Devices and Methods for Dosimetry of Personalized Photodynamic Therapy of Tumors: A Review on Recent Trends
Abstract
Simple Summary
Abstract
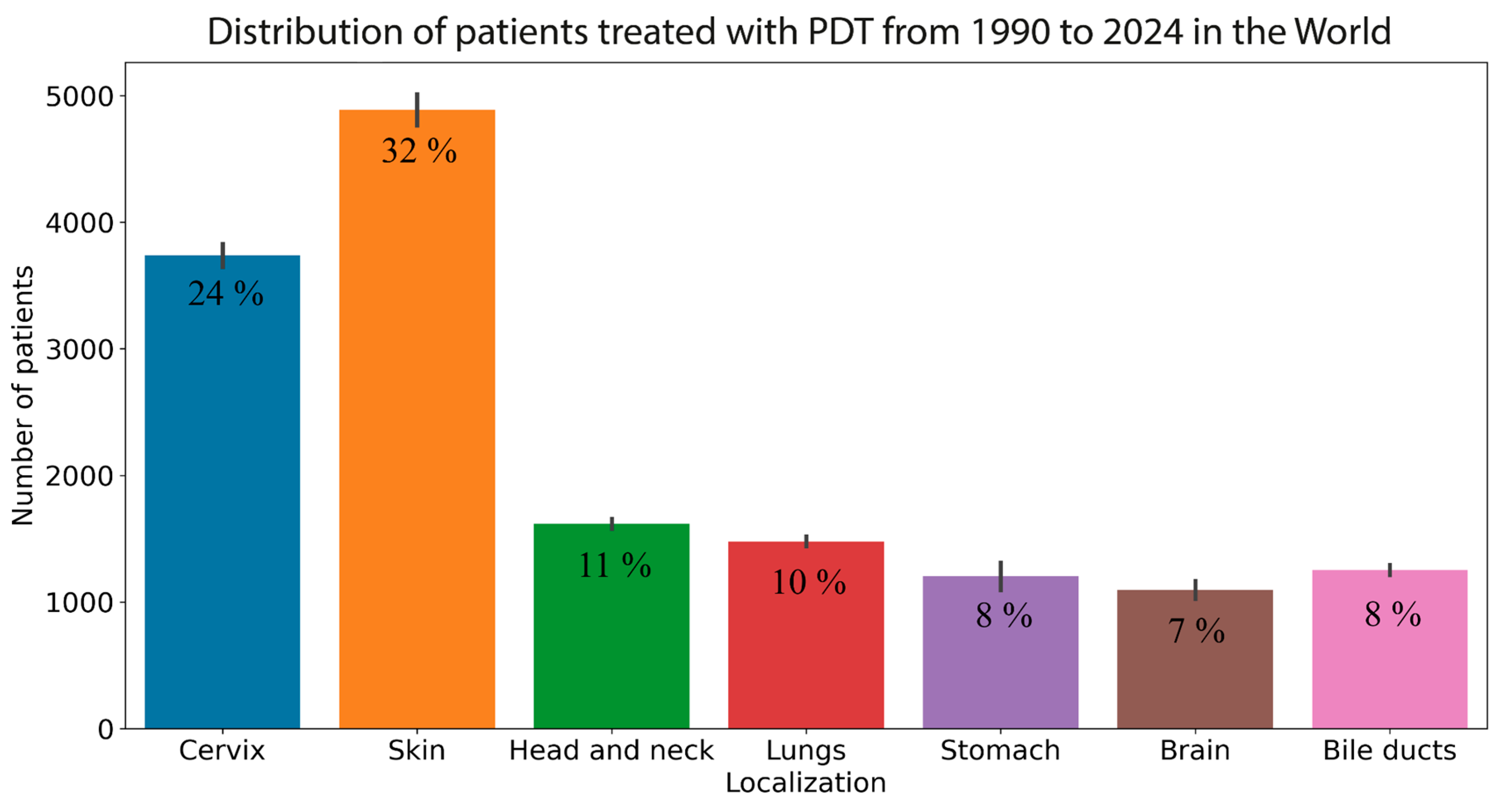
1. Introduction
2. Materials and Methods
3. Results and Discussion
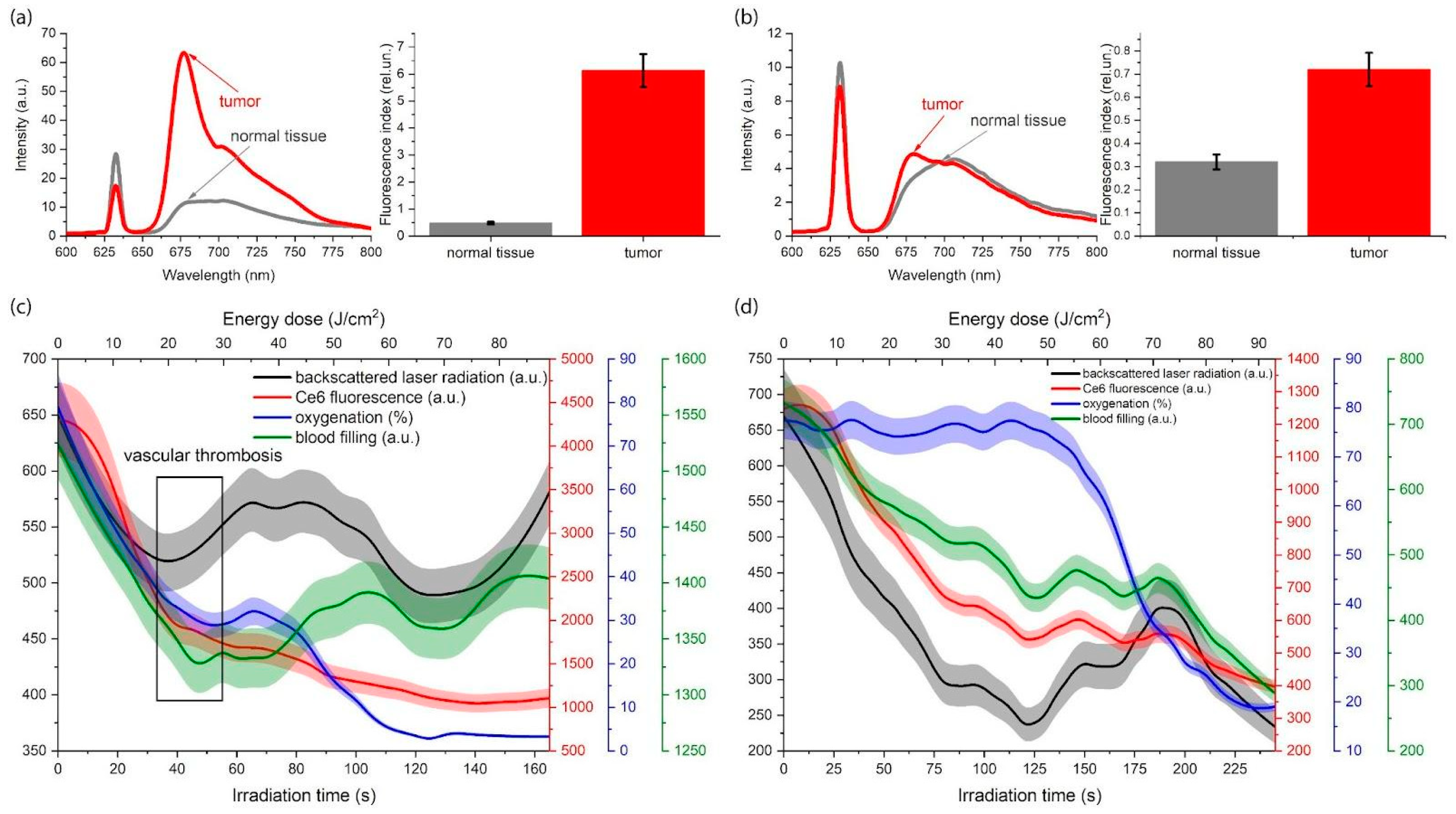
3.1. Optical Imaging
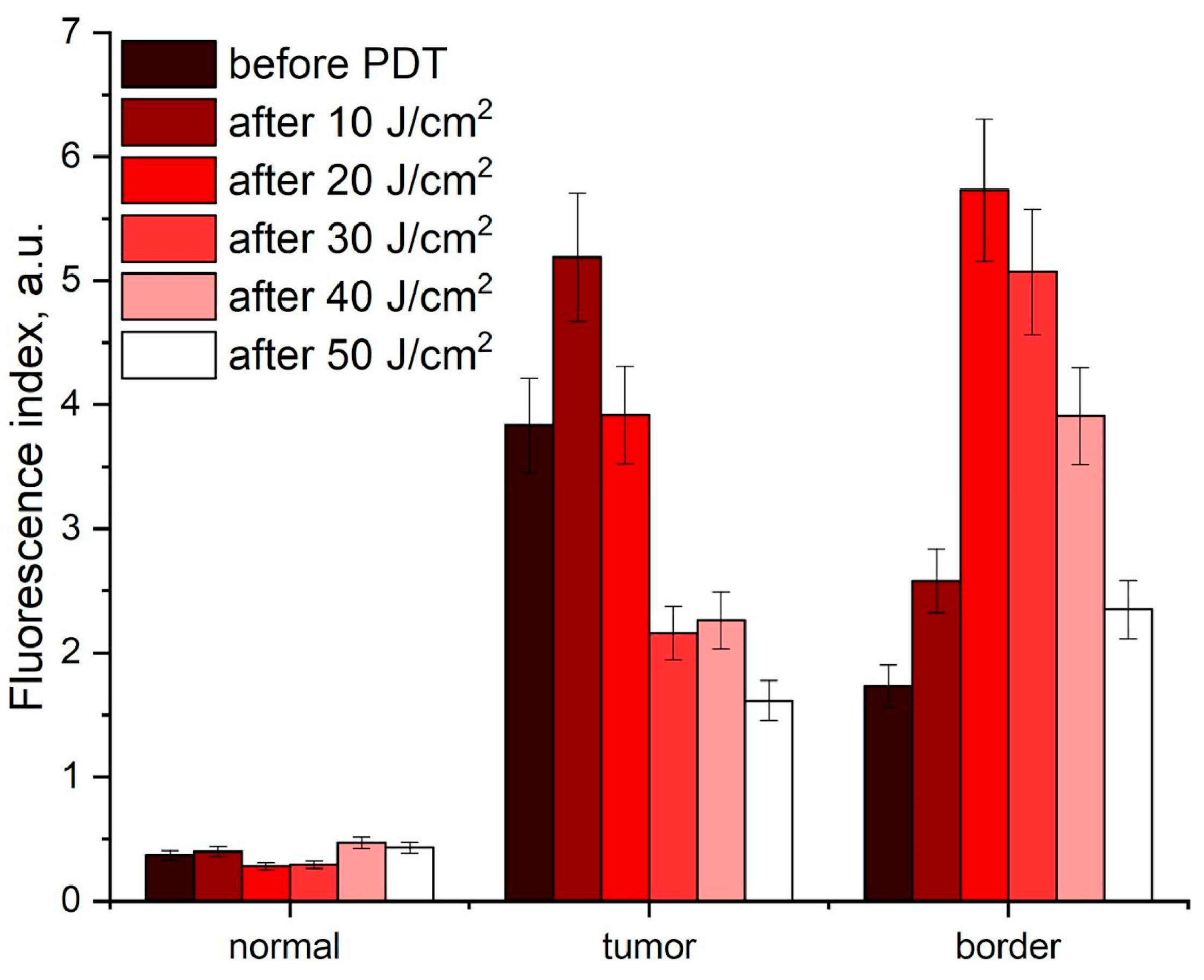
3.1.1. Fluorescence Imaging
3.1.2. Diffuse Reflectance Spectroscopy
3.1.3. Combined Method of Fluorescence Imaging and Diffuse Reflectance Spectroscopy
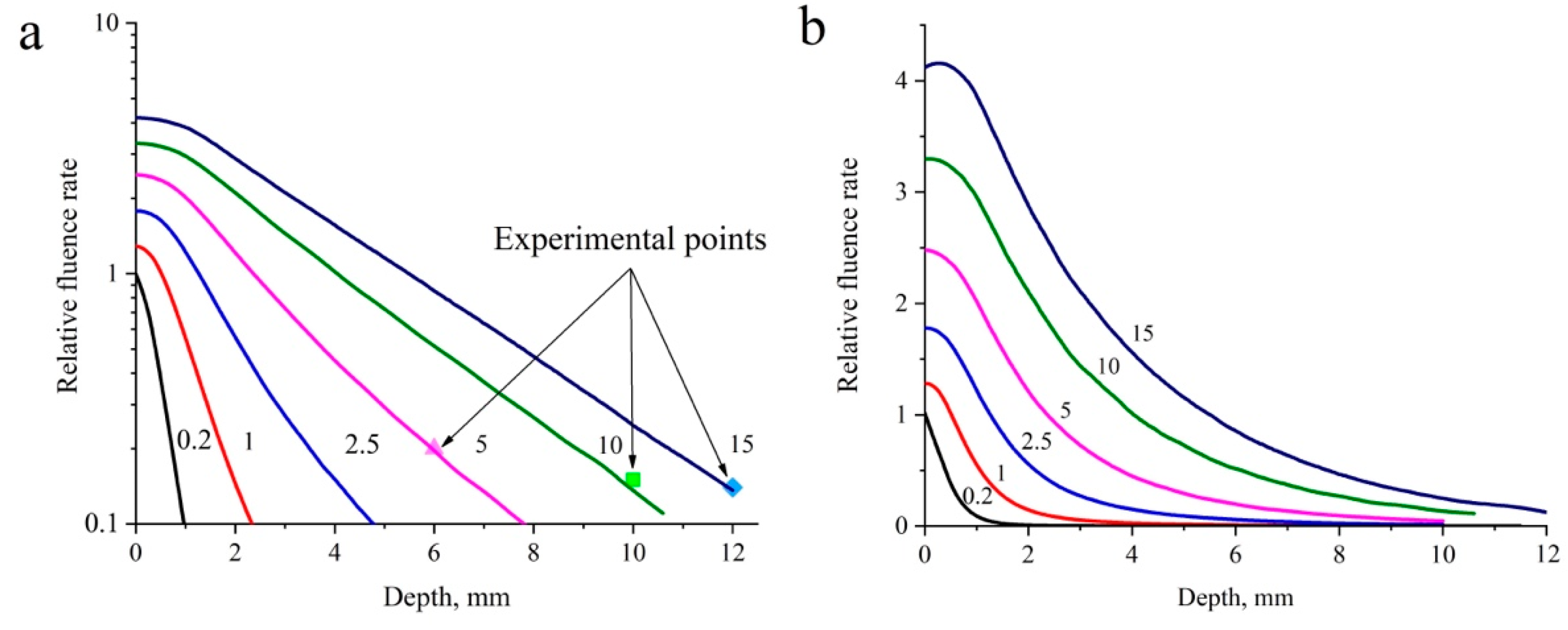
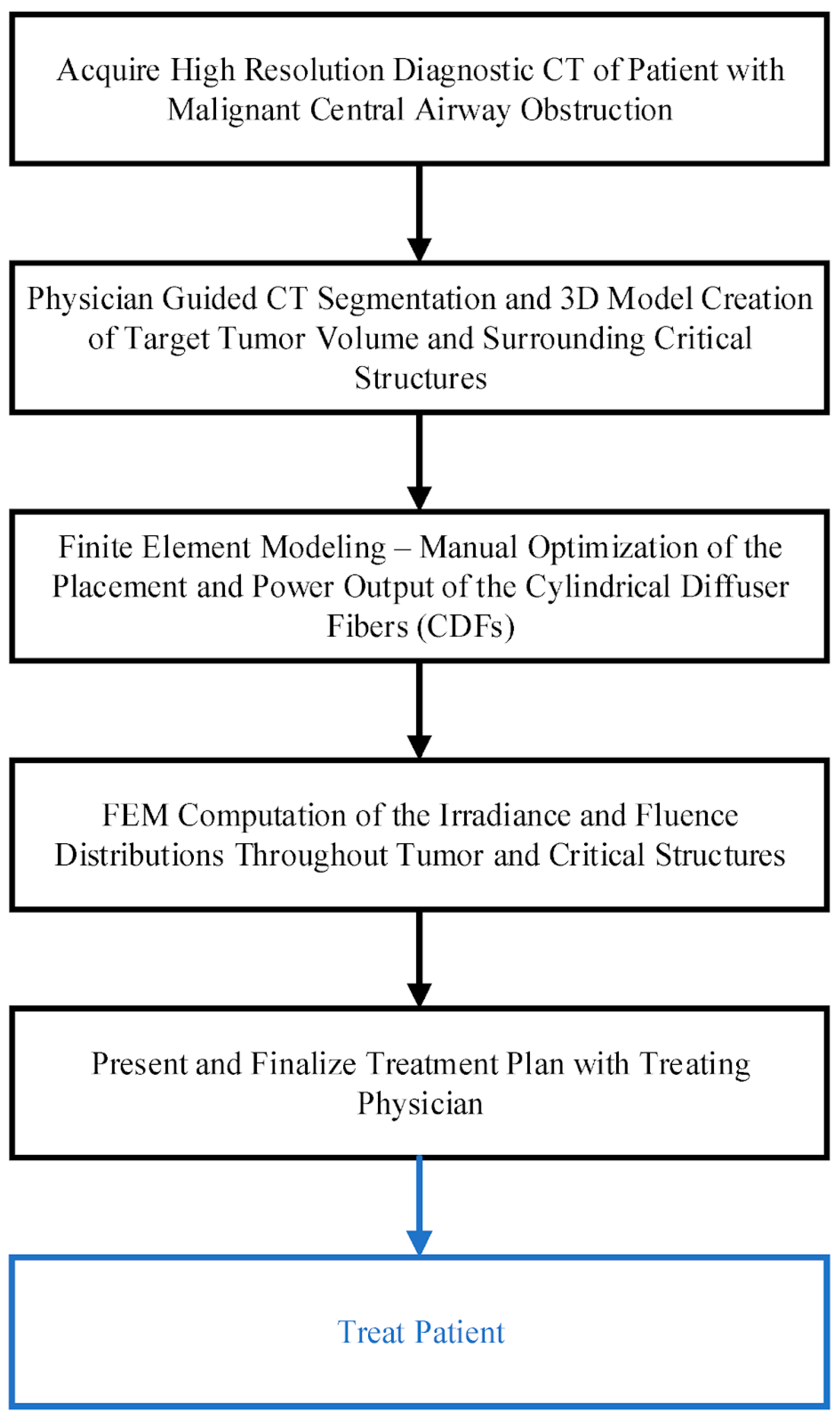
3.2. Computer Simulation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PDT | Photodynamic therapy |
| iPDT | Intraoperative photodynamic therapy |
| V-PDT | Vascular-targeted photodynamic therapy |
| PD | Photodynamic diagnostics |
| PS | Photosensitizer |
| ROS | Reactive oxygen species |
| 5-ALA | 5-aminolevulinic acid |
| PpIX | Protoporphyrin IX |
| Ce6 | Chlorin e6 |
| HAL | hexyl aminolevulinate |
| TRSO | Tumor reactive singlet oxygen |
| GPU | Graphics processing unit |
| PU | Perfusion units |
| OCT | Optical coherence tomography |
| LSCI | Laser speckle contrast imaging |
| LSI | Laser speckle imaging |
| PWSs | Port wine stains |
References
- Gierlich, P.; Donohoe, C.; Behan, K.; Kelly, D.J.; Senge, M.O.; Gomes-da-Silva, L.C. Antitumor Immunity Mediated by Photodynamic Therapy Using Injectable Chitosan Hydrogels for Intratumoral and Sustained Drug Delivery. Biomacromolecules 2023, 25, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Girotti, A.W.; Fahey, J.M.; Korbelik, M. Photodynamic Therapy as an Oxidative Anti-Tumor Modality: Negative Effects of Nitric Oxide on Treatment Efficacy. Pharmaceutics 2021, 13, 593. [Google Scholar] [CrossRef]
- Gurung, P.; Lim, J.; Shrestha, R.; Kim, Y.-W. Chlorin E6-Associated Photodynamic Therapy Enhances Abscopal Antitumor Effects via Inhibition of PD-1/PD-L1 Immune Checkpoint. Sci. Rep. 2023, 13, 4647. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Cao, Z.; Mao, K.; Wu, C.; Chen, H.; Wang, J.; Wang, X.; Cong, X.; Li, Y.; Meng, X. Photodynamic Therapy Produces Enhanced Efficacy of Antitumor Immunotherapy by Simultaneously Inducing Intratumoral Release of Sorafenib. Biomaterials 2020, 240, 119845. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Deng, J.; Liu, F.; Guo, T.; Liu, M.; Dai, P.; Fan, A.; Wang, Z.; Zhao, Y. Triggered All-Active Metal Organic Framework: Ferroptosis Machinery Contributes to the Apoptotic Photodynamic Antitumor Therapy. Nano Lett. 2019, 19, 7866–7876. [Google Scholar] [CrossRef] [PubMed]
- Nishie, H.; Kataoka, H.; Yano, S.; Yamaguchi, H.; Nomoto, A.; Tanaka, M.; Kato, A.; Shimura, T.; Mizoshita, T.; Kubota, E. Excellent Antitumor Effects for Gastrointestinal Cancers Using Photodynamic Therapy with a Novel Glucose Conjugated Chlorin E6. Biochem. Biochem. Biophys. Res. Commun. 2018, 496, 1204–1209. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.J.; Murray, K.S.; La Rosa, S.P.; Budhu, S.; Merghoub, T.; Somma, A.; Monette, S.; Kim, K.; Corradi, R.B.; Scherz, A. Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clin. Cancer Res. 2018, 24, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, L.J.; Li, J.W.; Li, J.H.; Wu, Z.M.; Zhang, L.X.; Chen, N.; Yan, Y.J.; Chen, Z.L. In Vitro and in Vivo Antitumor Activity of a Novel Chlorin Derivative for Photodynamic Therapy. Neoplasma 2016, 63, 37–43. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, L.-X.; Li, J.-W.; Huang, J.-Y.; Li, J.-H.; Zhang, L.-J.; O’Shea, D.; Chen, Z.-L. Antitumor Activity of Photodynamic Therapy with a Chlorin Derivative in Vitro and in Vivo. Tumor Biol. 2015, 36, 6839–6847. [Google Scholar] [CrossRef]
- Svyatchenko, V.A.; Nikonov, S.D.; Mayorov, A.P.; Gelfond, M.L.; Loktev, V.B. Antiviral Photodynamic Therapy: Inactivation and Inhibition of SARS-CoV-2 in Vitro Using Methylene Blue and Radachlorin. Photodiagnosis Photodyn. Ther. 2021, 33, 102112. [Google Scholar] [CrossRef]
- Afanasiev, M.S.; Dushkin, A.D.; Grishacheva, T.G.; Afanasiev, S.S.; Academician, A.V.K. Photodynamic Therapy for Early-Stage Cervical Cancer Treatment. Photodiagnosis Photodyn. Ther. 2022, 37, 102620. [Google Scholar] [CrossRef] [PubMed]
- Kines, R.; Çuburu, N.; Kobayashi, H.; MacDougall, J.; de los Pinos, E.; Schiller, J. HPV Based Photodynamic Therapy: A New Approach for Anti-Cancer Therapy (VAC12P. 1019). J. Immunol. 2014, 192, 206–208. [Google Scholar] [CrossRef]
- Gilyadova, A.; Ishchenko, A.; Shiryaev, A.; Alekseeva, P.; Efendiev, K.; Karpova, R.; Loshchenov, M.; Loschenov, V.; Reshetov, I. Phototheranostics of Cervical Neoplasms with Chlorin E6 Photosensitizer. Cancers 2022, 14, 211. [Google Scholar] [CrossRef] [PubMed]
- Gilyadova, A.; Ishchenko, A.; Ishenko, A.; Samoilova, S.; Shiryaev, A.; Kiseleva, A.; Petukhova, N.; Efendiev, K.; Alekseeva, P.; Stranadko, E.; et al. Analysis of the Results of Severe Intraepithelial Squamous Cell Lesions and Preinvasive Cervical Cancer Phototheranostics in Women of Reproductive Age. Biomedicines 2022, 10, 2521. [Google Scholar] [CrossRef] [PubMed]
- Reginato, E.; Wolf, P.; Hamblin, M.R. Immune Response after Photodynamic Therapy Increases Anti-Cancer and Anti-Bacterial Effects. World J. Immunol. 2014, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Efendiev, K.; Alekseeva, P.; Shiryaev, A.; Voitova, A.; Linkov, K.; Pisareva, T.; Reshetov, I.; Loschenov, V. Near-Infrared Phototheranostics of Tumors with Protoporphyrin IX and Chlorin E6 Photosensitizers. Photodiagnosis Photodyn. Ther. 2023, 42, 103566. [Google Scholar] [CrossRef] [PubMed]
- Finlay, J.C.; Zhu, T.C.; Dimofte, A.; Stripp, D.; Malkowicz, S.B.; Busch, T.M.; Hahn, S.M. Interstitial Fluorescence Spectroscopy in the Human Prostate during Motexafin Lutetium-mediated Photodynamic Therapy. Photochem. Photobiol. 2006, 82, 1270–1278. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ong, Y.H.; Kim, M.M.; Finlay, J.C.; Dimofte, A.; Cengel, K.A.; Zhu, T.C. Four-Channel PDT Dose Dosimetry for Pleural Photodynamic Therapy. Proc. SPIE 2017, 10047, 195–203. [Google Scholar] [CrossRef]
- Wilson, B.C.; Patterson, M.S.; Lilge, L. Implicit and Explicit Dosimetry in Photodynamic Therapy: A New Paradigm. Lasers Med. Sci. 1997, 12, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Elliott, J.T.; Kanick, S.C.; Davis, S.C.; Samkoe, K.S.; Maytin, E.V.; Pereira, S.P.; Hasan, T. Revisiting Photodynamic Therapy Dosimetry: Reductionist & Surrogate Approaches to Facilitate Clinical Success. Phys. Med. Biol. 2016, 61, R57–R89. [Google Scholar] [CrossRef]
- Nilsson, A.M.K.; Berg, R.; Andersson-Engels, S. Measurements of the Optical Properties of Tissue in Conjunction with Photodynamic Therapy. Appl. Opt. 1995, 34, 4609–4619. [Google Scholar] [CrossRef]
- Sandell, J.L.; Zhu, T.C. A Review of In-vivo Optical Properties of Human Tissues and Its Impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.; Ishii, K.; Terada, T.; Nanjo, T.; Awazu, K. Determination of the Tumor Tissue Optical Properties during and after Photodynamic Therapy Using Inverse Monte Carlo Method and Double Integrating Sphere between 350 and 1000 Nm. J. Biomed. Opt. 2011, 16, 58003. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wilson, B.C.; Shetty, S.D.; Patterson, M.S.; Cerny, J.C.; Hetzel, F.W. Changes in in Vivo Optical Properties and Light Distributions in Normal Canine Prostate during Photodynamic Therapy. Radiat. Res. 1997, 147, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Finlay, J.C.; Hahn, S.M. Determination of the Distribution of Light, Optical Properties, Drug Concentration, and Tissue Oxygenation in-Vivo in Human Prostate during Motexafin Lutetium-Mediated Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2005, 79, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.H.; Padawer-Curry, J.; Finlay, J.C.; Kim, M.M.; Dimofte, A.; Cengel, K.; Zhu, T.C. Determination of Optical Properties, Drug Concentration, and Tissue Oxygenation in Human Pleural Tissue before and after Photofrin-Mediated Photodynamic Therapy. Proc. SPIE 2018, 10476, 84–89. [Google Scholar] [CrossRef]
- Kuzin, M.I.; Zavodnov, V.Y.; Korablin, S.N.; Loshchenov, V.B. Method for Determining the Level of Oxygenation of the Organ Mucosa. Russian Federation Patent 1506357, 10 September 1987. [Google Scholar]
- Moan, J.; Sommer, S. Oxygen Dependence of the Photosensitizing Effect of Hematoporphyrin Derivative in NHIK 3025 Cells. Cancer Res. 1985, 45, 1608–1610. [Google Scholar]
- Mitchell, J.B.; McPherson, S.; DeGraff, W.; Gamson, J.; Zabell, A.; Russo, A. Oxygen Dependence of Hematoporphyrin Derivative-Induced Photoinactivation of Chinese Hamster Cells. Cancer Res. 1985, 45, 2008–2011. [Google Scholar] [PubMed]
- See, K.L.; Forbes, I.J.; Betts, W.H. Oxygen Dependency of Photocytotoxicity with Haematoporphyrin Derivative. Photochem. Photobiol. 1984, 39, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Nichols, M.G.; Foster, T.H. Oxygen Diffusion and Reaction Kinetics in the Photodynamic Therapy of Multicell Tumour Spheroids. Phys. Med. Biol. 1994, 39, 2161. [Google Scholar] [CrossRef]
- Georgakoudi, I.; Nichols, M.G.; Foster, T.H. The Mechanism of Photofrin Photobleaching and Its Consequences for Photodynamic Dosimetry. Photochem. Photobiol. 1997, 65, 135–144. [Google Scholar] [CrossRef]
- Sitnik, T.M.; Hampton, J.A.; Henderson, B.W. Reduction of Tumour Oxygenation during and after Photodynamic Therapy in Vivo: Effects of Fluence Rate. Br. J. Cancer 1998, 77, 1386–1394. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Vaughan, L.A.; Frawley, N.P.; Babich, D.; Sosa, T.A.; Zollo, J.D.; Dee, A.S.; Cooper, M.T.; Bellnier, D.A. Photofrin Photodynamic Therapy Can Significantly Deplete or Preserve Oxygenation in Human Basal Cell Carcinomas during Treatment, Depending on Fluence Rate. Cancer Res. 2000, 60, 525–529. [Google Scholar] [PubMed]
- Kareliotis, G.; Liossi, S.; Makropoulou, M. Assessment of Singlet Oxygen Dosimetry Concepts in Photodynamic Therapy through Computational Modeling. Photodiagnosis Photodyn. Ther. 2018, 21, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Busch, T.M.; Snyder, J.W. Fluence Rate as a Modulator of PDT Mechanisms. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2006, 38, 489–493. [Google Scholar] [CrossRef]
- Henderson, B.W.; Gollnick, S.O.; Snyder, J.W.; Busch, T.M.; Kousis, P.C.; Cheney, R.T.; Morgan, J. Choice of Oxygen-Conserving Treatment Regimen Determines the Inflammatory Response and Outcome of Photodynamic Therapy of Tumors. Cancer Res. 2004, 64, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Woodhams, J.H.; MacRobert, A.J.; Bown, S.G. The Role of Oxygen Monitoring during Photodynamic Therapy and Its Potential for Treatment Dosimetry. Photochem. Photobiol. Sci. 2007, 6, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Foster, T.H. Carbogen Breathing Significantly Enhances the Penetration of Red Light in Murine Tumours in Vivo. Phys. Med. Biol. 2004, 49, 1891. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.P.; Juzeniene, A.; Juzenas, P.; Stamnes, K.; Stamnes, J.J.; Moan, J. Choice of Optimal Wavelength for PDT: The Significance of Oxygen Depletion. Photochem. Photobiol. 2005, 81, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Shirmanova, M.V.; Gavrina, A.I.; Aksenova, N.A.; Glagolev, N.N.; Solovieva, A.B.; Shakhov, B.E.; Zagaynova, E.V. Comparative Study of Tissue Distribution of Chlorin E6 Complexes with Amphiphilic Polymers in Mice with Cervical Carcinoma. J. Anal. Bioanal. Tech. S 2014, 1. [Google Scholar] [CrossRef]
- Chin, W.W.L.; Heng, P.W.S.; Bhuvaneswari, R.; Lau, W.K.O.; Olivo, M. The Potential Application of Chlorin E6—Polyvinylpyrrolidone Formulation in Photodynamic Therapy. Photochem. Photobiol. Sci. 2006, 5, 1031–1037. [Google Scholar] [CrossRef]
- Soyama, T.; Sakuragi, A.; Oishi, D.; Kimura, Y.; Aoki, H.; Nomoto, A.; Yano, S.; Nishie, H.; Kataoka, H.; Aoyama, M. Photodynamic Therapy Exploiting the Anti-Tumor Activity of Mannose-Conjugated Chlorin E6 Reduced M2-like Tumor-Associated Macrophages. Transl. Oncol. 2021, 14, 101005. [Google Scholar] [CrossRef]
- Lukiyanets, E.A. Search for New Photosensitizers in Photodynamic Therapy. Biomed. Photonics 2013, 2, 3–16. [Google Scholar]
- Kiening, M.; Lange, N. A Recap of Heme Metabolism towards Understanding Protoporphyrin IX Selectivity in Cancer Cells. Int. J. Mol. Sci. 2022, 23, 7974. [Google Scholar] [CrossRef]
- Nakano, Y.; Kitagawa, T.; Osada, Y.; Tanaka, T.; Nishizawa, S.; Yamamoto, J. 5-Aminolevulinic Acid Suppresses Prostaglandin E2 Production by Murine Macrophages and Enhances Macrophage Cytotoxicity against Glioma. World Neurosurg. 2019, 127, e669–e676. [Google Scholar] [CrossRef] [PubMed]
- Ryabova, A.; Romanishkin, I.; Skobeltsin, A.; Markova, I.; Pominova, D.; Linkov, K.; Loschenov, V. Detection of Changes in Macrophage Polarization as a Result of 5-Aminolevulinic Acid Photodynamic Therapy Using Fluorescence-Lifetime Imaging Microscopy. Photonics 2022, 9, 961. [Google Scholar] [CrossRef]
- Kanick, S.C.; Davis, S.C.; Zhao, Y.; Hasan, T.; Maytin, E.V.; Pogue, B.W.; Chapman, M.S. Dual-Channel Red/Blue Fluorescence Dosimetry with Broadband Reflectance Spectroscopic Correction Measures Protoporphyrin IX Production during Photodynamic Therapy of Actinic Keratosis. J. Biomed. Opt. 2014, 19, 75002. [Google Scholar] [CrossRef]
- Tylcz, J.-B.; Bastogne, T.; Bourguignon, A.; Frochot, C.; Barberi-Heyob, M. Realtime Tracking of the Photobleaching Trajectory during Photodynamic Therapy. IEEE Trans. Biomed. Eng. 2016, 64, 1742–1749. [Google Scholar] [CrossRef]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F. Protoporphyrin IX Fluorescence and Photobleaching during Interstitial Photodynamic Therapy of Malignant Gliomas for Early Treatment Prognosis. Lasers Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Piffaretti, F.; Zellweger, M.; Kasraee, B.; Barge, J.; Salomon, D.; Van Den Bergh, H.; Wagnières, G. Correlation between Protoporphyrin IX Fluorescence Intensity, Photobleaching, Pain and Clinical Outcome of Actinic Keratosis Treated by Photodynamic Therapy. Dermatology 2013, 227, 214–225. [Google Scholar] [CrossRef]
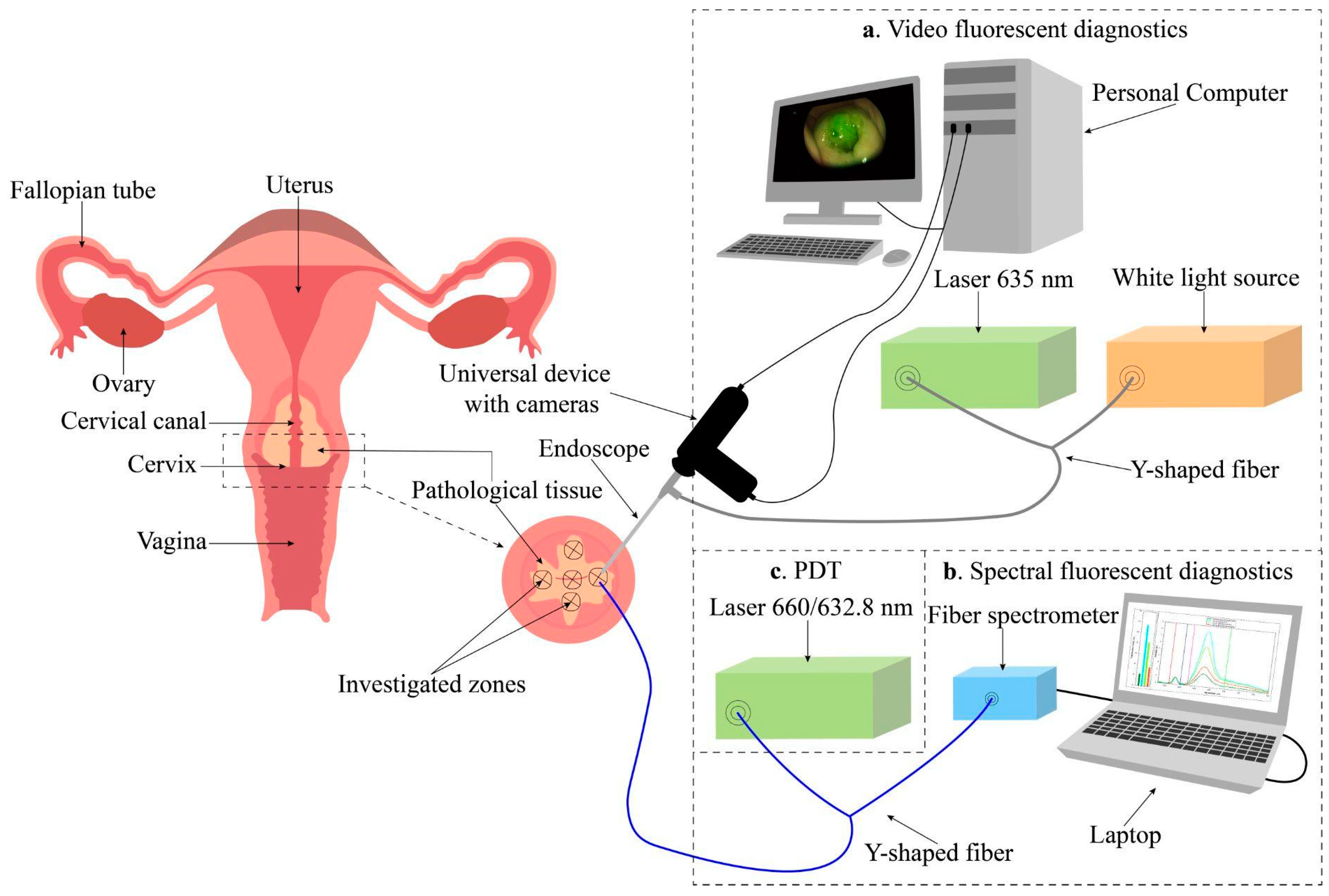
- Alekseeva, P.M.; Efendiev, K.T.; Loshchenov, M.V.; Shiryaev, A.A.; Ishchenko, A.A.; Gilyadova, A.V.; Karpova, R.V.; Reshetov, I.V.; Loschenov, V.B. Combined Spectral- and Video-Fluorescent Diagnostics of Cervical Neoplasms for Photodynamic Therapy. Laser Phys. Lett. 2020, 17, 105602. [Google Scholar] [CrossRef]
- Efendiev, K.T.; Alekseeva, P.M.; Bikmukhametova, I.R.; Piterskova, L.S.; Orudzhova, K.F.; Agabekova, U.D.; Slovokhodov, E.K.; Loschenov, V.B. Comparative Investigation of 5-Aminolevulinic Acid and Hexyl Aminolevulinate-Mediated Photodynamic Diagnostics and Therapy of Cervical Dysplasia and Vulvar Leukoplakia. Laser Phys. Lett. 2021, 18, 65601. [Google Scholar] [CrossRef]
- Kleshnin, M.S.; Fiks, I.I.; Plekhanov, V.I.; Gamayunov, S.V.; Turchin, I. V Compact and Fully Automated System for Monitoring Photodynamic Therapy, Based on Two LEDs and a Single CCD. Laser Phys. Lett. 2015, 12, 115602. [Google Scholar] [CrossRef]
- Munck, C.; Mordon, S.; Betrouni, N. Illumination Profile Characterization of a Light Device for the Dosimetry of Intra-Pleural Photodynamic Therapy for Mesothelioma. Photodiagnosis Photodyn. Ther. 2016, 16, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.J.; LaRochelle, E.P.M.; Gunn, J.R.; Hull, S.M.; Hasan, T.; Chapman, M.S.; Pogue, B.W. Smartphone Fluorescence Imager for Quantitative Dosimetry of Protoporphyrin-IX-Based Photodynamic Therapy in Skin. J. Biomed. Opt. 2020, 25, 63802. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.H.; Kim, M.M.; Finlay, J.C.; Dimofte, A.; Singhal, S.; Glatstein, E.; Cengel, K.A.; Zhu, T.C. PDT Dose Dosimetry for Photofrin-Mediated Pleural Photodynamic Therapy (PPDT). Phys. Med. Biol. 2017, 63, 15031. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.C.; Sun, H.; Ong, Y.-H.; Morales, R.H.; Dimofte, A.; Busch, T.; Singhal, S.; Cengel, K.A. Real-Time PDT Dose Dosimetry for Pleural Photodynamic Therapy. Proc. SPIE 2022, 11940, 1194002. [Google Scholar] [CrossRef]
- Wang, X.; Kang, W.-R.; Hu, X.-M.; Li, Q. Irradiance Uniformity Optimization for a Photodynamic Therapy Treatment Device with 3D Scanner. J. Biomed. Opt. 2021, 26, 78001. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, T.; Xiong, J.; Zhao, H.; Hu, X.; Li, Q.; Ren, J.; Zhao, Y. Three-Dimensional Image-Guided Topical Photodynamic Therapy System with Light Dosimetry Dynamic Planning and Monitoring. Biomed. Opt. Express 2023, 14, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.L.; Paquette, A.D.; Keymel, K.R.; Henderson, B.W.; Sunar, U. Monitoring Blood Flow Responses during Topical ALA-PDT. Biomed. Opt. Express 2011, 2, 123–130. [Google Scholar] [CrossRef]
- Dang, J.; He, H.; Chen, D.; Yin, L. Manipulating Tumor Hypoxia toward Enhanced Photodynamic Therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef]
- Chen, D.; Ren, J.; Wang, Y.; Li, B.; Gu, Y. Intraoperative Monitoring of Blood Perfusion in Port Wine Stains by Laser Doppler Imaging during Vascular Targeted Photodynamic Therapy: A Preliminary Study. Photodiagnosis Photodyn. Ther. 2016, 14, 142–151. [Google Scholar] [CrossRef]
- Dupre, P.J.; Ong, Y.H.; Friedberg, J.; Singhal, S.; Carter, S.; Simone, C.B.; Finlay, J.C.; Zhu, T.C.; Cengel, K.A.; Busch, T.M. Light Fluence Rate and Tissue Oxygenation (StO2) Distributions within the Thoracic Cavity of Patients Receiving Intraoperative Photodynamic Therapy for Malignant Pleural Mesothelioma. Photochem. Photobiol. 2020, 96, 417–425. [Google Scholar] [CrossRef]
- Chen, D.; Wang, Y.; Zhao, H.; Qiu, H.; Wang, Y.; Yang, J.; Gu, Y. Monitoring Perfusion and Oxygen Saturation in Port-Wine Stains during Vascular Targeted Photodynamic Therapy. Ann. Transl. Med. 2021, 9, 214. [Google Scholar] [CrossRef]
- Qiu, Z.; Chen, D.; Wang, Y.; Yao, G.; Gu, Y.; Li, B. Monitoring Blood Volume Fraction and Oxygen Saturation in Port-Wine Stains during Vascular Targeted Photodynamic Therapy with Diffuse Reflectance Spectroscopy: Results of a Preliminary Case Study. Photonics Lasers Med. 2014, 3, 273–280. [Google Scholar] [CrossRef]
- Qiu, Z.; Yao, G.; Chen, D.; Wang, Y.; Gu, Y.; Li, B. Determination of Optical and Microvascular Parameters of Port Wine Stains Using Diffuse Reflectance Spectroscopy. In Proceedings of the Oxygen Transport to Tissue XXXVIII, Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 923, pp. 359–365. [Google Scholar] [CrossRef]
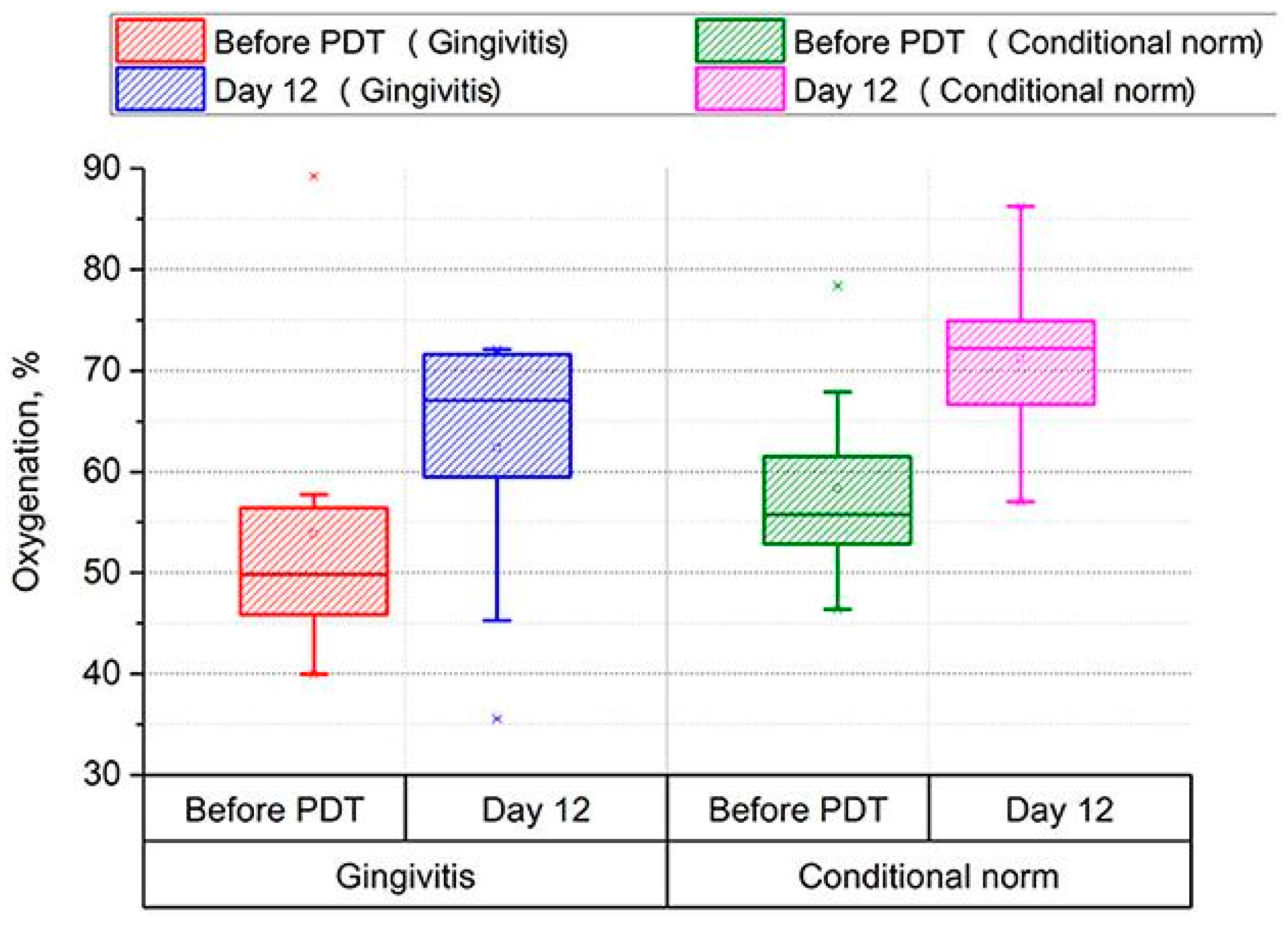
- Morozova, N.S.; Kozlitina, I.A.; Makarov, V.I.; Loschenov, V.B.; Grinin, V.M.; Ivanov, S.Y.; Kashtanova, M.S. Optical Spectral Diagnostics of the Oxygenation Level in Periodontal Tissues and Photodynamic Therapy Using Methylene Blue in Children with Cerebral Palsy. Front. Public Health 2023, 11, 961066. [Google Scholar] [CrossRef]
- Quintanar, L.; Fabila, D.; Stolik, S.; de la Rosa, J.M. An Irradiation System for Photodynamic Therapy with a Fiber-Optic Sensor for Measuring Tissue Oxygen. Proc. SPIE 2013, 8785, 1457–1464. [Google Scholar] [CrossRef]
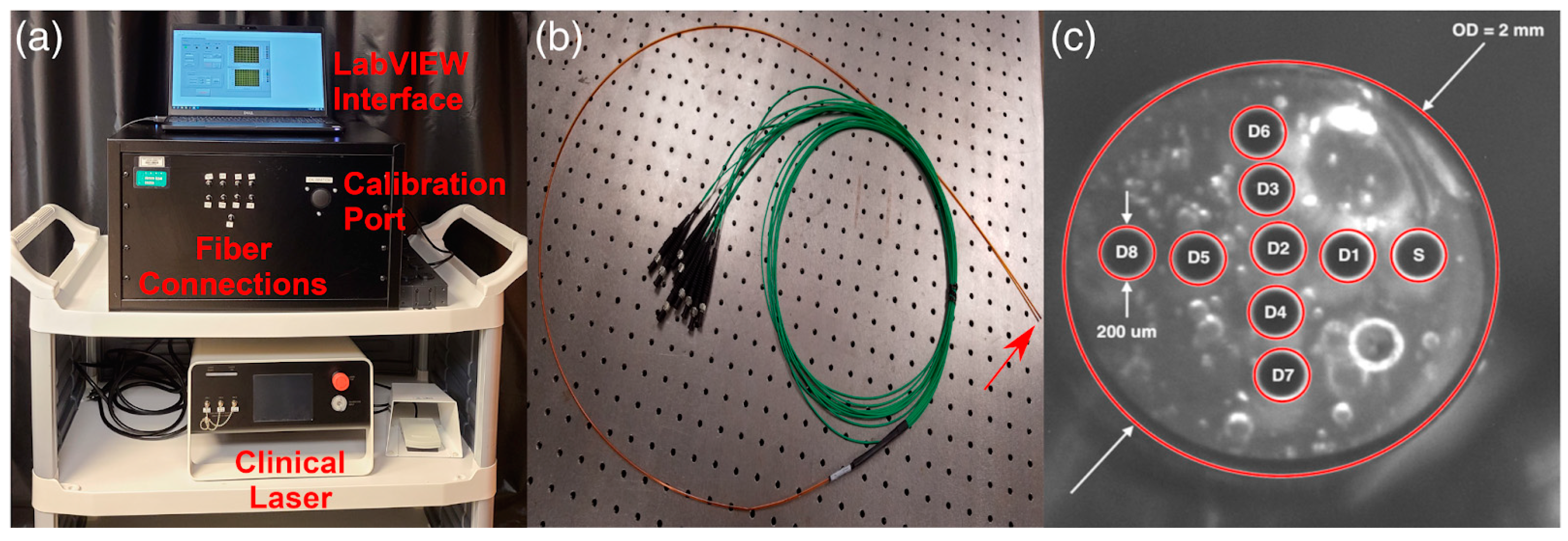
- Hannan, M.N.; Sharma, A.K.; Baran, T.M. First in Human Measurements of Abscess Cavity Optical Properties and Methylene Blue Uptake Prior to Photodynamic Therapy by in Vivo Diffuse Reflectance Spectroscopy. J. Biomed. Opt. 2024, 29, 27002. [Google Scholar] [CrossRef]
- McRobb, C.M.; Holt, D.W. Methylene Blue-Induced Methemoglobinemia during Cardiopulmonary Bypass? A Case Report and Literature Review. J. Extracorpor. Technol. 2008, 40, 206–214. [Google Scholar] [CrossRef]
- Hannan, M.N.; Sharma, A.K.; Baran, T.M. Preliminary Measurements of Optical Properties in Human Abscess Cavities Prior to Methylene Blue Photodynamic Therapy. Proc. SPIE 2023, 12359, 63–69. [Google Scholar]
- Hannan, M.N.; Baran, T.M. Application of Transfer Learning for Rapid Calibration of Spatially Resolved Diffuse Reflectance Probes for Extraction of Tissue Optical Properties. J. Biomed. Opt. 2024, 29, 27004. [Google Scholar] [CrossRef] [PubMed]
- Bridger, K.G.; Roccabruna, J.R.; Baran, T.M. Optical Property Recovery with Spatially-Resolved Diffuse Reflectance at Short Source-Detector Separations Using a Compact Fiber-Optic Probe. Biomed. Opt. Express 2021, 12, 7388–7404. [Google Scholar] [CrossRef]
- Morales, R.D.H.; Hong Ong, Y.; Finlay, J.; Dimofte, A.; Simone, C.B.; Friedberg, J.S.; Busch, T.M.; Cengel, K.A.; Zhu, T.C. In Vivo Spectroscopic Evaluation of Human Tissue Optical Properties and Hemodynamics during HPPH-Mediated Photodynamic Therapy of Pleural Malignancies. J. Biomed. Opt. 2022, 27, 105006. [Google Scholar] [CrossRef]
- Fredriksson, I.; Larsson, M.; Strömberg, T. Machine Learning for Direct Oxygen Saturation and Hemoglobin Concentration Assessment Using Diffuse Reflectance Spectroscopy. J. Biomed. Opt. 2020, 25, 112905. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, F.; Movasati, A.; Brunner, S.R.; Nguyen, L.; Priestley, P.; Cuppen, E.; Van Hoeck, A. Pan-Cancer Whole-Genome Comparison of Primary and Metastatic Solid Tumours. Nature 2023, 618, 333–341. [Google Scholar] [CrossRef]
- Efendiev, K.; Alekseeva, P.; Linkov, K.; Shiryaev, A.; Pisareva, T.; Gilyadova, A.; Reshetov, I.; Voitova, A.; Loschenov, V. Tumor Fluorescence and Oxygenation Monitoring during Photodynamic Therapy with Chlorin E6 Photosensitizer. Photodiagnosis Photodyn. Ther. 2024, 45, 103969. [Google Scholar] [CrossRef]
- Ahn, P.H.; Finlay, J.C.; Gallagher-Colombo, S.M.; Quon, H.; O’Malley Jr, B.W.; Weinstein, G.S.; Chalian, A.; Malloy, K.; Sollecito, T.; Greenberg, M. Lesion Oxygenation Associates with Clinical Outcomes in Premalignant and Early Stage Head and Neck Tumors Treated on a Phase 1 Trial of Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2018, 21, 28–35. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Rigual, N.; Arshad, H.; Tracy, E.C.; Cooper, M.T.; Shafirstein, G.; Wilding, G.; Merzianu, M.; Baumann, H.; Henderson, B.W. Intraoperative Optical Assessment of Photodynamic Therapy Response of Superficial Oral Squamous Cell Carcinoma. J. Biomed. Opt. 2016, 21, 18002. [Google Scholar] [CrossRef]
- Rohrbach, D.J.; Rigual, N.; Tracy, E.; Keymel, K.; Cooper, M.T.; Baumann, H.; Henderson, B.W.; Sunar, U. Monitoring PDT Response of Head and Neck Lesions with Diffuse Optical Spectroscopies. Proc. SPIE 2013, 8568, 202–208. [Google Scholar]
- Gallagher-Colombo, S.M.; Quon, H.; Malloy, K.M.; Ahn, P.H.; Cengel, K.A.; Simone, C.B.; Chalian, A.A.; O’Malley, B.W.; Weinstein, G.S.; Zhu, T.C. Measuring the Physiologic Properties of Oral Lesions Receiving Fractionated Photodynamic Therapy. Photochem. Photobiol. 2015, 91, 1210–1218. [Google Scholar] [CrossRef]
- Efendiev, K.T.; Alekseeva, P.M.; Shiryaev, A.A.; Skobeltsin, A.S.; Solonina, I.L.; Fatyanova, A.S.; Reshetov, I.V.; Loschenov, V.B. Preliminary Low-Dose Photodynamic Exposure to Skin Cancer with Chlorin E6 Photosensitizer. Photodiagnosis Photodyn. Ther. 2022, 38, 102894. [Google Scholar] [CrossRef]
- Wang, L.; Li, G.; Cao, L.; Shao, K.; Li, Y.; Zhang, X.; Zhao, J.; Zhao, W. Novel Water-Soluble Chlorin-Based Photosensitizer for Low-Fluence Photodynamic Therapy. ACS Pharmacol. Transl. Sci. 2022, 5, 110–117. [Google Scholar] [CrossRef]
- Kim, M.M.; Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294. [Google Scholar] [CrossRef]
- Lisenko, A.S.; Kugeiko, M.M. Method for Estimating Optimal Spectral and Energy Parameters of Laser Irradiation in Photodynamic Therapy of Biological Tissue. Quantum Electron. 2015, 45, 358. [Google Scholar] [CrossRef]
- Lisenko, A.S.; Kugeiko, M.M.; Firago, V.A.; Sobchuk, A.N. Noninvasive Fast Analysis of Hemoglobin Levels in Blood Using a Fiber Optic Spectrophotometer. J. Appl. Spectrosc. 2014, 81, 118–126. [Google Scholar] [CrossRef]
- Alekseeva, P.M.; Efendiev, K.T.; Savelieva, T.A.; Moskalev, A.S.; Steiner, R.; Loschenov, V.B. Optimization of Energy Parameters for Laser-Induced PDT of Cervical Tissues Using Numerical Simulation and Fluorescent Monitoring. Laser Phys. 2023, 33, 065602. [Google Scholar] [CrossRef]
- Pickard, D.C.O.; Lovat, L.B.; Novelli, M.; Ripley, P.M.; Kelly, C.; Bigio, I.J.; Bown, S.G. Diagnosis of Dysplasia in Barrett’s Oesophagus with in-Situ Elastic-Scattering Spectroscopy. Proc. SPIE 2000, 4161, 122–130. [Google Scholar] [CrossRef]
- Torricelli, A.; Pifferi, A.; Spinelli, L.; Cubeddu, R.; Martelli, F.; Del Bianco, S.; Zaccanti, G. Time-Resolved Reflectance at Null Source-Detector Separation: Improving Contrast and Resolution in Diffuse Optical Imaging. Phys. Rev. Lett. 2005, 95, 78101. [Google Scholar] [CrossRef]
- Welch, A.J.; Van Gemert, M.J.C. Optical-Thermal Response of Laser-Irradiated Tissue; Springer: New York, NY, USA, 2011; Volume 2. [Google Scholar]
- Wang, L.; Jacques, S.L.; Zheng, L. MCML—Monte Carlo Modeling of Light Transport in Multi-Layered Tissues. Comput. Methods Programs Biomed. 1995, 47, 131–146. [Google Scholar] [CrossRef]
- Cassidy, J.; Betz, V.; Lilge, L. Treatment Plan Evaluation for Interstitial Photodynamic Therapy in a Mouse Model by Monte Carlo Simulation with FullMonte. Front. Phys. 2015, 3, 6. [Google Scholar] [CrossRef]
- Cassidy, J.; Betz, V.; Lilge, L. Monte Carlo Fluence Simulation for Prospective Evaluation of Interstitial Photodynamic Therapy Treatment Plans. Proc. SPIE 2015, 9308, 57–64. [Google Scholar] [CrossRef]
- Baran, T.M.; Choi, H.W.; Flakus, M.J.; Sharma, A.K. Photodynamic Therapy of Deep Tissue Abscess Cavities: Retrospective Image-based Feasibility Study Using Monte Carlo Simulation. Med. Phys. 2019, 46, 3259–3267. [Google Scholar] [CrossRef]
- Ramadan, K.T.; McFadden, C.; Gomes, B.; Schwiegelshohn, F.; Ribeiro, R.V.P.; Chan, H.H.L.; Betz, V.; Cypel, M.; Lilge, L. Determination of Optical Properties and Photodynamic Threshold of Lung Tissue for Treatment Planning of in Vivo Lung Perfusion Assisted Photodynamic Therapy. Photodiagnosis Photodyn. Ther. 2021, 35, 102353. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.M.; Brown, C.T.A.; Moseley, H.; Ibbotson, S.; Wood, K. Monte Carlo Modeling of in Vivo Protoporphyrin IX Fluorescence and Singlet Oxygen Production during Photodynamic Therapy for Patients Presenting with Superficial Basal Cell Carcinomas. J. Biomed. Opt. 2011, 16, 48002. [Google Scholar] [CrossRef] [PubMed]
- Valentine, R.M.; Wood, K.; Brown, C.T.A.; Ibbotson, S.H.; Moseley, H. Monte Carlo Simulations for Optimal Light Delivery in Photodynamic Therapy of Non-Melanoma Skin Cancer. Phys. Med. Biol. 2012, 57, 6327. [Google Scholar] [CrossRef] [PubMed]
- Lilge, L.; Wu, J.; Xu, Y.; Manalac, A.; Molenhuis, D.; Schwiegelshohn, F.; Vesselov, L.; Embree, W.; Nesbit, M.; Betz, V. Minimal Required PDT Light Dosimetry for Nonmuscle Invasive Bladder Cancer. J. Biomed. Opt. 2020, 25, 68001. [Google Scholar] [CrossRef]
- van Doeveren, T.E.M.; Bouwmans, R.; Wassenaar, N.P.M.; Schreuder, W.H.; van Alphen, M.J.A.; van der Heijden, F.; Tan, I.B.; Karakullukçu, M.B.; van Veen, R.L.P. On the Development of a Light Dosimetry Planning Tool for Photodynamic Therapy in Arbitrary Shaped Cavities: Initial Results. Photochem. Photobiol. 2020, 96, 405–416. [Google Scholar] [CrossRef]
- Campbell, C.L.; Wood, K.; Brown, C.T.A.; Moseley, H. Monte Carlo Modelling of Photodynamic Therapy Treatments Comparing Clustered Three Dimensional Tumour Structures with Homogeneous Tissue Structures. Phys. Med. Biol. 2016, 61, 4840. [Google Scholar] [CrossRef]
- LaRochelle, E.P.M.; Marra, K.; LeBlanc, R.E.; Chapman, M.S.; Maytin, E.V.; Pogue, B.W. Modeling PpIX Effective Light Fluence at Depths into the Skin for PDT Dose Comparison. Photodiagnosis Photodyn. Ther. 2019, 25, 425–435. [Google Scholar] [CrossRef]
- Yassine, A.-A.; Lilge, L.; Betz, V. Optimizing Interstitial Photodynamic Therapy Planning with Reinforcement Learning-Based Diffuser Placement. IEEE Trans. Biomed. Eng. 2021, 68, 1668–1679. [Google Scholar] [CrossRef]
- Finlayson, L.; McMillan, L.; Suveges, S.; Steele, D.; Eftimie, R.; Trucu, D.; Brown, C.T.A.; Eadie, E.; Hossain-Ibrahim, K.; Wood, K. Simulating Photodynamic Therapy for the Treatment of Glioblastoma Using Monte Carlo Radiative Transport. J. Biomed. Opt. 2024, 29, 25001. [Google Scholar] [CrossRef] [PubMed]
- Lopez, N.; Mulet, R.; Rodríguez, R. Tumor Reactive Ringlet Oxygen Approach for Monte Carlo Modeling of Photodynamic Therapy Dosimetry. J. Photochem. Photobiol. B Biol. 2016, 160, 383–391. [Google Scholar] [CrossRef]
- Li, Z.; Nguyen, L.; Bass, D.A.; Baran, T.M. Effects of Patient-Specific Treatment Planning on Eligibility for Photodynamic Therapy of Deep Tissue Abscess Cavities: Retrospective Monte Carlo Simulation Study. J. Biomed. Opt. 2022, 27, 83007. [Google Scholar] [CrossRef] [PubMed]
- Oakley, E.; Parilov, E.; Beeson, K.; Potasek, M.; Ivanick, N.; Tworek, L.; Hutson, A.; Shafirstein, G. Computational Optimization of Irradiance and Fluence for Interstitial Photodynamic Therapy Treatment of Patients with Malignant Central Airway Obstruction. Cancers 2023, 15, 2636. [Google Scholar] [CrossRef]
- Savelieva, T.A.; Stratonnikov, A.A.; Loschenov, V.B. Multi-Spectral Imaging of Oxygen Saturation. Proc. SPIE 2008, 7022, 702205. [Google Scholar] [CrossRef]
- Dupont, C.; Baert, G.; Mordon, S.; Vermandel, M. Parallelized Monte-Carlo Dosimetry Using Graphics Processing Units to Model Cylindrical Diffusers Used in Photodynamic Therapy: From Implementation to Validation. Photodiagnosis Photodyn. Ther. 2019, 26, 351–360. [Google Scholar] [CrossRef]
- Wang, S.; Dai, X.Y.; Ji, S.; Saeidi, T.; Schwiegelshohn, F.; Yassine, A.-A.; Lilge, L.; Betz, V. Scalable and Accessible Personalized Photodynamic Therapy Optimization with FullMonte and PDT-SPACE. J. Biomed. Opt. 2022, 27, 83006. [Google Scholar] [CrossRef]
- Dupont, C.; Vignion, A.; Mordon, S.; Reyns, N.; Vermandel, M. Photodynamic Therapy for Glioblastoma: A Preliminary Approach for Practical Application of Light Propagation Models. Lasers Surg. Med. 2018, 50, 523–534. [Google Scholar] [CrossRef]
- Wang, S.; Saeidi, T.; Lilge, L.; Betz, V. Integrating Clinical Access Limitations into IPDT Treatment Planning with PDT-SPACE. Biomed. Opt. Express 2023, 14, 714–738. [Google Scholar] [CrossRef]
- Yassine, A.-A.; Lo, W.C.Y.; Saeidi, T.; Ferguson, D.; Whyne, C.M.; Akens, M.K.; Betz, V.; Lilge, L. Photodynamic Therapy Outcome Modelling for Patients with Spinal Metastases: A Simulation-Based Study. Sci. Rep. 2021, 11, 17871. [Google Scholar] [CrossRef]
- Lan, Q.; McClarren, R.G.; Vishwanath, K. Neural Network Forward Model and Transfer Learning Calibration from Monte Carlo to Diffuse Reflectance Spectroscopy. Proc. SPIE 2021, 11639, 49–57. [Google Scholar] [CrossRef]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Bärnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023, 9, 465. [Google Scholar] [CrossRef]
- Ward, Z.J.; Scott, A.M.; Hricak, H.; Atun, R. Global Costs, Health Benefits, and Economic Benefits of Scaling up Treatment and Imaging Modalities for Survival of 11 Cancers: A Simulation-Based Analysis. Lancet Oncol. 2021, 22, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Lauwerends, L.J.; van Driel, P.B.A.A.; Baatenburg de Jong, R.J.; Hardillo, J.A.U.; Koljenovic, S.; Puppels, G.; Mezzanotte, L.; Löwik, C.W.G.M.; Rosenthal, E.L.; Vahrmeijer, A.L.; et al. Real-Time Fluorescence Imaging in Intraoperative Decision Making for Cancer Surgery. Lancet Oncol. 2021, 22, e186–e195. [Google Scholar] [CrossRef] [PubMed]
- Moritz, T.J.; Zhao, Y.; Hinds, M.F.; Gunn, J.R.; Shell, J.R.; Pogue, B.W.; Davis, S.J. Multispectral Singlet Oxygen and Photosensitizer Luminescence Dosimeter for Continuous Photodynamic Therapy Dose Assessment during Treatment. J. Biomed. Opt. 2020, 25, 063810. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Moritz, T.; Hinds, M.F.; Gunn, J.R.; Shell, J.R.; Pogue, B.W.; Davis, S.J. High Optical-throughput Spectroscopic Singlet Oxygen and Photosensitizer Luminescence Dosimeter for Monitoring of Photodynamic Therapy. J. Biophotonics 2021, 14, e202100088. [Google Scholar] [CrossRef] [PubMed]
- Ong, Y.H.; Dimofte, A.; Kim, M.M.; Finlay, J.C.; Sheng, T.; Singhal, S.; Cengel, K.A.; Yodh, A.G.; Busch, T.M.; Zhu, T.C. Reactive Oxygen Species Explicit Dosimetry for Photofrin-mediated Pleural Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Hussain, M.A.B.; Khan, A.P.; Liu, H.; Siddiqui, S.; Mallidi, S.; Leon, P.; Daly, L.; Rudd, G.; Cuckov, F. Clinical Evaluation of Smartphone-Based Fluorescence Imaging for Guidance and Monitoring of ALA-PDT Treatment of Early Oral Cancer. J. Biomed. Opt. 2020, 25, 63813. [Google Scholar] [CrossRef] [PubMed]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef] [PubMed]
- Aumiller, M.; Heckl, C.; Quach, S.; Stepp, H.; Ertl-Wagner, B.; Sroka, R.; Thon, N.; Rühm, A. Interrelation between Spectral Online Monitoring and Postoperative T1-Weighted Mri in Interstitial Photodynamic Therapy of Malignant Gliomas. Cancers 2021, 14, 120. [Google Scholar] [CrossRef]
- Kirillin, M.; Khilov, A.; Kurakina, D.; Orlova, A.; Perekatova, V.; Shishkova, V.; Malygina, A.; Mironycheva, A.; Shlivko, I.; Gamayunov, S. Dual-Wavelength Fluorescence Monitoring of Photodynamic Therapy: From Analytical Models to Clinical Studies. Cancers 2021, 13, 5807. [Google Scholar] [CrossRef]
- Abrahamse, H.; Hamblin, M.R. New Photosensitizers for Photodynamic Therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef]
- Beigzadeh, A.M.; Rashidian Vaziri, M.R.; Ziaie, F.; Sharif, S. A New Optical Method for Online Monitoring of the Light Dose and Dose Profile in Photodynamic Therapy. Lasers Surg. Med. 2020, 52, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Horgan, C.C.; Bergholt, M.S.; Nagelkerke, A.; Thin, M.Z.; Pence, I.J.; Kauscher, U.; Kalber, T.L.; Stuckey, D.J.; Stevens, M.M. Integrated Photodynamic Raman Theranostics for Cancer Diagnosis, Treatment, and Post-Treatment Molecular Monitoring. arXiv 2020, arXiv:2009.04222. [Google Scholar] [CrossRef]
- Horgan, C.C.; Bergholt, M.S.; Nagelkerke, A.; Thin, M.Z.; Pence, I.J.; Kauscher, U.; Kalber, T.L.; Stuckey, D.J.; Stevens, M.M. Integrated Photodynamic Raman Theranostic System for Cancer Diagnosis, Treatment, and Post-Treatment Molecular Monitoring. Theranostics 2021, 11, 2006. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.; Fontana, L.C.; Raniero, L.; Ferreira-Strixino, J. In Vivo Raman Spectroscopy of Breast Tumors Prephotodynamic and Postphotodynamic Therapy. J. Raman Spectrosc. 2018, 49, 786–791. [Google Scholar] [CrossRef]
- Hamdoon, Z.; Jerjes, W.; Rashed, D.; Kawas, S.; abdul Sattar, A.; Samsudin, R.; Hopper, C. In Vivo Optical Coherence Tomography-Guided Photodynamic Therapy for Skin Pre-Cancer and Cancer. Photodiagnosis Photodyn. Ther. 2021, 36, 102520. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Winkelmann, J.A.; Spicer, G.; Zhu, Y.; Eid, A.; Ameer, G.A.; Backman, V.; Yi, J. Single Capillary Oximetry and Tissue Ultrastructural Sensing by Dual-Band Dual-Scan Inverse Spectroscopic Optical Coherence Tomography. Light Sci. Appl. 2018, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, L.; Zhang, X.; Nadkarni, N.A.; Cai, Z.; Batra, A.; Sullivan, D.P.; Muller, W.A.; Sun, C.; Kuranov, R.; Zhang, H.F. Longitudinal Deep-Brain Imaging in Mouse Using Visible-Light Optical Coherence Tomography through Chronic Microprism Cranial Window. Biomed. Opt. Express 2019, 10, 5235–5250. [Google Scholar] [CrossRef]
- Sirotkina, M.A.; Matveev, L.A.; Shirmanova, M.V.; Zaitsev, V.Y.; Buyanova, N.L.; Elagin, V.V.; Gelikonov, G.V.; Kuznetsov, S.S.; Kiseleva, E.B.; Moiseev, A.A. Photodynamic Therapy Monitoring with Optical Coherence Angiography. Sci. Rep. 2017, 7, 41506. [Google Scholar] [CrossRef]
- Sirotkina, M.A.; Moiseev, A.A.; Matveev, L.A.; Zaitsev, V.Y.; Elagin, V.V.; Kuznetsov, S.S.; Gelikonov, G.V.; Ksenofontov, S.Y.; Zagaynova, E.V.; Feldchtein, F.I. Accurate Early Prediction of Tumour Response to PDT Using Optical Coherence Angiography. Sci. Rep. 2019, 9, 6492. [Google Scholar] [CrossRef]
- Logsdon, E.A.; Finley, S.D.; Popel, A.S.; Gabhann, F. Mac A Systems Biology View of Blood Vessel Growth and Remodelling. J. Cell. Mol. Med. 2014, 18, 1491–1508. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, W.; Park, H.-C.; Li, X. In Vivo Assessment of Vascular-Targeted Photodynamic Therapy Effects on Tumor Microvasculature Using Ultrahigh-Resolution Functional Optical Coherence Tomography. Biomed. Opt. Express 2020, 11, 4316–4325. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, S.; Zhang, Z.; Luo, Q. Laser Speckle Contrast Imaging: Monitoring Blood Flow Dynamics and Vascular Structure of Photodynamic Therapy. Proc. SPIE 2005, 5630, 26–33. [Google Scholar] [CrossRef]
- Ren, C.; Chen, T.; Chen, M.; Shen, Y.; Li, B. Enhancing Laser Speckle Contrast Imaging Based on Adaptive Scale and Directional Kernel during V-PDT. Proc. SPIE 2023, 12745, 50–56. [Google Scholar] [CrossRef]
- Qiu, H.; Zhou, Y.; Gu, Y.; Ang, Q.; Zhao, S.; Wang, Y.; Zeng, J.; Huang, N. Monitoring Microcirculation Changes in Port Wine Stains during Vascular Targeted Photodynamic Therapy by Laser Speckle Imaging. Photochem. Photobiol. 2012, 88, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, P.; Zhao, H.; Chen, D.; Zhen, J.; Wang, Y.; Wang, Y.; Gu, Y. Assessment of Tissue Perfusion Changes in Port Wine Stains after Vascular Targeted Photodynamic Therapy: A Short-Term Follow-up Study. Lasers Med. Sci. 2014, 29, 781–788. [Google Scholar] [CrossRef]
- Choi, B.; Tan, W.; Jia, W.; White, S.M.; Moy, W.J.; Yang, B.Y.; Zhu, J.; Chen, Z.; Kelly, K.M.; Nelson, J.S. The Role of Laser Speckle Imaging in Port-Wine Stain Research: Recent Advances and Opportunities. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Sourvanos, D.; Sun, H.; Zhu, T.C.; Dimofte, A.; Yang, W.; Busch, T.M.; Cengel, K.A.; Byrd, B.; Neiva, R.; Fiorellini, J.P. Enhanced Photodynamic Therapy Delivery for Malignant Pleural Mesothelioma Using 3D-Printed Models and Optical Scanning Technology. Proc. SPIE 2024, 12823, 45–54. [Google Scholar]
- Moskalev, A.; Kalyagina, N.; Kozlikina, E.; Kustov, D.; Loshchenov, M.; Amouroux, M.; Daul, C.; Blondel, W. Validation of a White Light and Fluorescence Augmented Panoramic Endoscopic Imaging System on a Bimodal Bladder Wall Experimental Model. Photonics 2024, 11, 514. [Google Scholar] [CrossRef]
- Yassine, A.-A.; Lilge, L.; Betz, V. Machine Learning for Real-Time Optical Property Recovery in Interstitial Photodynamic Therapy: A Stimulation-Based Study. Biomed. Opt. Express 2021, 12, 5401–5422. [Google Scholar] [CrossRef]




| Method | Advantages | Limitations |
|---|---|---|
| Video fluorescence imaging |
|
|
| Spectral fluorescence imaging |
|
|
| Diffuse reflectance spectroscopy |
|
|
| Computer simulation |
|
|
| Singlet oxygen dosimetry |
|
|
| Raman spectroscopy |
|
|
| Optical coherence tomography |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alekseeva, P.; Makarov, V.; Efendiev, K.; Shiryaev, A.; Reshetov, I.; Loschenov, V. Devices and Methods for Dosimetry of Personalized Photodynamic Therapy of Tumors: A Review on Recent Trends. Cancers 2024, 16, 2484. https://doi.org/10.3390/cancers16132484
Alekseeva P, Makarov V, Efendiev K, Shiryaev A, Reshetov I, Loschenov V. Devices and Methods for Dosimetry of Personalized Photodynamic Therapy of Tumors: A Review on Recent Trends. Cancers. 2024; 16(13):2484. https://doi.org/10.3390/cancers16132484
Chicago/Turabian StyleAlekseeva, Polina, Vladimir Makarov, Kanamat Efendiev, Artem Shiryaev, Igor Reshetov, and Victor Loschenov. 2024. "Devices and Methods for Dosimetry of Personalized Photodynamic Therapy of Tumors: A Review on Recent Trends" Cancers 16, no. 13: 2484. https://doi.org/10.3390/cancers16132484
APA StyleAlekseeva, P., Makarov, V., Efendiev, K., Shiryaev, A., Reshetov, I., & Loschenov, V. (2024). Devices and Methods for Dosimetry of Personalized Photodynamic Therapy of Tumors: A Review on Recent Trends. Cancers, 16(13), 2484. https://doi.org/10.3390/cancers16132484







