A Novel Prognostic Indicator for Immunotherapy Response: Lymphocyte-to-Albumin (LA) Ratio Predicts Survival in Metastatic NSCLC Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcomes
2.2. Statistical Analysis
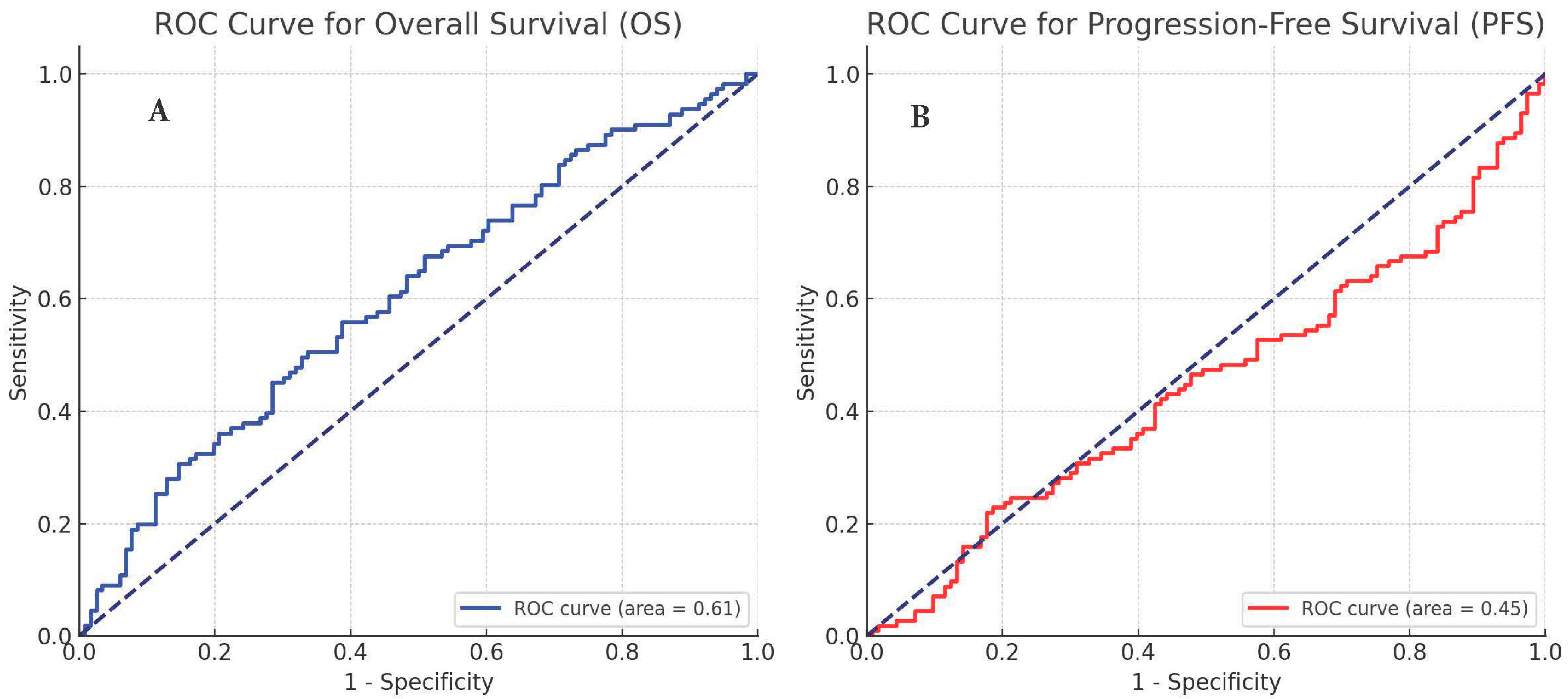
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- WHO; Classification of Tumours Editorial Board. Thoracic Tumours. In WHO Classification of Tumours, 5th ed.; IARC Publications: Lyon, France, 2021; Volume 5. [Google Scholar]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Garon, E.B.; Kim, D.W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Rittmeyer, A.; Gadgeel, S.; Hida, T.; Gandara, D.R.; Cortinovis, D.L.; Barlesi, F.; Yu, W.; Matheny, C.; Ballinger, M.; et al. Atezolizumab versus Docetaxel in Pretreated Patients with NSCLC: Final Results from the Randomized Phase 2 POPLAR and Phase 3 OAK Clinical Trials. J. Thorac. Oncol. 2021, 16, 140–150. [Google Scholar] [CrossRef]
- Sankar, K.; Ye, J.C.; Li, Z.; Zheng, L.; Song, W.; Hu-Lieskovan, S. The role of biomarkers in personalized immunotherapy. Biomark Res. 2022, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Tostes, K.; Siqueira, A.P.; Reis, R.M.; Leal, L.F.; Arantes, L.M.R.B. Biomarkers for Immune Checkpoint Inhibitor Response in NSCLC: Current Developments and Applicability. Int. J. Mol. Sci. 2023, 24, 11887. [Google Scholar] [CrossRef] [PubMed]
- Shirasawa, M.; Yoshida, T.; Ohe, Y. Biomarkers of immunotherapy for non-small cell lung cancer. Jpn. J. Clin. Oncol. 2024, 54, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pabst, L.; Lopes, S.; Bertrand, B.; Creusot, Q.; Kotovskaya, M.; Pencreach, E.; Beau-Faller, M.; Mascaux, C. Prognostic and Predictive Biomarkers in the Era of Immunotherapy for Lung Cancer. Int. J. Mol. Sci. 2023, 24, 7577. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat. Immunol. 2017, 18, 843–850. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, Y.; Peng, Y.; Wang, M.; Zhou, Y.; Gu, W.; Li, Y.; Zou, J.; Zhu, N.; Chen, L. Advanced lung cancer inflammation index combined with geriatric nutritional risk index predict all-cause mortality in heart failure patients. BMC Cardiovasc. Disord. 2023, 23, 565. [Google Scholar] [CrossRef]
- Zhang, C.L.; Gao, M.Q.; Jiang, X.C.; Pan, X.; Zhang, X.Y.; Li, Y.; Shen, Q.; Chen, Y.; Pang, B. Research progress and value of albumin-related inflammatory markers in the prognosis of non-small cell lung cancer: A review of clinical evidence. Ann. Med. 2023, 55, 1294–1307. [Google Scholar] [CrossRef]
- Zhao, S.T.; Chen, X.X.; Yang, X.M.; He, S.C.; Qian, F.H. Application of Monocyte-to-Albumin Ratio and Neutrophil Percentage-to-Hemoglobin Ratio on Distinguishing Non-Small Cell Lung Cancer Patients from Healthy Subjects. Int. J. Gen. Med. 2023, 16, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Huai, Q.; Luo, C.; Song, P.; Bie, F.; Bai, G.; Li, Y.; Liu, Y.; Chen, X.; Zhou, B.; Sun, X.; et al. Peripheral blood inflammatory biomarkers dynamics reflect treatment response and predict prognosis in non-small cell lung cancer patients with neoadjuvant immunotherapy. Cancer Sci. 2023, 114, 4484–4498. [Google Scholar] [CrossRef] [PubMed]
- Savioli, F.; Morrow, E.S.; Dolan, R.D.; Romics, L.; Lannigan, A.; Edwards, J.; McMillan, D.C. Prognostic role of preoperative circulating systemic inflammatory response markers in primary breast cancer: Meta-analysis. Br. J. Surg. 2022, 109, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Portale, G.; Bartolotta, P.; Azzolina, D.; Gregori, D.; Fiscon, V. Prognostic role of platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte, and lymphocyte-to-monocyte ratio in operated rectal cancer patients: Systematic review and meta-analysis. Langenbecks Arch. Surg. 2023, 408, 85. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Hida, K.; Matsusue, R.; Itatani, Y.; Mizuno, R.; Yamaguchi, T.; Ikai, I.; Sakai, Y. Combination of lymphocyte count and albumin concentration as a new prognostic biomarker for rectal cancer. Sci. Rep. 2021, 11, 5027. [Google Scholar] [CrossRef]
- David, S.E.; Mckinney, K.; Oken, M.; Lee, H.K.; Crumbaker, M.; Beg, M.S.; Holland, J.; American Society of Clinical Oncology. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer. Version 2. (NCCN.org., 2024); National Comprehensive Cancer Network, Inc.: Plymouth Meeting, PA, USA, 2024. [Google Scholar]
- Matsuura, S.; Serizawa, S.; Yamashita, R.; Morikawa, K.; Ito, Y.; Hiramatsu, T.; Mochizuki, E.; Tanaka, K.; Akiyama, N.; Tsukui, M.; et al. The Prognostic Nutritional Index before durvalumab after chemoradiation predict the overall survival in patients with stage III non-small cell lung cancer. Ann. Med. 2023, 55, 2196089. [Google Scholar] [CrossRef]
- Manjarrez-Orduño, N.; Menard, L.C.; Kansal, S.; Fischer, P.; Kakrecha, B.; Jiang, C.; Cunningham, M.; Greenawalt, D.; Patel, V.; Yang, M.; et al. Circulating T Cell Subpopulations Correlate with Immune Responses at the Tumor Site and Clinical Response to PD1 Inhibition in Non-Small Cell Lung Cancer. Front. Immunol. 2018, 9, 1613. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, P.C.; Wang, C.Y.; Kuo, P.L. Evaluation of cytotoxic T lymphocyte-mediated anticancer response against tumor interstitium-simulating physical barriers. Sci. Rep. 2020, 10, 13662. [Google Scholar] [CrossRef]
- Zheng, Q.M.; Li, Y.Y.; Wang, Y.P.; Li, G.X.; Zhao, M.M.; Sun, Z.G. Association between CD8+ tumor-infiltrating lymphocytes and prognosis of non-small cell lung cancer patients treated with PD-1/PD-L1 inhibitors: A systematic review and meta-analysis. Expert Rev. Anticancer Ther. 2023, 23, 643–659. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Chen, P.; Xu, W.; Wu, Y.; Che, G. Prognostic value of the pretreatment systemic immune-inflammation index (SII) in patients with non-small cell lung cancer: A meta-analysis. Ann. Transl. Med. 2019, 7, 433. [Google Scholar] [CrossRef]
- Wang, M.D.; Duan, F.F.; Hua, X.; Cao, L.; Xia, W.; Chen, J.Y. A Novel Albumin-Related Nutrition Biomarker Predicts Breast Cancer Prognosis in Neoadjuvant Chemotherapy: A Two-Center Cohort Study. Nutrients 2023, 15, 4292. [Google Scholar] [CrossRef]
- Heppner, B.; Untch, M.; Denkert, C.; Pfitzner, B.; Lederer, B.; Schmitt, W.; Eidtmann, H.; Fasching, P.; Tesch, H.; Solbach, C.; et al. Tumor-Infiltrating Lymphocytes: A Predictive and Prognostic Biomarker in Neoadjuvant-Treated HER2-Positive Breast Cancer. Clin. Cancer Res. 2016, 22, 5747–5754. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhao, S.T.; Yang, X.M.; He, S.C.; Qian, F.H. Additional diagnostic value of the monocyte to red blood cell count ratio and the product of lymphocyte count and albumin concentration in lung cancer management. Oncol. Lett. 2023, 25, 135. [Google Scholar] [CrossRef]

| Variables | N (227) | (%) |
|---|---|---|
| Age (years) | ||
| Median (min–max) | 63 (24–88) | |
| <65 | 125 | 55.1 |
| ≥65 | 102 | 44.9 |
| Gender | ||
| Female | 31 | 13.7 |
| Male | 196 | 86.3 |
| ECOG score | ||
| 0 | 161 | 70.9 |
| 1 | 49 | 21.6 |
| 2 | 17 | 7.5 |
| Histopathology | ||
| Squamous | 89 | 39.2 |
| Non-squamous | 138 | 60.8 |
| Initial stage | ||
| Early stage at diagnosis | 102 | 44.9 |
| Metastatic at diagnosis | 125 | 55.1 |
| Number of lines of chemotherapy received before nivolumab | ||
| 1 | 149 | 65.6 |
| 2 | 57 | 25.1 |
| 3 or more | 21 | 9.3 |
| Initial radiological response evaluation to nivolumab | ||
| Complete Response | 12 | 5.3 |
| Partial response | 69 | 30.4 |
| Stable disease | 56 | 24.7 |
| Confirmed progression | 89 | 39.2 |
| Pseudoprogression | 1 | 0.4 |
| Progression | ||
| No | 98 | 43.2 |
| Yes | 129 | 56.8 |
| Status | ||
| Alive | 130 | 57.3 |
| Exitus | 97 | 42.7 |
| Average Follow-Up Time | 9.35 ± 5.82 | |
| Overall Survival | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| n | 1-Year OS (%) | p | HR | %95 CI | p | ||
| Age | <65 | 125 | 59.0 | 0.902 | |||
| ≥65 | 102 | 55.2 | |||||
| Sex | Woman | 31 | 51.7 | 0.878 | |||
| Man | 196 | 58.5 | |||||
| ECOG score | 0 | 161 | 58.0 | <0.001 | ref | 0.011 | |
| 1 | 49 | 68.0 | 1.08 | 0.65–1.79 | 0.755 | ||
| 2 | 17 | 0.0 | 2.80 | 1.43–5.50 | 0.003 | ||
| Histopathology | Squamous | 89 | 57.3 | 0.653 | |||
| Non-squamous | 138 | 57.6 | |||||
| Number of lines of CT received before nivolumab | 1 | 149 | 53.7 | 0.210 | |||
| 2 | 57 | 66.8 | |||||
| ≥3 | 21 | 55.0 | |||||
| LA | >52.87 | 122 | 82.0 | <0.001 | ref | ||
| ≤52.87 | 105 | 29.8 | 6.24 | 3.46–11.25 | <0.001 | ||
| NLR | <3.61 | 118 | 73.0 | <0.001 | ref | ||
| ≥3.61 | 109 | 39.5 | 0.97 | 0.55–1.70 | 0.923 | ||
| MLR | <0.48 | 113 | 71.2 | <0.001 | ref | ||
| ≥0.48 | 114 | 43.8 | 0.76 | 0.42–1.36 | 0.359 | ||
| PLR | <196.48 | 105 | 70.7 | <0.001 | ref | ||
| ≥196.48 | 122 | 43.0 | 0.89 | 0.53–1.49 | 0.669 | ||
| SII | <921.57 | 118 | 70.6 | <0.001 | ref | ||
| ≥921.57 | 109 | 43.2 | 1.75 | 1.01–3.03 | 0.045 | ||
| MAR | <0.017 | 99 | 62.7 | 0.011 | ref | ||
| ≥0.017 | 128 | 54.0 | 2.07 | 1.22–3.51 | 0.006 | ||
| PFS | |||||||
|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | ||||||
| n | 1-Year PFS (%) | p | HR | %95 CI | p | ||
| Age | <65 | 125 | 39.5 | 0.885 | |||
| ≥65 | 102 | 36.8 | |||||
| Sex | Woman | 31 | 35.2 | 0.572 | |||
| Man | 196 | 39.0 | |||||
| ECOG score | 0 | 161 | 36.7 | <0.001 | ref | 0.022 | |
| 1 | 49 | 52.5 | 0.88 | 0.56–1.37 | 0.581 | ||
| 2 | 17 | 0.0 | 2.27 | 1.22–4.21 | 0.009 | ||
| Histopathology | Squamous | 89 | 35.2 | 0.868 | |||
| Non-squamous | 138 | 40.4 | |||||
| Number of lines of CT received before nivolumab | 1 | 149 | 36.5 | 0.581 | |||
| 2 | 57 | 44.0 | |||||
| ≥3 | 21 | 34.4 | |||||
| LA | >57.67 | 106 | 65.2 | <0.001 | ref | ||
| ≤57.67 | 121 | 15.4 | 4.47 | 2.73–7.34 | <0.001 | ||
| NLR | <3.35 | 109 | 51.6 | <0.001 | ref | ||
| ≥3.35 | 118 | 26.5 | 0.79 | 0.48–1.30 | 0.356 | ||
| MLR | <0.46 | 105 | 54.2 | <0.001 | ref | ||
| ≥0.46 | 122 | 24.9 | 0.84 | 0.51–1.39 | 0.507 | ||
| PLR | <190.30 | 110 | 50.2 | 0.001 | ref | ||
| ≥190.30 | 117 | 27.5 | 1.00 | 0.64–1.57 | 0.971 | ||
| SII | <876.85 | 110 | 50.3 | <0.001 | ref | ||
| ≥876.85 | 117 | 27.7 | 1.68 | 1.04–2.71 | 0.032 | ||
| MAR | <0.016 | 87 | 49.2 | 0.003 | ref | ||
| ≥0.016 | 140 | 31.3 | 2.03 | 1.30–3.16 | 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildirim, S.; Dogan, A.; Akdag, G.; Cavdar, E.; Kinikoglu, O.; Oksuz, S.; Yildiz, H.S.; Kucukoz Uzun, A.; Isik, D.; Surmeli, H.; et al. A Novel Prognostic Indicator for Immunotherapy Response: Lymphocyte-to-Albumin (LA) Ratio Predicts Survival in Metastatic NSCLC Patients. Cancers 2024, 16, 2512. https://doi.org/10.3390/cancers16142512
Yildirim S, Dogan A, Akdag G, Cavdar E, Kinikoglu O, Oksuz S, Yildiz HS, Kucukoz Uzun A, Isik D, Surmeli H, et al. A Novel Prognostic Indicator for Immunotherapy Response: Lymphocyte-to-Albumin (LA) Ratio Predicts Survival in Metastatic NSCLC Patients. Cancers. 2024; 16(14):2512. https://doi.org/10.3390/cancers16142512
Chicago/Turabian StyleYildirim, Sedat, Akif Dogan, Goncagul Akdag, Eyyup Cavdar, Oguzcan Kinikoglu, Sila Oksuz, Hacer Sahika Yildiz, Aysun Kucukoz Uzun, Deniz Isik, Heves Surmeli, and et al. 2024. "A Novel Prognostic Indicator for Immunotherapy Response: Lymphocyte-to-Albumin (LA) Ratio Predicts Survival in Metastatic NSCLC Patients" Cancers 16, no. 14: 2512. https://doi.org/10.3390/cancers16142512
APA StyleYildirim, S., Dogan, A., Akdag, G., Cavdar, E., Kinikoglu, O., Oksuz, S., Yildiz, H. S., Kucukoz Uzun, A., Isik, D., Surmeli, H., Basoglu, T., Sever, O. N., Odabas, H., Yildirim, M. E., & Turan, N. (2024). A Novel Prognostic Indicator for Immunotherapy Response: Lymphocyte-to-Albumin (LA) Ratio Predicts Survival in Metastatic NSCLC Patients. Cancers, 16(14), 2512. https://doi.org/10.3390/cancers16142512





