New Frontiers in the Treatment of Patients with HER2+ Cancer and Brain Metastases: Is Radiotherapy Always Useful?
Abstract
Simple Summary
Abstract
1. Introduction
2. Traditional Medical Treatments
| Study/Reference | Treatment Method | Local Control Rate (%) | Median OS (months) | Neurocognitive Function (NCF) Impact | Comments |
|---|---|---|---|---|---|
| [18] | SRS (Gamma Knife—GK) | 87 | 7 | / | High local control rate |
| [18] | SRS + WBRT | 91 | 5 | / | Better local control than SRS alone |
| [18] | WBRT | 62 | 9 | / | Lower local control rate compared to SRS and SRS + WBRT |
| [33,34] | WBRT vs. SRS (5–15 metastases) | / | 10.4 (SRS), 8.4 (WBRT) | No significant difference between groups | Study suggests WBRT may be avoidable for patients with multiple metastases |
| [16,23] | WBRT + SRS; SRS alone | Limited [16], The 1-year local tumour control rate: 67% (SRS), 100% (SRS+ WBRT). | 7.5 (WBRT + SRS) vs. 8 (SRS) [16]. | Decline typically occurs 3–6 months after treatment and it can be irreversible and progressive [16]. Patients receiving SRS+ show a decline in learning and memory function (mean posterior probability of decline 52%) at 4 months compared to those receiving SRS alone (mean posterior probability of decline 24%) [23]. | Highly effective for symptom relief |
| [17] | WBRT in improvement functional independence after surgery or radiosurgery for brain metastases | / | 10.9 (WBRT) vs 10.7 (OBS). Note: WBRT reduced the 2-year relapse rate both at initial sites (surgery: 59% to 27%, radiosurgery: 31% to 19%, and at new sites (surgery: 42% to 23%, radiosurgery: 48% to 33%). | / | After radiosurgery or surgery for a limited number of brain metastases, WBRT reduces intracranial relapses but it does not improve functional independence or OS. |
| [19] | WBRT + SIB | >75 | 14.5 | / | Novel technique to improve local control rates |
| [20] | WBRT + SIB; WBRT + SRS | 39.4 (outside boost area), 60.6 (boost area) | 24.3 (WBRT + SIB) vs. 20.3 (WBRT + SRS) Note: median intracranial PFS (WBRT + SIB): 9.1 vs. 5.9 (WBRT + SRS) | / | Lower progression outside boost area, higher progression within boost area compared to WBRT + SRS |
| [26] | HA-WBRT | / | 6.8 | Helps preserve neurocognitive function and quality of life compared to historical controls (p < 0.001) | Demonstrated efficacy in preserving neurocognitive function and quality of life |
| [27] | HA-WBRT + memantine | / | 6.3 | Lower risk of neurocognitive failure compared to WBRT + memantine (HR 0.74) | No differences in intracranial progression-free survival (PFS), overall |
| [28] | HA-WBRT without memantine | / | 13.3 | HA-WBRT patients without memantine show better memory preservation at 6 months, but not improved verbal fluency or executive function. Those with longer life expectancy may benefit more from this treatment. | Effective in preserving memory function survival and toxicity |
| [35] | FLC followed by LRT | 47.5% achieved rCR | 54 months | / | No significant survival advantage in any subgroup, but slight trend for better recurrence outcomes in triple-positive tumors. |
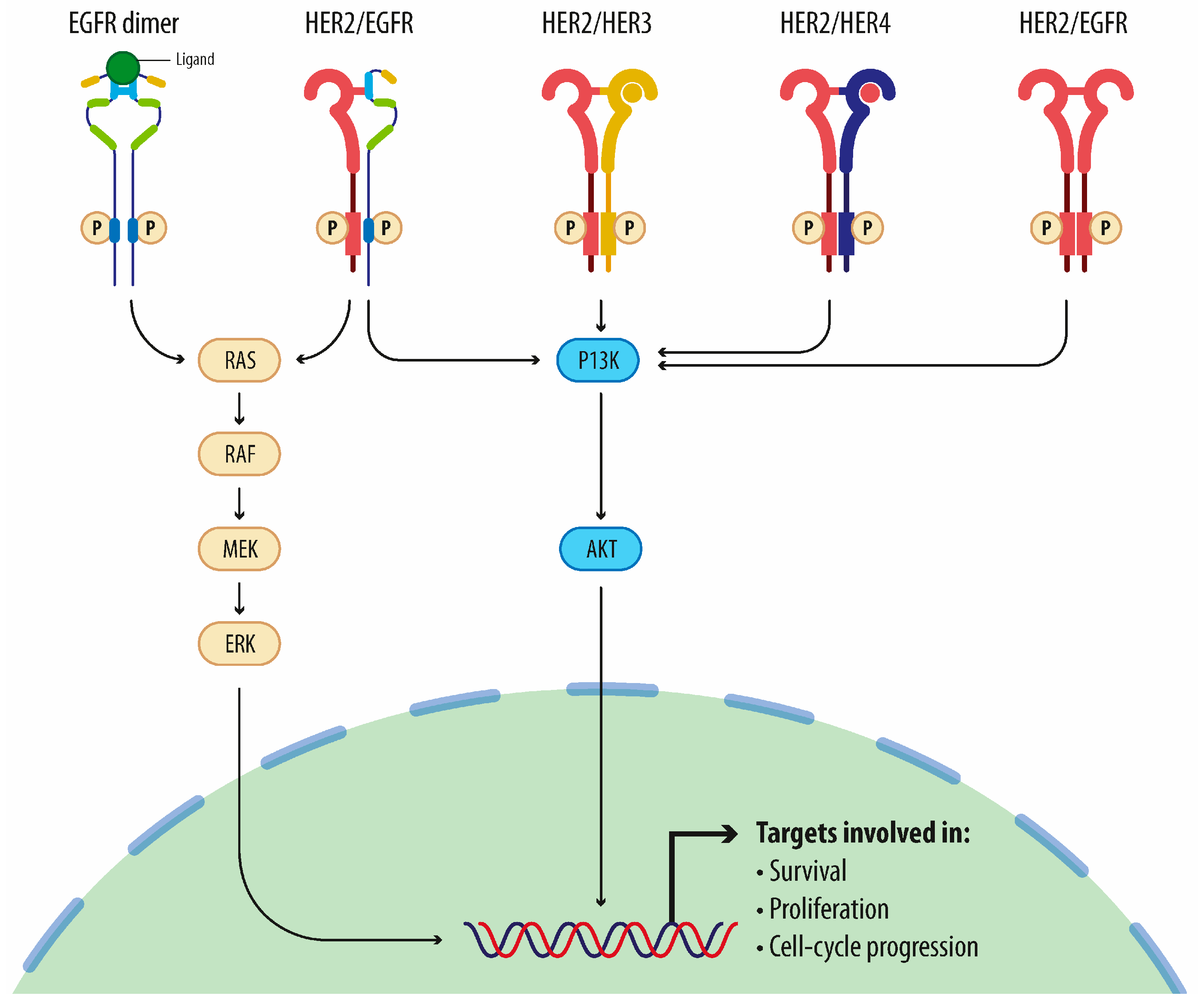
3. Molecular-Targeted Therapy
3.1. Therapy with Monoclonal Antibodies
3.2. Therapy with Antibody-Drug Conjugated (ADC)
3.3. Therapy with Tyrosine Kinase Inhibitors
3.4. Tucatinib: A New Hope in Cancer Treatment
3.5. Therapeutic Synergy: Combination of Treatments
4. Innovations in HER2-Targeted Therapy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cronin, K.A.; Harlan, L.C.; Dodd, K.W.; Abrams, J.S.; Ballard-Barbash, R. Population-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the US (Nov). Cancer Investig. 2010, 28, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, C.; Schiff, R. HER2: Biology.; detection.; and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Suppan, C.; Balic, M. Current standards and future outlooks in metastatic Her2-positive breast cancer. Breast Care 2023, 18, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Leone, J.P.; Lin, N.U. Systemic therapy of central nervous system metastases of breast cancer. Curr. Oncol. Rep. 2019, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Frisk, G.; Svensson, T.; Backlund, L.M.; Lidbrink, E.; Blomqvist, P.; Smedby, K.E. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br. J. Cancer 2012, 106, 1850–1853. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.I.; Grandis, J.R. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin. Cancer Res. 2015, 21, 526–533. [Google Scholar] [CrossRef]
- Valiente, M.; Ahluwalia, M.S.; Boire, A.; Brastianos, P.K.; Goldberg, S.B.; Lee, E.Q.; Le Rhun, E.; Preusser, M.; Winkler, F.; Soffietti, R. The evolving landscape of brain metastasis. Trends Cancer 2018, 4, 176–196. [Google Scholar] [CrossRef]
- Sevenich, L.; Bowman, R.L.; Mason, S.D.; Quail, D.F.; Rapaport, F.; Elie, B.T.; Brogi, E.; Brastianos, P.K.; Hahn, W.C.; Holsinger, L.J.; et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 2014, 16, 876–888. [Google Scholar] [CrossRef]
- Bailleux, C.; Eberst, L.; Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 2021, 124, 142–155. [Google Scholar] [CrossRef]
- Neman, J.; Termini, J.; Wilczynski, S.; Vaidehi, N.; Choy, C.; Kowolik, C.M.; Li, H.; Hambrecht, A.C.; Roberts, E.; Jandial, R. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl. Acad. Sci. USA 2014, 111, 984–989. [Google Scholar] [CrossRef]
- Kann, B.H.; Park, H.S.; Johnson, S.B.; Chiang, V.L.; Yu, J.B. Radiosurgery for brain metastases: Changing practice patterns and disparities in the United States. J. Natl. Compr. Canc Netw. 2017, 15, 1494–1502. [Google Scholar] [CrossRef] [PubMed]
- Akagunduz, O.O.; Yilmaz, S.G.; Tavlayan, E.; Baris, M.E.; Afrashi, F.; Esassolak, M. Radiation-induced ocular surface disorders and retinopathy: Ocular structures and radiation dose-volume effect. Cancer Res. Treat. 2022, 54, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhong, X.; Xu, H.; Wang, J.; Liu, X.; Wang, Y.; He, L.; Ma, J.; Li, G.; Liu, L. Survival analysis of palliative radiotherapy in patients with HER-2+ metastatic breast cancer. Front. Endocrinol. 2024, 14, 1305429. [Google Scholar] [CrossRef] [PubMed]
- Mintz, A.H.; Kestle, J.; Rathbone, M.P.; Gaspar, L.; Hugenholtz, H.; Fisher, B.; Duncan, G.; Skingley, P.; Foster, G.; Levine, M. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer 1996, 78, 1470–1476. [Google Scholar] [CrossRef]
- Ippolito, E.; Silipigni, S.; Matteucci, P.; Greco, C.; Carrafiello, S.; Palumbo, V.; Tacconi, C.; Talocco, C.; Fiore, M.; D’Angelillo, R.M.; et al. Radiotherapy for HER 2 Positive Brain Metastases: Urgent Need for a Paradigm Shift. Cancers 2022, 14, 1514. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, H.; Shirato, H.; Tago, M.; Nakagawa, K.; Toyoda, T.; Hatano, K.; Kenjyo, M.; Oya, N.; Hirota, S.; Shioura, H.; et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006, 295, 2483–2491. [Google Scholar] [CrossRef] [PubMed]
- Kocher, M.; Soffietti, R.; Abacioglu, U.; Villà, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J. Clin. Oncol. 2011, 29, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Chougule, P.; Burton-Williams, M.; Saris, S.; Zheng, Z.; Ponte, B.; Noren, G.; Alderson, L.; Friehs, G.; Wazer, D.; Epstein, M. Randomized treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 114. [Google Scholar] [CrossRef]
- Casanova, N.; Mazouni, Z.; Bieri, S.; Combescure, C.; Pica, A.; Weber, D.C. Whole brain radiotherapy with a conformational external beam radiation boost for lung cancer patients with 1-3 brain metastasis: A multi institutional study. Radiat. Oncol. 2010, 5, 13. [Google Scholar] [CrossRef]
- Lin, B.; Huang, D.; Du, H.; Fan, J.; Zhang, Y.; Feng, G.; Gao, F.; Du, X.B. Whole-Brain Radiation Therapy with Simultaneous Integrated Boost Versus Whole-Brain Radiation Therapy Plus Stereotactic Radiosurgery for the Treatment of Brain Metastasis from Lung Cancer. Front. Oncol. 2021, 11, 631422. [Google Scholar] [CrossRef]
- Roman, D.D.; Sperduto, P.W. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 983–998. [Google Scholar] [CrossRef] [PubMed]
- Soffietti, R.; Kocher, M.; Abacioglu, U.M.; Villa, S.; Fauchon, F.; Baumert, B.G.; Fariselli, L.; Tzuk-Shina, T.; Kortmann, R.D.; Carrie, C.; et al. AEuropean Organisation for Research Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: Quality-of-life results. J. Clin. Oncol. 2013, 31, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.L.; Wefel, J.S.; Hess, K.R.; Allen, P.K.; Lang, F.F.; Kornguth, D.G.; Arbuckle, R.B.; Swint, J.M.; Shiu, A.S.; Maor, M.H.; et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009, 10, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular pathways: Radiation-induced cognitive impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef]
- Gondi, V.; Tomé, W.A.; Mehta, M.P. Why avoid the hippocampus? A comprehensive review. Radiother. Oncol. 2010, 97, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Pugh, S.L.; Tome, W.A.; Caine, C.; Corn, B.; Kanner, A.; Rowley, H.; Kundapur, V.; DeNittis, A.; Greenspoon, J.N.; et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J. Clin. Oncol. 2014, 32, 3810–3816. [Google Scholar] [CrossRef]
- Brown, P.D.; Gondi, V.; Pugh, S.; Tome, W.A.; Wefel, J.S.; Armstrong, T.S.; Bovi, J.A.; Robinson, C.; Konski, A.; Khuntia, D.; et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients with Brain Metastases: Phase IIITrial NRGOncology CC001. J. Clin. Oncol. 2020, 38, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Chen, Y.F.; Yang, C.C.; Wu, P.F.; Chan, H.M.; Chen, J.L.; Chen, G.Y.; Cheng, J.C.; Kuo, S.H.; Hsu, F.M. Hippocampal avoidance whole-brain radiotherapy without memantine in preserving neurocognitive function for brain metastases: A phase II blinded randomized trial. Neuro Oncol. 2021, 23, 478–486, Erratum in Neuro Oncol. 2021, 23, 2125. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.D.; Pugh, S.; Laack, N.N.; Wefel, J.S.; Khuntia, D.; Meyers, C.; Choucair, A.; Fox, S.; Suh, J.H.; Roberge, D.; et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013, 15, 1429–1437. [Google Scholar] [CrossRef]
- Hall, E.J.; Brenner, D.J. The radiobiology of radiosurgery: Rationale for different treatment regimes for AVMs and malignancies. Int. J. Radiat. Oncol. Biol. Phys. 1993, 25, 381–385. [Google Scholar] [CrossRef]
- Lupattelli, M.; Alì, E.; Ingrosso, G.; Saldi, S.; Fulcheri, C.; Borghesi, S.; Tarducci, R.; Aristei, C. Stereotactic Radiotherapy for Brain Metastases: Imaging Tools and Dosimetric Predictive Factors for Radionecrosis. J. Pers. Med. 2020, 10, 59. [Google Scholar] [CrossRef]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Kline, R.; Loeffler, J.; Farnan, N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Shuto, T.; Akabane, A.; Higuchi, Y.; Kawagishi, J.; Yamanaka, K.; Sato, Y.; Jokura, H.; Yomo, S.; et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014, 15, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Serizawa, T.; Higuchi, Y.; Sato, Y.; Kawagishi, J.; Yamanaka, K.; Shuto, T.; Akabane, A.; Jokura, H.; Yomo, S.; et al. AMulti-institutional Prospective Observational Study of Stereotactic Radiosurgery for Patients with Multiple Brain Metastases (JLGK0901 Study Update): Irradiation-related Complications Long-term Maintenance of Mini-Mental State Examination Scores. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Tinterri, C.; Sagona, A.; Barbieri, E.; Di Maria Grimaldi, S.; Jacobs, F.; Zambelli, A.; Trimboli, R.M.; Bernardi, D.; Vinci, V.; Gentile, D. Loco-Regional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancer Patients Undergoing Front-Line Chemotherapy. Cancers 2022, 14, 6237. [Google Scholar] [CrossRef] [PubMed]
- Pons-Tostivint, E.; Kirova, Y.; Lusque, A.; Campone, M.; Geffrelot, J.; Mazouni, C.; Mailliez, A.; Pasquier, D.; Madranges, N.; Firmin, N.; et al. Survival Impact of Locoregional Treatment of the Primary Tumor in De Novo Metastatic Breast Cancers in a Large Multicentric Cohort Study: A Propensity Score-Matched Analysis. Ann. Surg. Oncol. 2019, 26, 356–365. [Google Scholar] [CrossRef]
- Marks, C.E.; Thomas, S.M.; Fayanju, O.M.; DiLalla, G.; Sammons, S.; Hwang, E.S.; Plichta, J.K. Metastatic Breast Cancer: Who Benefits from Surgery? Am. J. Surg. 2022, 223, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Huang, K.; Zeng, Q.; Zhang, J.; Song, C. Impact of Breast Surgery on Survival of Patients with Stage IV Breast Cancer: A SEER Population-Based Propensity Score Matching Analysis. PeerJ 2020, 8, e8694. [Google Scholar] [CrossRef]
- Harris, E.; Barry, M.; Kell, M.R. Meta-Analysis to Determine If Surgical Resection of the Primary Tumour in the Setting of Stage IV Breast Cancer Impacts on Survival. Ann. Surg. Oncol. 2013, 20, 2828–2834. [Google Scholar] [CrossRef]
- Petrelli, F.; Barni, S. Surgery of Primary Tumors in Stage IV Breast Cancer: An Updated Meta-Analysis of Published Studies with Meta-Regression. Med. Oncol. 2012, 29, 3282–3290. [Google Scholar] [CrossRef]
- Hosonaga, M.; Saya, H.; Arima, Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020, 39, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Shen, G.; Wang, T.; Li, J.; Xie, Q.; Liu, Z.; Wang, M.; Zhao, F.; Ren, D.; Zhao, J. Treatment options for patients with human epidermal growth factor 2-positive breast cancer brain metastases: A systematic review and meta-analysis. Front. Oncol. 2023, 13, 1003565. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J. Treatment of HER2-overexpressing breast cancer. Ann. Oncol. 2010, 21, vii36–vii40. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Norton, L.; Albanell, J.; Kim, Y.M.; Mendelsohn, J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the anti-tumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998, 58, 2825–2831. [Google Scholar] [PubMed]
- Pegram, M.; Hsu, S.; Lewis, G.; Pietras, R.; Beryt, M.; Sliwkowski, M.; Coombs, D.; Baly, D.; Kabbinavar, F.; Slamon, D. Inhibitory effects of combinations of HER-2/neu antibody and chemotherapeutic agents used for treatment of human breast cancers. Oncogene 1999, 18, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Pietras, R.J.; Fendly, B.M.; Chazin, V.R.; Pegram, M.D.; Howell, S.B.; Slamon, D.J. Antibody to HER-2/neu receptor blocks DNA repair after cisplatin in human breast and ovarian cancer cells. Oncogene 1994, 9, 1829–1838. [Google Scholar] [PubMed]
- Pietras, R.J.; Pegram, M.D.; Finn, R.S.; Maneval, D.A.; Slamon, D.J. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene 1988, 17, 2235–2249. [Google Scholar] [CrossRef] [PubMed]
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.; Jackisch, C.; et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2012, CD006243. [Google Scholar] [PubMed]
- Nahta, R.; Esteva, F.J. Herceptin: Mechanisms of action and resistance. Cancer Lett. 2006, 232, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Drebin, J.A.; Link, V.C.; Greene, M.I. Monoclonal antibodies reactive with distinct domains of the neu oncogene-encoded p185 molecule exert synergistic anti-tumor effects in vivo. Oncogene 1988, 2, 273–277. [Google Scholar] [PubMed]
- Ishii, K.; Morii, N.; Yamashiro, H. Pertuzumab in the treatment of HER2-positive breast cancer: 671 an evidence-based review of its safety.; efficacy.; and place in therapy. Core Evid. 2019, 14, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Agus, D.B.; Akita, R.W.; Fox, W.D.; Lewis, G.D.; Higgins, B.; Pisacane, P.I.; Lofgren, J.A.; Tindell, C.; Evans, D.P.; Maiese, K.; et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002, 2, 127–137. [Google Scholar] [CrossRef]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A central role for HER3 in HER2-amplified breast cancer: Implications for targeted therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef] [PubMed]
- Nahta, R.; Hung, M.C.; Esteva, F.J. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004, 64, 2343–2346. [Google Scholar] [CrossRef] [PubMed]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly enhanced anti-tumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef]
- Mamidi, S.; Cinci, M.; Hasmann, M.; Fehring, V.; Kirschfink, M. Lipoplex mediated silencing of membrane regulators (CD46.; CD55 and CD59) enhances complement dependent anti-tumor activity of trastuzumab and pertuzumab. Mol. Oncol. 2013, 7, 580–594. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Kim, S.B.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Heeson, S.; et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N. Engl. J. Med. 2015, 372, 724–734. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Bergen, E.S.; Binter, A.; Starzer, A.M.; Heller, G.; Kiesel, B.; Tendl-Schulz, K.; Bago-Horvath, Z.; Furtner, J.; Leitner, J.; Exner, R.; et al. Favourable outcome of patients with breast cancer brain metastases treated with dual HER2 blockade of trastuzumab and pertuzumab. Ther. Adv. Med. Oncol. 2021, 13, 17588359211009002. [Google Scholar] [CrossRef]
- Lin, N.U.; Pegram, M.; Sahebjam, S.; Ibrahim, N.; Fung, A.; Cheng, A.; Nicholas, A.; Kirschbrown, W.; Kumthekar, P. Pertuzumab Plus High-Dose Trastuzumab in Patients with Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study. J. Clin. Oncol. 2021, 39, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Ji, J.; Liu, H.; He, X. The evolving role of trastuzumab emtansine (TDM1) in HER2-positive breast cancer with brain metastases. Crit. Rev. Oncol. Hematol. 2019, 143, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Barok, M.; Joensuu, H.; Isola, J. Trastuzumab emtansine: Mechanisms of action and drug resistance. Breast Cancer Res. 2014, 16, 209. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective.; exploratory analysis in EMILIA. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2- positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, F.; Delaloge, S.; Barrios, C.H.; Wuerstlein, R.; Anton, A.; Brain, E.; Hatschek, T.; Kelly, C.M.; Peña-Murillo, C.; Yilmaz, M.; et al. Trastuzumab emtansine (TDM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA.; a single-arm phase IIIb clinical trial. Ann. Oncol. 2020, 31, 1350–1358. [Google Scholar] [CrossRef]
- Dormann, C. Metastatic Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Current Treatment Standards and Future Perspectives. Breast Care 2020, 15, 570–578. [Google Scholar] [CrossRef]
- Modi, S.; Saura, C.; Yamashita, T.; Park, Y.H.; Kim, S.B.; Tamura, K.; Andre, F.; Iwata, H.; Ito, Y.; Tsurutani, J.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N. Engl. J. Med. 2020, 382, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, R.; Berghoff, A.S.; Furtner, J.; Marhold, M.; Bergen, E.S.; Roider-Schur, S.; Starzer, A.M.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: A single-arm.; phase 2 trial. Nat. Med. 2022, 28, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.B.; Chung, W.P.; Im, S.A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.M.; Petry, V.; Chung, C.F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Modi, S.; Li, W.; Park, Y.H.; Chung, W.; Kim, S.-B.; Cortés, J.; Yamashita, T.; Pedrini, J.; Im, S.-A.; et al. A pooled analysis of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-positive (HER2+) metastatic breast cancer (mBC) with brain metastases (BMs) from DESTINY-Breast (DB) -01.; -02.; and -03. Ann. Oncol. 2023, 34, S335–S336. [Google Scholar] [CrossRef]
- Yamanaka, T. Trastuzumab deruxtecan for the treatment of patients with HER2-positive breast cancer with brain and/or leptomeningeal metastases: A multicenter retrospective study (ROSET-BM study). In Proceedings of the San Antonio Breast Cancer Symposium, San Antonio, TX, USA, 6–10 December 2022. [Google Scholar]
- Pérez-García, J.M.; Vaz Batista, M.; Cortez, P.; Ruiz-Borrego, M.; Cejalvo, J.M.; de la Haba-Rodriguez, J.; Garrigós, L.; Racca, F.; Servitja, S.; Blanch, S.; et al. Trastuzumab Deruxtecan in Patients with Central Nervous System Involvement from HER2-Positive Breast Cancer: The DEBBRAH Trial. Neuro Oncol. 2023, 25, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E.; Pegram, M.D.; Venkatesan, N.; Finn, R.; Yang, G.; Rahmeh, M.; Untch, M.; Rusnak, D.W.; Spehar, G.; Mullin, R.J.; et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2- overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006, 66, 1630–1639. [Google Scholar] [CrossRef]
- Nahta, R.; Yuan, L.X.; Du, Y.; Esteva, F.J. Lapatinib induces apoptosis in trastuzumabresistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol. Cancer Ther. 2007, 6, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.Y.; Gonçalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, J.F.; Wright-Hughes, A.; Pottinger, A.; Velikova, G.; Oughton, J.B.; Murden, G.; Rizwanullah, M.; Price, C.; Passant, H.; Heudtlass, P.; et al. Lapatinib plus Capecitabine versus Trastuzumab plus Capecitabine in the Treatment of Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer with Central Nervous System Metastases for Patients Currently or Previously Treated with Trastuzumab (LANTERN): A Phase II Randomised Trial. Clin. Oncol. 2020, 32, 656–664. [Google Scholar]
- Saura, C.; Oliveira, M.; Feng, Y.H.; Dai, M.S.; Chen, S.W.; Hurvitz, S.A.; Kim, S.B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated with ≥2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Rabindran, S.K.; Discafani, C.M.; Rosfjord, E.C.; Baxter, M.; Floyd, M.B.; Golas, J.; Hallett, W.A.; Johnson, B.D.; Nilakantan, R.; Overbeek, E.; et al. Anti-tumor activity of HKI-272.; an orally active.; irreversible inhibitor of the HER-2 tyrosine kinase. Cancer Res. 2004, 64, 3958–3965. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Gijsen, M.; Mullooly, M.; Bennett, R.; Bouguern, N.; Pedersen, K.; O’Brien, N.A.; Roxanis, I.; Li, J.L.; Bridge, E.; et al. Neratinib overcomes trastuzumab resistance in HER2 amplified breast cancer. Oncotarget 2013, 4, 1592–1605. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A Phase II Journal Pre-proof Trial of Neratinib and Capecitabine for Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; Lowry, M.C.; O’Driscoll, L. Neratinib resistance and cross-resistance to other HER2-targeted drugs due to increased activity of metabolism enzyme cytochrome P4503A4. Br. J. Cancer 2017, 116, 620–625. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, V.; Raimondo, L.; Formisano, L.; Giuliano, M.; De Placido, S.; Rosa, R.; Bianco, R. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat. Rev. 2015, 41, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre.; randomised.; double-blind.; placebo-controlled.; phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised.; open-label.; multicentre.; phase 3 trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef]
- Moulder, S.L.; Borges, V.F.; Baetz, T.; McSpadden, T.; Fernetich, G.; Murthy, R.K.; Chavira, R.; Guthrie, K.; Barrett, E.; Chia, S.K. Phase I study of ONT-380.; a HER2 inhibitor.; in patients with HER2(+)-advanced solid tumors.; with an expansion cohort in HER2(+) metastatic breast cancer (MBC). Clin. Cancer Res. 2017, 23, 3529–3536. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib.; Trastuzumab.; and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- O’Brien, N.A.; Huang, H.K.T.; McDermott, M.S.J.; Madrid, A.M.; Luo, T.; Ayala, R.; Issakhanian, S.; Gong, K.W.; Lu, M.; Zhang, J.; et al. Tucatinib has Selective Activity in HER2-Positive Cancers and Significant Combined Activity with Approved and Novel Breast Cancer-Targeted Therapies. Mol. Cancer Ther. 2022, 21, 751–761. [Google Scholar] [CrossRef] [PubMed]
- Liang, K.; Lu, Y.; Jin, W.; Ang, K.K.; Milas, L.; Fan, Z. Sensitization of breast cancer cells to radiation by trastuzumab. Mol. Cancer Ther. 2003, 2, 1113–1120. [Google Scholar] [PubMed]
- Chargari, C.; Idrissi, H.R.; Pierga, J.Y.; Bollet, M.A.; Dieras, V.; Campana, F.; Cottu, P.; Fourquet, A.; Kirova, Y.M. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Yu, X.; Cai, G.; Yang, Z.; Cao, L.; Hu, C.; Guo, X.; Sun, J.; Chen, J. Survival benefit of anti-HER2 therapy after whole-brain radiotherapy in HER2-positive breast cancer patients with brain metastasis. Breast Cancer 2016, 23, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.J.; Rugo, H.S. Tyrosine kinase inhibitors for human epidermal growth factor receptor 2-positive metastatic breast cancer: Is personalizing therapy within reach. J. Clin. Oncol. 2017, 35, 3089–3091. [Google Scholar] [CrossRef] [PubMed]
- Fauquette, W.; Amourette, C.; Dehouck, M.P.; Diserbo, M. Radiation-induced blood-brain barrier damages: An in vitro study. Brain Res. 2012, 1433, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, P.K.; Cittelly, D.M.; Robin, T.P.; Carlson, J.A.; Stuhr, K.A.; Contreras-Zarate, M.J.; Lai, S.; Ormond, D.R.; Rusthoven, C.G.; Gaspar, L.E.; et al. Combination of trastuzumab emtansine and stereotactic radiosurgery results in high rates of clinically significant radionecrosis and dysregulation of Aquaporin-4. Clin. Cancer Res. 2019, 25, 3946–3953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, J.; Yu, X.; Ma, J.; Cai, G.; Yang, Z.; Cao, L.; Chen, X.; Guo, X.; Chen, J. Systemic treatment after whole-brain radiotherapy may improve survival in RPA class II/III breast cancer patients with brain metastasis. J. Neurooncol. 2013, 114, 181–189. [Google Scholar] [CrossRef]
- Le Scodan, R.; Jouanneau, L.; Massard, C.; Gutierrez, M.; Kirova, Y.; Cherel, P.; Gachet, J.; Labib, A.; Mouret-Fourme, E. Brain metastases from breast cancer: Prognostic significance of HER-2 overexpression.; effect of trastuzumab and cause of death. BMC Cancer 2011, 11, 395. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, I.A. Evolving treatment strategies of brain metastases from breast cancer: Current status and future direction. Ther. Adv. Med. Oncol. 2020, 12, 1758835920936117. [Google Scholar] [CrossRef]
- Stavrou, E.; Winer, E.P.; Lin, N.U. How we treat HER2-positive brain metastases. ESMO Open 2021, 6, 100256. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.-A.; Wright, G.L.S.; Escriva-de-Romani, S.; DeLaurentiis, M.; Cortes, J.; Bahadur, S.W.; Haley, B.B.; Oyola, R.H.; Riseberg, D.A.; et al. SOPHIA primary analysis: A phase 3 (P3) study of margetuximab (M) + chemotherapy (C) versus trastuzumab (T) + C in patients (pts) with HER2+ metastatic (met) breast cancer (MBC) after prior anti-HER2 therapies (Tx). J. Clin. Oncol. 2019, 37, 1000. [Google Scholar] [CrossRef]
- Saura Manich, C.; O’Shaughnessy, J.; Aftimos, P.; van den Tweel, E.; Oesterholt, M.; Escrivá-de- Romaní, S.; Quenel Tueux, N.; Tan, T.J.; Lim, J.S.; Ladoire, S.; et al. LBA15 Primary outcome of the phase III SYD985. 002/TULIP trial comparing [vic-] trastuzumab duocarmazine to physician’s choice treatment in patients with pre-treated HER2-positive locally advanced or metastatic breast cancer. Ann. Oncol. 2021, 32, S1288. [Google Scholar] [CrossRef]
- Ma, F.; Ouyang, Q.; Li, W.; Jiang, Z.; Tong, Z.; Liu, Y.; Liu, Y.; Li, H.; Yu, S.; Feng, J.; et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes.; anthracyclines.; and/or trastuzumab: A randomized.; phase II study. J. Clin. Oncol. 2019, 37, 2610–2619. [Google Scholar] [CrossRef]
- Krop, I.E.; Carey, L.A.; Ramos, J.; Chen, Y.; Hamilton, E.P. Phase 2 trial of tucatinib plus trastuzumab deruxtecan in patients with HER2+ locally advanced or metastatic breast cancer with and without brain metastases (HER2CLIMB-04.; trial in progress). J. Clin. Oncol. 2022, 40, TPS1111. [Google Scholar] [CrossRef]
| Clinical Trials on Combinational Radiotherapy | Description | Outcome | Patients | Study Status |
|---|---|---|---|---|
| Liang et al. [93] | Study of the impact of HER2 on BC cells’ radiosensitivity and involvement of trastuzumab | Trastuzumab increased radiation-induced cell death in high HER2+ cells and sensitized cells to radiation. Inhibiting the PI3-K pathway enhanced trastuzumab’s radiosensitizing effects | six breast cancer cell lines | Completed |
| Chargari et al. [94] | Trastuzumab and WBRT | ORR: 74.2%; median survival time: 18 months; median intracranial disease control: 10.5 months | 31 | Completed |
| Zhang et al. [95] | Survival benefit in BM patients after WBRT in combination with anti-HER2 therapy | The median OS longer in patients who received chemotherapy or anti-HER2 therapy after WBRT than in those who did not receive (16 vs. 6 months and 21 vs. 9 months) | 60 | Completed |
| Chien and Rugo, Fauquette et al. [96,97] | BBB challenge | TKIs can penetrate BBB [96]. RT enhances BBB permeability, improving drug effectiveness [97] | [96] Ionizing radiation were studied on an in vitro BBB model [97] | Completed Completed |
| Stumpf et al. [98] | Combination of T-DM1 and SRS | Patients receiving T-DM1: 39.1% developed CSRN. In contrast, only 4.5% of patients who did not receive T-DM1 experienced CSRN | 45 | Completed |
| Clinical Study | Description | Outcome | Study Status | Patients |
|---|---|---|---|---|
| CLEOPATRA Trial [61] | Investigated pertuzumab addition to docetaxel and trastuzumab for HER2+ breast cancer patients | Median OS: 56.5 months, (pertzumab, trastuzumab and docetaxel group) Median OS: 40.8 months (trastuzumab and docetaxel group) | Ongoing | 808 |
| Retrospective Study by Bergen et al. [63] | Combination of pertuzumab and trastuzumab in HER2+ BCBM patients | TP OS: 44 months Other-HER2-targeted therapy: 17 months No-HER2-targeted therapy: 3 months | Completed | 252 |
| PATRICIA Trial [64] | Safety and efficacy of pertuzumab plus high-dose trastuzumab in HER2+ BCBM | ORR: 11%, 68% of patients experienced clinical benefit | Completed | 40 |
| Clinical Trials on Antibody-Drug Conjugates (ADC) | ||||
| EMILIA Trial [66] | Phase 3 trial comparing T-DM1 to lapatinib/capecitabine therapy in advanced HER2+ BC patients | Prolonged median OS (26.8 months), higher CNS ORR with T-DM1, higher probability of bleeding events Median in XL patients: 12.9 months | Completed | 991 |
| KAMILLA Trial [69] | Single-arm phase 3b trial assessing T-DM1 in stable HER2+ BCBM patients | PFS in patients with baseline BM: 5.5 months PFS in patients without BM: 7.7 months. Median OS in patients with baseline BM: 18.9 months Median OS in patients without BM: 30.0 months | Ongoing | 398 |
| TUXEDO-1 Trial [72] | Investigated T-DXd in HER2+ BC patients with untreated BM | Response Rate of 73.3%, PFS: 14 months, OS not reached | Completed | 15 |
| DESTINY-Breast01 Study [71] | Demonstrated superiority of T-DXd over T-DM1 in HER2+ BC | Median OS T-DXd patients: 29.1 months Median PFS T-DXt patients: 19.4 months Confirmed ORR in 62% of patients | Completed | 184 |
| DESTINY-Breast03 Study [73] | Showed superiority of T-DXd over T-DM1 | Confirmed ORR of 67.4%. OS: at 12 months, 94.1% of T-DXt patients were alive, compared to 85.9% T-DM1 patients. PFS: at 12 months, 75.8% of T-DXd patients were alive without disease progression, compared to 34.1% on T-DM1. | Completed | 524 |
| Pooled Analysis by Hurvitz et al. [74] | Combined data from DESTINY-Breast -01, -02, -03 trials to assess the efficacy of T-DXd in HER2+ BC patients, particularly those with BMs. | CNS PFS T- DXd vs. comparator: Stable BMs: 12.3 vs. 8.7 months Active BMs: 18.5 vs. 4.0 months. Stable BMs IC-ORR T-DXd vs. comparator: 45.2% vs. 27.6% Active BMs IC-ORR T-DXd vs. comparator: 45.5% vs. 12.0% | Completed | 148 |
| ROSET-BM Study [75] | Demonstrated T-DXd efficacy in HER2+ BC with brain or leptomeningeal metastases | Median PFS of 16.1 months and one-year OS rate of 74.9% | Completed | 104 |
| DEBBRAH Study [76] | Showed IC-ORR in patients with active BM treated with T-DXd | IC-ORR of HER2+ ABC patients with asymptomatic untreated and progressing BMs was 50.0% and 44.4%, respectively. HER2+ ABC patients with stable BMs who received T-DXd had 16-week PFS rate of 87.5%. | Ongoing | 21 |
| Clinical Trials on Tyrosine Kinase Inhibitors (TKIs) | ||||
| LANDSCAPE Study [79] | Evaluated lapatinib/capecitabine therapy in HER2+ BC patients without prior WBRT | 65.9% of patients achieved partial CNS responses. 84% of patients had a reduction in tumour volume from baseline. | Ongoing | 44 |
| LANTERN Trial [80] | Compared lapatinib/capecitabine to trastuzumab/capecitabine in HER2+ BC | CNS disease progression: 41.8% in lap-cap and 41.2% in tars-cap. PFS: 44.4% in lap-cap and 50.0% in tras-cap arms. | Completed | 30 |
| NALA Trial [81] | Investigated neratinib/capecitabine (N1C) therapy versus lapatinib/capecitabine (L1C) therapy in patients with HER2+ BC and metastases | PFC: N1C showed a 24% reduction in the risk of disease progression or death compared to L1C. OS: not statistically significant. However, N1C showed a trend toward improved OS. ORR: N1C: 32.8% L1C: 26.7% | Ongoing | 621 |
| TBCRC022, Co3 [84] | Assessed neratinib/capecitabine therapy in patients with CNS progression following prior treatment. Patients were divided in Cohort 3A (lapatinib-naïve) and Cohort 3B (lapatinib-treated). | CNS ORR 3A: 49%, CNS ORR 3B: 33%. Median PFS 3A: 5.5 months, median PFS 3B: 3.1 months. Median OS 3A: 13.3 months median OS 3B: 15.1 months. | Completed | 49 |
| Clinical Trials on Tucatinib | ||||
| O’Brien et al. [92] | Analyzed cell lines to assess tucatinib efficacy in HER2+ cancers | Selectivity for HER2; dependence on activated HER2 signaling | Completed | 456 molecularly characterized human cancer cell lines associated with 16 different malignant histologies |
| HER2CLIMB Clinical Trial [91] | Investigated tucatinib with trastuzumab and capecitabine for HER2+ metastatic BM | PFS in tucatinib-combination group at 1 year: 33.1%, PFS in the placebo-combination group at 1 year: 12.3%. OS in tucatinib-combination group at 2 years: 44.9, OS in the placebo-combination group at 2 years: 26.6%. | Completed | 612 |
| Clinical Trials on Investigational Agents | ||||
| Margetuximab | SOPHIA Trial (Phase III) [103] | PFS in margetuximab/chemotherapy patients: 5.8 months. PFS in trastuzumab/chemotherapy patients: 4.9 months, numerical but not statistically significant OS benefit in the first group rather than in the second one (21.9 vs. 19.8 months). | Completed | 536 |
| Trastuzumab duocarmazine | TULIP Trial (Phase III) [104] | Improved PFS in patients who received trastuzumab duocarmazine rather the PC (7.0 vs. 4.9 months) and OS (20.4 vs. 16.3 months) with manageable adverse events | Completed | 437 |
| Pyrotinib | Phase II Trial [105] | Pyrotinib ORR: 78.5%, Lapatinib ORR: 57.1%. Pyrotinib median PFS: 18.1 months, Lapatinib median PFS: 7.0 months. | Completed | 128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scandurra, G.; Lombardo, V.; Scibilia, G.; Sambataro, D.; Gebbia, V.; Scollo, P.; Pecorino, B.; Valerio, M.R. New Frontiers in the Treatment of Patients with HER2+ Cancer and Brain Metastases: Is Radiotherapy Always Useful? Cancers 2024, 16, 2466. https://doi.org/10.3390/cancers16132466
Scandurra G, Lombardo V, Scibilia G, Sambataro D, Gebbia V, Scollo P, Pecorino B, Valerio MR. New Frontiers in the Treatment of Patients with HER2+ Cancer and Brain Metastases: Is Radiotherapy Always Useful? Cancers. 2024; 16(13):2466. https://doi.org/10.3390/cancers16132466
Chicago/Turabian StyleScandurra, Giuseppa, Valentina Lombardo, Giuseppe Scibilia, Daniela Sambataro, Vittorio Gebbia, Paolo Scollo, Basilio Pecorino, and Maria Rosaria Valerio. 2024. "New Frontiers in the Treatment of Patients with HER2+ Cancer and Brain Metastases: Is Radiotherapy Always Useful?" Cancers 16, no. 13: 2466. https://doi.org/10.3390/cancers16132466
APA StyleScandurra, G., Lombardo, V., Scibilia, G., Sambataro, D., Gebbia, V., Scollo, P., Pecorino, B., & Valerio, M. R. (2024). New Frontiers in the Treatment of Patients with HER2+ Cancer and Brain Metastases: Is Radiotherapy Always Useful? Cancers, 16(13), 2466. https://doi.org/10.3390/cancers16132466








