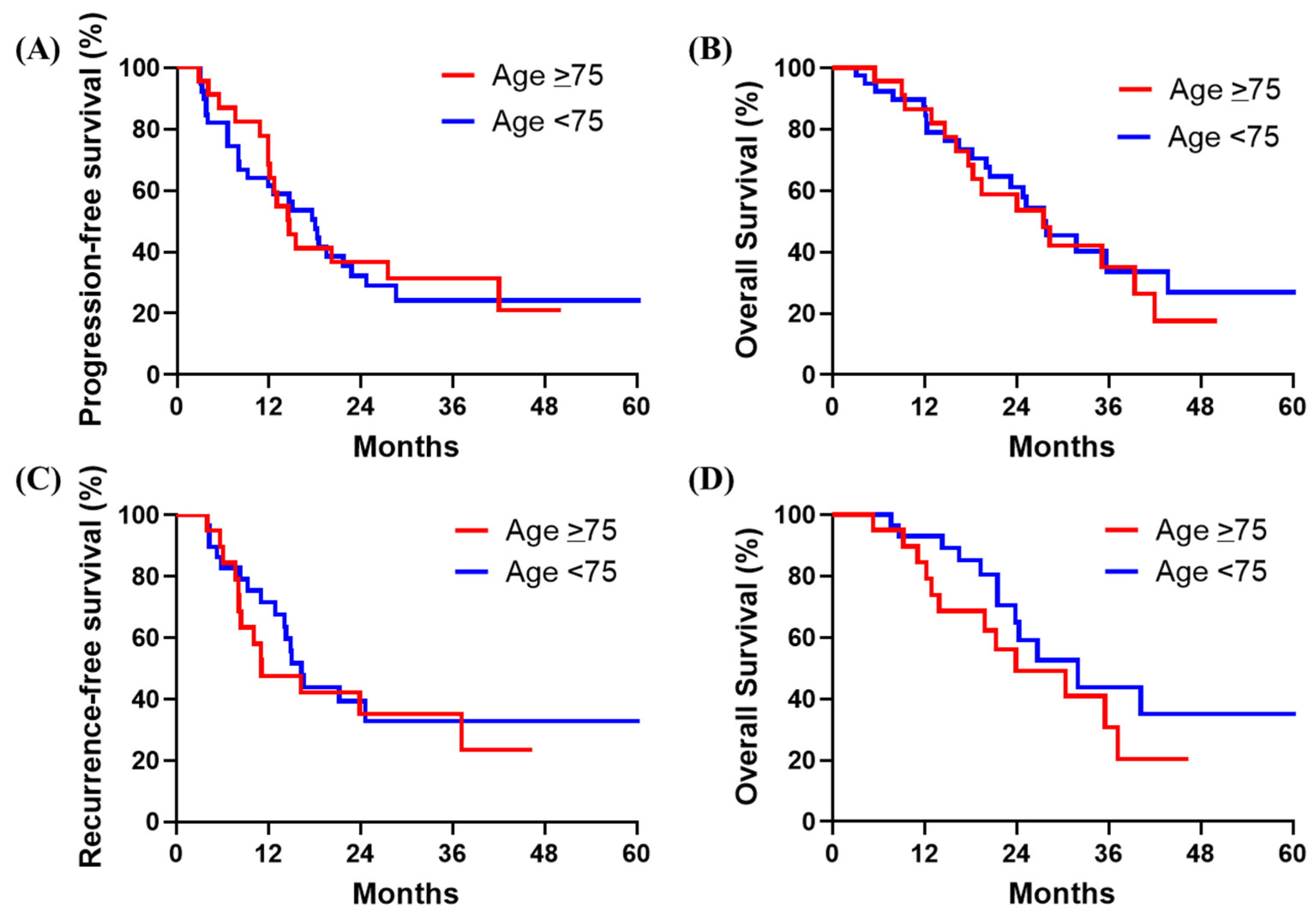
3.5. Survival Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. G8 Screening Tool
| Items | Score | |
|---|---|---|
| 1 | Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0 = severe decrease in food intake |
| 1 = moderate decrease in food intake | ||
| 2 = no decrease in food intake | ||
| 2 | Weight loss during the last 3 months? | 0 = weight loss >3 kg |
| 1 = does not know | ||
| 2 = weight loss between 1 and 3 kg | ||
| 3 = no weight loss | ||
| 3 | Mobility | 0 = bed or chair bound |
| 1 = able to get out of bed/chair but does not go out | ||
| 2 = goes out | ||
| 4 | Neuropsychological problems | 0 = severe dementia or depression |
| 1 = mild dementia or depression | ||
| 2 = no psychological problems | ||
| 5 | BMI | 0 = BMI <19 |
| 1 = BMI 19 to <21 | ||
| 2 = BMI 21 to <23 | ||
| 3 = BMI ≥ 23 | ||
| 6 | Takes more than three mediations per day? | 0 = yes |
| 1 = no | ||
| 7 | In comparison with other people of the same age, how does the patient consider his/her health status? | 0 = not as good |
| 0.5 = does not know | ||
| 1 = as good | ||
| 2 = better | ||
| 8 | Age | 0: >85 |
| 1: 80–85 | ||
| 2: <80 | ||
| Total Score | 0–17 | |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Niesen, W.; Hank, T.; Büchler, M.; Strobel, O. Local radicality and survival outcome of pancreatic cancer surgery. Ann. Gastroenterol. Surg. 2019, 3, 464–475. [Google Scholar] [CrossRef]
- Khorana, A.A.; Mangu, P.B.; Berlin, J.; Engebretson, A.; Hong, T.S.; Maitra, A.; Mohile, S.G.; Mumber, M.; Schulick, R.; Shapiro, M.; et al. Potentially Curable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2016, 34, 2541–2556. [Google Scholar] [CrossRef]
- Motoi, F.; Kosuge, T.; Ueno, H.; Yamaue, H.; Satoi, S.; Sho, M.; Honda, G.; Matsumoto, I.; Wada, K.; Furuse, J.; et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn. J. Clin. Oncol. 2019, 49, 190–194. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Dhir, M.; Zenati, M.S.; Hamad, A.; Singhi, A.D.; Bahary, N.; Hogg, M.E.; Zeh, H.J., 3rd; Zureikat, A.H. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann. Surg. Oncol. 2018, 25, 1896–1903. [Google Scholar] [CrossRef]
- Yamaguchi, J.; Yokoyama, Y.; Fujii, T.; Yamada, S.; Takami, H.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Maeda, O.; Ogawa, H.; et al. Results of a Phase II Study on the Use of Neoadjuvant Chemotherapy (FOLFIRINOX or GEM/nab-PTX) for Borderline-resectable Pancreatic Cancer (NUPAT-01). Ann. Surg. 2022, 275, 1043–1049. [Google Scholar] [CrossRef]
- Ozaka, M.; Ishii, H.; Sato, T.; Ueno, M.; Ikeda, M.; Uesugi, K.; Sata, N.; Miyashita, K.; Mizuno, N.; Tsuji, K.; et al. A phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 2018, 81, 1017–1023. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Franchi, R.; Okoye, C.; Antognoli, R.; Pompilii, I.M.; Taverni, I.; Landi, T.; Ghilli, M.; Roncella, M.; Calsolaro, V.; Monzani, F.; et al. Multidimensional Oncological Frailty Scale (MOFS): A New Quick-To-Use Tool for Detecting Frailty and Stratifying Risk in Older Patients with Cancer-Development and Validation Pilot Study. Cancers 2023, 15, 1553. [Google Scholar] [CrossRef]
- Bassi, C.; Marchegiani, G.; Dervenis, C.; Sarr, M.; Abu Hilal, M.; Adham, M.; Allen, P.; Andersson, R.; Asbun, H.J.; Besselink, M.G.; et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery 2017, 161, 584–591. [Google Scholar] [CrossRef]
- Wente, M.N.; Bassi, C.; Dervenis, C.; Fingerhut, A.; Gouma, D.J.; Izbicki, J.R.; Neoptolemos, J.P.; Padbury, R.T.; Sarr, M.G.; Traverso, L.W.; et al. Delayed gastric emptying (DGE) after pancreatic surgery: A suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007, 142, 761–768. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Huan, L.; Yu, F.; Cao, D.; Zhou, H.; Qin, M.; Cao, Y. Comparison of neoadjuvant treatment and surgery first for resectable or borderline resectable pancreatic carcinoma: A systematic review and network meta-analysis of randomized controlled trials. PLoS ONE 2024, 19, e0295983. [Google Scholar] [CrossRef]
- Rieser, C.J.; Zenati, M.; Narayanan, S.; Bahary, N.; Lee, K.K.; Paniccia, A.; Bartlett, D.L.; Zureikat, A.H. Optimal Management of Resectable Pancreatic Head Cancer in the Elderly Patient: Does Neoadjuvant Therapy Offer a Survival Benefit? Ann. Surg. Oncol. 2021, 28, 6264–6272. [Google Scholar] [CrossRef]
- Akahori, T.; Terai, T.; Nagai, M.; Nakamura, K.; Kohara, Y.; Yasuda, S.; Matsuo, Y.; Doi, S.; Sakata, T.; Sho, M. Total neoadjuvant therapy improves survival of patients with borderline resectable pancreatic cancer with arterial involvement. Ann. Gastroenterol. Surg. 2024, 8, 151–162. [Google Scholar] [CrossRef]
- Igarashi, T.; Fukasawa, M.; Watanabe, T.; Kimura, N.; Itoh, A.; Tanaka, H.; Shibuya, K.; Yoshioka, I.; Hirabayashi, K.; Fujii, T. Evaluating staging laparoscopy indications for pancreatic cancer based on resectability classification and treatment strategies for patients with positive peritoneal washing cytology. Ann. Gastroenterol. Surg. 2024, 8, 124–132. [Google Scholar] [CrossRef]
- Tanaka, N.; Takami, H.; Hayashi, M.; Inokawa, Y.; Kurimoto, K.; Hattori, N.; Kanda, M.; Tanaka, C.; Nakayama, G.; Kodera, Y. Predictive impacts of peritoneal washing cytology for surgical resection-intended pancreatic cancer cases: Establishment of planned staging laparoscopy criteria. J. Hepatobiliary Pancreat. Sci. 2023, 30, 1273–1281. [Google Scholar] [CrossRef]
- Miura, J.T.; Krepline, A.N.; George, B.; Ritch, P.S.; Erickson, B.A.; Johnston, F.M.; Oshima, K.; Christians, K.K.; Evans, D.B.; Tsai, S. Use of neoadjuvant therapy in patients 75 years of age and older with pancreatic cancer. Surgery 2015, 158, 1545–1555. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Rogers, J.E.; Hess, K.R.; Wolff, R.A.; Varadhachary, G.R.; Javle, M.M.; Shroff, R.T.; Ho, L.; Fogelman, D.R.; Raghav, K.P.S.; et al. Modified FOLFIRINOX in pancreatic cancer patients Age 75 or older. Pancreatology 2020, 20, 501–504. [Google Scholar] [CrossRef]
- Oba, A.; Wu, Y.H.A.; Lieu, C.H.; Meguid, C.; Colborn, K.L.; Beaty, L.; Al-Musawi, M.H.; Davis, S.L.; Leal, A.D.; Purcell, T.; et al. Outcome of neoadjuvant treatment for pancreatic cancer in elderly patients: Comparative, observational cohort study. Br. J. Surg. 2021, 108, 976–982. [Google Scholar] [CrossRef]
- Okabayashi, T.; Sui, K.; Murokawa, T.; Kimura, J.; Iwata, J.; Morita, S.; Iiyama, T.; Shimada, Y. Indications for pancreaticoduodenectomy affected postoperative outcomes in octogenarians. Ann. Gastroenterol. Surg. 2021, 5, 102–110. [Google Scholar] [CrossRef]
- Vining, C.C.; Kuchta, K.; Schuitevoerder, D.; Paterakos, P.; Berger, Y.; Roggin, K.K.; Talamonti, M.S.; Hogg, M.E. Risk factors for complications in patients undergoing pancreaticoduodenectomy: A NSQIP analysis with propensity score matching. J. Surg. Oncol. 2020, 122, 183–194. [Google Scholar] [CrossRef]
- Nymo, L.S.; Kleive, D.; Waardal, K.; Bringeland, E.A.; Søreide, J.A.; Labori, K.J.; Mortensen, K.E.; Søreide, K.; Lassen, K. Centralizing a national pancreatoduodenectomy service: Striking the right balance. BJS Open 2020, 4, 904–913. [Google Scholar] [CrossRef]
- Sukharamwala, P.; Thoens, J.; Szuchmacher, M.; Smith, J.; DeVito, P. Advanced age is a risk factor for post-operative complications and mortality after a pancreaticoduodenectomy: A meta-analysis and systematic review. HPB 2012, 14, 649–657. [Google Scholar] [CrossRef]
- Kim, S.Y.; Weinberg, L.; Christophi, C.; Nikfarjam, M. The outcomes of pancreaticoduodenectomy in patients aged 80 or older: A systematic review and meta-analysis. HPB 2017, 19, 475–482. [Google Scholar] [CrossRef]
- Huang, Y.; Damodaran Prabha, R.; Chua, T.C.; Arena, J.; Kotecha, K.; Mittal, A.; Gill, A.J.; Samra, J.S. Safety and Efficacy of Pancreaticoduodenectomy in Octogenarians. Front. Surg. 2021, 8, 617286. [Google Scholar] [CrossRef]
- Beltrame, V.; Gruppo, M.; Pastorelli, D.; Pedrazzoli, S.; Merigliano, S.; Sperti, C. Outcome of pancreaticoduodenectomy in octogenarians: Single institution’s experience and review of the literature. J. Visc. Surg. 2015, 152, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sho, M.; Murakami, Y.; Kawai, M.; Motoi, F.; Satoi, S.; Matsumoto, I.; Honda, G.; Uemura, K.; Yanagimoto, H.; Kurata, M.; et al. Prognosis after surgical treatment for pancreatic cancer in patients aged 80 years or older: A multicenter study. J. Hepatobiliary Pancreat. Sci. 2016, 23, 188–197. [Google Scholar] [CrossRef]
- Sugiura, T.; Okamura, Y.; Ito, T.; Yamamoto, Y.; Ashida, R.; Uesaka, K. Impact of Patient Age on the Postoperative Survival in Pancreatic Head Cancer. Ann. Surg. Oncol. 2017, 24, 3220–3228. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: A phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Conroy, T.; Castan, F.; Lopez, A.; Turpin, A.; Ben Abdelghani, M.; Wei, A.C.; Mitry, E.; Biagi, J.J.; Evesque, L.; Artru, P.; et al. Five-Year Outcomes of FOLFIRINOX vs. Gemcitabine as Adjuvant Therapy for Pancreatic Cancer: A Randomized Clinical Trial. JAMA Oncol. 2022, 8, 1571–1578. [Google Scholar] [CrossRef]
- Nassoiy, S.; Christopher, W.; Marcus, R.; Keller, J.; Weiss, J.; Chang, S.C.; Essner, R.; Foshag, L.; Fischer, T.; Goldfarb, M. Evolving management of early stage pancreatic adenocarcinoma in older patients. Am. J. Surg. 2023, 225, 212–219. [Google Scholar] [CrossRef] [PubMed]
| Factors | Age ≥ 75 Years (n = 23) | Age < 75 Years (n = 39) | p-Value |
|---|---|---|---|
| Age (years) | 79 (75–82) | 67 (37–74) | <0.0001 |
| Sex (Male) | 12 (52) | 23 (59) | 0.7913 |
| ASA-PS | 0.2383 | ||
| 1 | 0 | 4 (10) | |
| 2 | 18 (78) | 25 (64) | |
| 3 | 5 (22) | 10 (26) | |
| BMI (kg/m2) | 21 (16–26) | 22 (15–32) | 0.1101 |
| CCI | 0.9125 | ||
| 0 | 6 (26) | 12 (31) | |
| 1 | 9 (39) | 15 (38) | |
| >2 | 8 (35) | 12 (31) | |
| G8 score | 0.3957 | ||
| <14 | 18 (78) | 26 (67) | |
| >14 | 5 (22) | 13 (33) | |
| Tumor location | 0.1164 | ||
| Head | 9 (39) | 24 (62) | |
| Body/tail | 14 (61) | 15 (38) | |
| Resectability status | 0.5082 | ||
| R | 20 (87) | 30 (77) | |
| BR | 3 (13) | 9 (23) | |
| Initial tumor size (mm) | 24 (13–44) | 23 (11–57) | 0.9509 |
| Initial CA19-9 (U/mL) | 81 (2–4701) | 281 (2–19072) | 0.4493 |
| Initial SUV-max | 5 (1.6–14.5) | 5.5 (1.6–12.7) | 0.9625 |
| UGT1A1 | 0.7406 | ||
| Wild | 12 (52) | 20 (51) | |
| 6 hetero/28 hetero | 11 (48) | 18 (46) | |
| 6 homo | 0 (0) | 1 (3) |
| Age ≥ 75 Years (n = 23) | Age < 75 Years (n = 39) | p-Value | |
|---|---|---|---|
| Completion of NAC | 17 (74) | 30 (77) | >0.9999 |
| Dose reduction | 21 (91) | 28 (72) | 0.1063 |
| ARDI | 65.3 (44.3–100.0) | 77.0 (45.2–100.0) | 0.0665 |
| Surgical resection feasible | 20 (87) | 29 (74) | 0.3381 |
| Reason for not undergoing surgical resection | 0.7008 | ||
| Disease progression | 2 (9) | 7 (18) | |
| Poor general condition | 0 (0) | 1 (3) | |
| Adverse event | 0 (0) | 1 (3) | |
| Others | 1 (4) | 1 (3) |
| Age ≥ 75 Years (n = 23) | Age < 75 Years (n = 39) | p-Value | ||||
|---|---|---|---|---|---|---|
| Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | Any Grade | Grade ≥ 3 | |
| Neutropenia | 18 (78) | 11 (48) | 26 (67) | 18 (46) | 0.3957 | >0.9999 |
| Anemia | 21 (91) | 0 (0) | 32 (82) | 2 (5) | 0.4639 | 0.5256 |
| Thrombocytopenia | 16 (70) | 0 (0) | 28 (72) | 2 (5) | >0.9999 | 0.5256 |
| Febrile neutropenia | 1 (4) | 1 (4) | 1 (3) | 1 (3) | >0.9999 | >0.9999 |
| AST increased | 5 (22) | 0 (0) | 21 (54) | 2 (5) | 0.0173 | 0.5256 |
| ALT increased | 7 (30) | 0 (0) | 23 (59) | 2 (5) | 0.0378 | 0.5256 |
| Creatinine increased | 2 (9) | 0 ( 0) | 2 (5) | 2 (5) | 0.6232 | 0.5256 |
| Fatigue | 10 (43) | 2 (9) | 23 (59) | 1 (3) | 0.2962 | 0.5494 |
| Nausea | 6 (26) | 0 (0) | 23 (59) | 2 (5) | 0.0177 | 0.5256 |
| Biliary tract infection | 0 (0) | 0 (0) | 4 (10) | 4 (10) | 0.2871 | 0.2871 |
| Diarrhea | 8 (35) | 2 (9) | 13 (33) | 2 (5) | >0.9999 | 0.6232 |
| Stomatitis | 3 (13) | 0 (0) | 7 (18) | 0 (0) | 0.7313 | >0.9999 |
| Sensory neuropathy | 8 (35) | 0 (0) | 21 (54) | 0 (0) | 0.1910 | >0.9999 |
| Pneumonitis | 0 (0) | 0 (0) | 1 (3) | 1 (3) | >0.9999 | >0.9999 |
| Age ≥ 75 Years (n = 20) | Age < 75 Years (n = 29) | p-Value | |
|---|---|---|---|
| Operative procedure | 0.4981 | ||
| PD | 9 (45) | 18 (62) | |
| DP | 10 (50) | 10 (34) | |
| TP | 1 (5) | 1 (3) | |
| Vascular resection | 2 (10) | 3 (10) | >0.9999 |
| Operation time (min) | 451 (280–850) | 523 (273–805) | 0.2447 |
| Blood loss (mL) | 340 (25–2800) | 475 (10–4450) | 0.3098 |
| pT stage | 0.7145 | ||
| 0 | 1 (5) | 0 (0) | |
| 1 | 11 (55) | 19 (66) | |
| 2 | 8 (40) | 8 (28) | |
| 3 | 0 (0) | 2 (7) | |
| pN1 | 9 (45) | 6 (21) | 0.1142 |
| R0 resection | 18 (90) | 25 (86) | >0.9999 |
| Clavien–Dindo ≥ IIIA | 7 (35) | 16 (55) | 0.2450 |
| POPF of ≥grade B | 3 (15) | 6 (21) | 0.7199 |
| DGE of ≥grade B | 1 (5) | 2 (7) | >0.9999 |
| Mortality | 0 (0) | 0 (0) | >0.9999 |
| Postoperative hospital stay (days) | 19 (10–79) | 24 (10–70) | 0.3496 |
| Adjuvant chemotherapy | 18 (90) | 27 (93) | >0.9999 |
| Univariate Analysis | p-Value | Multivariate Analysis | p-Value | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |||
| Age (≥75 vs. <75 years) | 1.155 | 0.593–2.248 | 0.6723 | 1.247 | 0.608–2.556 | 0.5475 |
| Sex (male vs. female) | 1.307 | 0.658–2.593 | 0.4443 | |||
| ASA-PS (3 vs. 1/2) | 1.678 | 0.803–3.508 | 0.1687 | 3.515 | 1.422–8.691 | 0.0065 |
| BMI (<22 vs. ≥22) | 1.182 | 0.613–2.277 | 0.6178 | |||
| CCI (≥2 vs. <2) | 1.620 | 0.831–3.160 | 0.1566 | |||
| G8 (≤14 vs. >14) | 1.586 | 0.722–3.482 | 0.2507 | |||
| Tumor location (Head vs. Body/tail) | 1.404 | 0.721–2.733 | 0.3183 | |||
| Resectability status (BR vs. R) | 2.663 | 1.198–5.923 | 0.0163 | |||
| Initial tumor size (≥20 vs. <20 mm) | 1.442 | 0.677–3.073 | 0.3430 | |||
| Initial CA19-9 (≥37 vs. <37 U/mL) | 1.855 | 0.843–4.085 | 0.1247 | 2.302 | 0.936–5.661 | 0.0695 |
| Initial SUV-max (≥5 vs. <5) | 1.709 | 0.880–3.319 | 0.1137 | |||
| Completion of NAC (no vs. yes) | 1.918 | 0.906–4.062 | 0.0887 | |||
| Dose reduction (no vs. yes) | 1.044 | 0.456–2.388 | 0.9195 | |||
| ARDI (≤70 vs. >70) | 1.585 | 0.818–3.073 | 0.1725 | |||
| Surgical resection feasible (no vs. yes) | 14.603 | 5.910–36.089 | <0.0001 | 26.878 | 7.955–90.819 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shindo, Y.; Ioka, T.; Tokumitsu, Y.; Matsui, H.; Nakajima, M.; Kimura, Y.; Watanabe, Y.; Tomochika, S.; Nakagami, Y.; Tsunedomi, R.; et al. Safety and Feasibility of Neoadjuvant-Modified FOLFIRINOX in Elderly Patients with Pancreatic Cancer. Cancers 2024, 16, 2522. https://doi.org/10.3390/cancers16142522
Shindo Y, Ioka T, Tokumitsu Y, Matsui H, Nakajima M, Kimura Y, Watanabe Y, Tomochika S, Nakagami Y, Tsunedomi R, et al. Safety and Feasibility of Neoadjuvant-Modified FOLFIRINOX in Elderly Patients with Pancreatic Cancer. Cancers. 2024; 16(14):2522. https://doi.org/10.3390/cancers16142522
Chicago/Turabian StyleShindo, Yoshitaro, Tatsuya Ioka, Yukio Tokumitsu, Hiroto Matsui, Masao Nakajima, Yuta Kimura, Yusaku Watanabe, Shinobu Tomochika, Yuki Nakagami, Ryouichi Tsunedomi, and et al. 2024. "Safety and Feasibility of Neoadjuvant-Modified FOLFIRINOX in Elderly Patients with Pancreatic Cancer" Cancers 16, no. 14: 2522. https://doi.org/10.3390/cancers16142522
APA StyleShindo, Y., Ioka, T., Tokumitsu, Y., Matsui, H., Nakajima, M., Kimura, Y., Watanabe, Y., Tomochika, S., Nakagami, Y., Tsunedomi, R., Iida, M., Takahashi, H., & Nagano, H. (2024). Safety and Feasibility of Neoadjuvant-Modified FOLFIRINOX in Elderly Patients with Pancreatic Cancer. Cancers, 16(14), 2522. https://doi.org/10.3390/cancers16142522







