Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. Bokhman’s Pathogenetic Model
3. The WHO Classification
3.1. Historical Perspectives
3.2. Current WHO Classification
4. The Molecular Classification
4.1. Advantages
4.2. Limitations
4.2.1. Immunohistochemical Staining as a Surrogate
4.2.2. Diffusion and Accessibility
4.2.3. Non-Endometrioid Histotypes
4.2.4. NSMP
4.2.5. MMRd
4.2.6. The Prognostic Extremes: p53mut and POLEmut
4.3. Main Applications
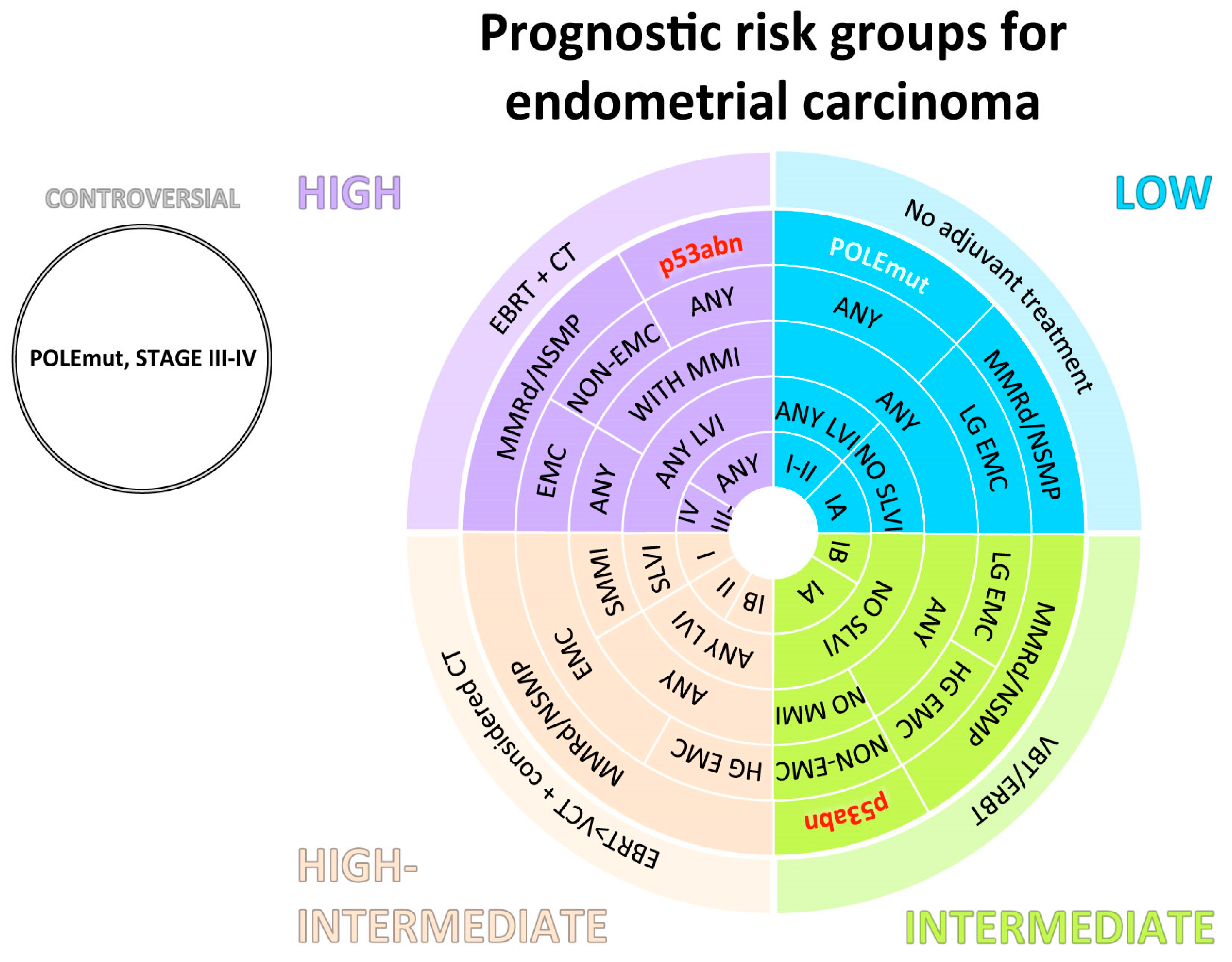
5. Standard Treatment
5.1. Surgical Therapy
5.2. Medical and Radiation Therapy
5.2.1. Adjuvant Setting
5.2.2. Recurrent and Metastatic Settings
6. Ongoing and Future Perspectives
6.1. Immunotherapy
6.2. PARP Inhibitors
6.3. Antibody–Drug Conjugates
6.4. ARID1A Inhibition
6.5. PI3K/AKT/mTOR Pathway Inhibitors
6.6. Other Drugs
6.7. Ongoing Trials Testing Tailored Therapies in Neoadjuvant/Adjuvant Setting
7. Fertility Preservation
8. Discussion
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.E. Histological Typing of Female Genital Tract Tumours, 2nd ed.; International histological classification of tumours; Springer: Berlin, Germany; New York, NY, USA, 1994; 189p. [Google Scholar]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Female Genital Tumours, 5th ed.; WHO Classification of Tumours Vol. 4; World Health Organization: Lyon, France; Geneva, Switzerland, 2020; 632p. [Google Scholar]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Shih, I.M.; Kurman, R.J. Ovarian tumorigenesis: A proposed model based on morphological and molecular genetic analysis. Am. J. Pathol. 2004, 164, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Kurman, R.J.; Shih, I.M. Ovarian Cancer Is an Imported Disease: Fact or Fiction? Curr. Obstet. Gynecol. Rep. 2012, 1, 1–9. [Google Scholar] [CrossRef]
- Kuhn, E.; Ayhan, A. Diagnostic immunohistochemistry in gynaecological neoplasia: A brief survey of the most common scenarios. J. Clin. Pathol. 2018, 71, 98–109. [Google Scholar] [CrossRef]
- Ross, D.S.; Devereaux, K.A.; Jin, C.; Lin, D.Y.; Zhang, Y.; Marra, A.; Makker, V.; Weigelt, B.; Ellenson, L.H.; Chui, M.H. Histopathologic features and molecular genetic landscape of HER2-amplified endometrial carcinomas. Mod. Pathol. 2022, 35, 962–971. [Google Scholar] [CrossRef]
- Vermij, L.; Horeweg, N.; Leon-Castillo, A.; Rutten, T.A.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Powell, M.E.; Singh, N.; Crosbie, E.J.; et al. HER2 Status in High-Risk Endometrial Cancers (PORTEC-3): Relationship with Histotype, Molecular Classification, and Clinical Outcomes. Cancers 2020, 13, 44. [Google Scholar] [CrossRef]
- Soslow, R.A.; Bissonnette, J.P.; Wilton, A.; Ferguson, S.E.; Alektiar, K.M.; Duska, L.R.; Oliva, E. Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: Similar outcomes belie distinctive biologic differences. Am. J. Surg. Pathol. 2007, 31, 979–987. [Google Scholar] [CrossRef]
- Poulsen, H.E.; Taylor, C.W.; Sobin, L.H. Histological Typing of Female Genital Tract Tumours; International Histological Classification of Tumours; World Health Organization: Geneva, Switzerland, 1975; 89p. [Google Scholar]
- World Health Organization; Tavassoli, F.A.; Devilee, P.; International Agency for Research on Cancer. Pathology and Genetics of Tumours of the Breast and Female Genital Organs; World Health Organisation Classification of Tumours; IARC Press: Lyon, France, 2003; 432p. [Google Scholar]
- Kurman, R.J.; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumours of Female Reproductive Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2014; Volume 6, p. 307. [Google Scholar]
- Wong, R.W.; Talia, K.L.; McCluggage, W.G. Endometrial Gastric-type Carcinoma: An Aggressive and Morphologically Heterogenous New Histotype Arising From Gastric Metaplasia of the Endometrium. Am. J. Surg. Pathol. 2020, 44, 1736–1737. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.; Clarke, B.A.; Akbari, M.; Makretsov, N.; Lim, P.; Miller, D.; Magliocco, A.; Coldman, A.; van de Rijn, M.; Huntsman, D.; et al. Identification of prognostically relevant and reproducible subsets of endometrial adenocarcinoma based on clustering analysis of immunostaining data. Mod. Pathol. 2007, 20, 1156–1165. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.; Köbel, M.; Kalloger, S.E.; Gilks, C.B. High-grade endometrial carcinoma: Serous and grade 3 endometrioid carcinomas have different immunophenotypes and outcomes. Int. J. Gynecol. Pathol. 2010, 29, 343–350. [Google Scholar] [CrossRef]
- Han, G.; Sidhu, D.; Duggan, M.A.; Arseneau, J.; Cesari, M.; Clement, P.B.; Ewanowich, C.A.; Kalloger, S.E.; Köbel, M. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod. Pathol. 2013, 26, 1594–1604. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Ding, J.; Cheang, M.C.; Wiegand, K.; Senz, J.; Tone, A.; Yang, W.; Prentice, L.; Tse, K.; Zeng, T.; et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012, 228, 20–30. [Google Scholar] [CrossRef]
- Catasus, L.; D’Angelo, E.; Pons, C.; Espinosa, I.; Prat, J. Expression profiling of 22 genes involved in the PI3K-AKT pathway identifies two subgroups of high-grade endometrial carcinomas with different molecular alterations. Mod. Pathol. 2010, 23, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining prognosis and identifying targetable pathways for high-risk endometrial cancer; a TransPORTEC initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef]
- Plotkin, A.; Kuzeljevic, B.; De Villa, V.; Thompson, E.F.; Gilks, C.B.; Clarke, B.A.; Köbel, M.; McAlpine, J.N. Interlaboratory Concordance of ProMisE Molecular Classification of Endometrial Carcinoma Based on Endometrial Biopsy Specimens. Int. J. Gynecol. Pathol. 2020, 39, 537–545. [Google Scholar] [CrossRef]
- Stelloo, E.; Nout, R.A.; Naves, L.C.; Ter Haar, N.T.; Creutzberg, C.L.; Smit, V.T.; Bosse, T. High concordance of molecular tumor alterations between pre-operative curettage and hysterectomy specimens in patients with endometrial carcinoma. Gynecol. Oncol. 2014, 133, 197–204. [Google Scholar] [CrossRef]
- Gilks, C.B.; Oliva, E.; Soslow, R.A. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am. J. Surg. Pathol. 2013, 37, 874–881. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; Gilvazquez, E.; Nout, R.; Smit, V.T.; McAlpine, J.N.; McConechy, M.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; et al. Clinicopathological and molecular characterisation of ‘multiple-classifier’ endometrial carcinomas. J. Pathol. 2020, 250, 312–322. [Google Scholar] [CrossRef]
- Kuhn, E.; Kurman, R.J.; Vang, R.; Sehdev, A.S.; Han, G.; Soslow, R.; Wang, T.L.; Shih Ie, M. TP53 mutations in serous tubal intraepithelial carcinoma and concurrent pelvic high-grade serous carcinoma—Evidence supporting the clonal relationship of the two lesions. J. Pathol. 2012, 226, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Léon-Castillo, A.; Singh, N.; Powell, M.E.; Edmondson, R.J.; Genestie, C.; Khaw, P.; Pyman, J.; McLachlin, C.M.; Ghatage, P.; et al. p53 immunohistochemistry in endometrial cancer: Clinical and molecular correlates in the PORTEC-3 trial. Mod. Pathol. 2022, 35, 1475–1483. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Talhouk, A.; Li-Chang, H.H.; Leung, S.; Huntsman, D.G.; Gilks, C.B.; McAlpine, J.N. Detection of DNA mismatch repair (MMR) deficiencies by immunohistochemistry can effectively diagnose the microsatellite instability (MSI) phenotype in endometrial carcinomas. Gynecol. Oncol. 2015, 137, 306–310. [Google Scholar] [CrossRef]
- Stelloo, E.; Jansen, A.M.L.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Ruano, D.; Church, D.N.; Morreau, H.; Smit, V.T.H.B.; van Wezel, T.; et al. Practical guidance for mismatch repair-deficiency testing in endometrial cancer. Ann. Oncol. 2017, 28, 96–102. [Google Scholar] [CrossRef]
- Bou Farhat, E.; Adib, E.; Daou, M.; Naqash, A.R.; Matulonis, U.; Ng, K.; Kwiatkowski, D.J.; Sholl, L.M.; Nassar, A.H. Benchmarking mismatch repair testing for patients with cancer receiving immunotherapy. Cancer Cell 2024, 42, 6–7. [Google Scholar] [CrossRef]
- Köbel, M.; Ronnett, B.M.; Singh, N.; Soslow, R.A.; Gilks, C.B.; McCluggage, W.G. Interpretation of P53 Immunohistochemistry in Endometrial Carcinomas: Toward Increased Reproducibility. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S123–S131. [Google Scholar] [CrossRef]
- Sari, A.; Pollett, A.; Eiriksson, L.R.; Lumsden-Johanson, B.; Van de Laar, E.; Kazerouni, H.; Salehi, A.; Sur, M.; Lytwyn, A.; Ferguson, S.E. Interobserver Agreement for Mismatch Repair Protein Immunohistochemistry in Endometrial and Nonserous, Nonmucinous Ovarian Carcinomas. Am. J. Surg. Pathol. 2019, 43, 591–600. [Google Scholar] [CrossRef]
- Ryan, N.; Wall, J.; Crosbie, E.J.; Arends, M.; Bosse, T.; Arif, S.; Faruqi, A.; Frayling, I.; Ganesan, R.; Hock, Y.L.; et al. Lynch syndrome screening in gynaecological cancers: Results of an international survey with recommendations for uniform reporting terminology for mismatch repair immunohistochemistry results. Histopathology 2019, 75, 813–824. [Google Scholar] [CrossRef]
- Riedinger, C.J.; Esnakula, A.; Haight, P.J.; Suarez, A.A.; Chen, W.; Gillespie, J.; Villacres, A.; Chassen, A.; Cohn, D.E.; Goodfellow, P.J.; et al. Characterization of mismatch-repair/microsatellite instability-discordant endometrial cancers. Cancer 2024, 130, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Bragantini, E.; Castiglione, F.; Ganesan, R.; Matias-Guiu, X.; Frattini, M.; Gallotta, V.; Garcia, P.; Pattni, Y.; Tsiampali-Laprell, J.; et al. Biomarker characterization in endometrial cancer in Europe: First survey data analysis from 69 pathological academic and hospital labs. Pathologica 2024, 116, 32–45. [Google Scholar] [CrossRef]
- Gotoh, O.; Sugiyama, Y.; Takazawa, Y.; Kato, K.; Tanaka, N.; Omatsu, K.; Takeshima, N.; Nomura, H.; Hasegawa, K.; Fujiwara, K.; et al. Clinically relevant molecular subtypes and genomic alteration-independent differentiation in gynecologic carcinosarcoma. Nat. Commun. 2019, 10, 4965. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Raffone, A.; Raimondo, D.; Arciuolo, D.; Angelico, G.; Valente, M.; Scaglione, G.; D’alessandris, N.; Casadio, P.; Inzani, F.; et al. Prognostic value of the TCGA molecular classification in uterine carcinosarcoma. Int. J. Gynaecol. Obstet. 2022, 158, 13–20. [Google Scholar] [CrossRef] [PubMed]
- DeLair, D.F.; Burke, K.A.; Selenica, P.; Lim, R.S.; Scott, S.N.; Middha, S.; Mohanty, A.S.; Cheng, D.T.; Berger, M.F.; Soslow, R.A.; et al. The genetic landscape of endometrial clear cell carcinomas. J. Pathol. 2017, 243, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Dong, F.; Vivero, M.; Shah, V.; Lindeman, N.; Schoolmeester, J.K.; Baltay, M.; MacConaill, L.; Sholl, L.M.; Nucci, M.R.; et al. Molecular Characterization of Neuroendocrine Carcinomas of the Endometrium: Representation in All 4 TCGA Groups. Am. J. Surg. Pathol. 2020, 44, 1541–1548. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, I.; Lee, C.H.; D’Angelo, E.; Palacios, J.; Prat, J. Undifferentiated and Dedifferentiated Endometrial Carcinomas with POLE Exonuclease Domain Mutations Have a Favorable Prognosis. Am. J. Surg. Pathol. 2017, 41, 1121–1128. [Google Scholar] [CrossRef]
- Nigon, E.; Lefeuvre-Plesse, C.; Martinez, A.; Chauleur, C.; Lortholary, A.; Favier, L.; Bats, A.S.; Guille, A.; AdélaÏde, J.; Finetti, P.; et al. Clinical, pathological, and comprehensive molecular analysis of the uterine clear cell carcinoma: A retrospective national study from TMRG and GINECO network. J. Transl. Med. 2023, 21, 408. [Google Scholar] [CrossRef]
- Kim, S.R.; Cloutier, B.T.; Leung, S.; Cochrane, D.; Britton, H.; Pina, A.; Storness-Bliss, C.; Farnell, D.; Huang, L.; Shum, K.; et al. Molecular subtypes of clear cell carcinoma of the endometrium: Opportunities for prognostic and predictive stratification. Gynecol. Oncol. 2020, 158, 3–11. [Google Scholar] [CrossRef]
- Reijnen, C.; Vrede, S.W.; Eijkelenboom, A.; Draak, R.; Sweegers, S.; Snijders, M.P.L.M.; van Gestel, P.; Pijnenborg, J.M.A.; Bulten, J.; Küsters-Vandevelde, H.V.N. Pure and mixed clear cell carcinoma of the endometrium: A molecular and immunohistochemical analysis study. Cancer Med. 2023, 12, 12365–12376. [Google Scholar] [CrossRef] [PubMed]
- McConechy, M.K.; Hoang, L.N.; Chui, M.H.; Senz, J.; Yang, W.; Rozenberg, N.; Mackenzie, R.; McAlpine, J.N.; Huntsman, D.G.; Clarke, B.A.; et al. In-depth molecular profiling of the biphasic components of uterine carcinosarcomas. J. Pathol. Clin. Res. 2015, 1, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, A.D.; Shen, H.; Walter, V.; Stewart, C.; Murray, B.A.; Bowlby, R.; Hu, X.; Ling, S.; Soslow, R.A.; Broaddus, R.R.; et al. Integrated Molecular Characterization of Uterine Carcinosarcoma. Cancer Cell 2017, 31, 411–423. [Google Scholar] [CrossRef]
- Huvila, J.; Jamieson, A.; Pors, J.; Hoang, L.; Mirkovic, J.; Cochrane, D.; McAlpine, J.N.; Gilks, C.B. Endometrial Carcinosarcomas are Almost Exclusively of p53abn Molecular Subtype After Exclusion of Mimics. Int. J. Gynecol. Pathol. 2024, 10–97. [Google Scholar] [CrossRef] [PubMed]
- Saijo, M.; Nakamura, K.; Ida, N.; Nasu, A.; Yoshino, T.; Masuyama, H.; Yanai, H. Histologic Appearance and Immunohistochemistry of DNA Mismatch Repair Protein and p53 in Endometrial Carcinosarcoma: Impact on Prognosis and Insights Into Tumorigenesis. Am. J. Surg. Pathol. 2019, 43, 1493–1500. [Google Scholar] [CrossRef]
- Kuhn, E.; Ayhan, A.; Bahadirli-Talbott, A.; Zhao, C.; Shih, I.M. Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am. J. Surg. Pathol. 2014, 38, 660–665. [Google Scholar] [CrossRef]
- Silva, E.G.; Deavers, M.T.; Malpica, A. Undifferentiated carcinoma of the endometrium: A review. Pathology 2007, 39, 134–138. [Google Scholar] [CrossRef]
- Rosa-Rosa, J.M.; Leskelä, S.; Cristóbal-Lana, E.; Santón, A.; López-García, M.; Muñoz, G.; Pérez-Mies, B.; Biscuola, M.; Prat, J.; Esther, O.E.; et al. Molecular genetic heterogeneity in undifferentiated endometrial carcinomas. Mod. Pathol. 2016, 29, 1594. [Google Scholar] [CrossRef]
- Köbel, M.; Hoang, L.N.; Tessier-Cloutier, B.; Meng, B.; Soslow, R.A.; Stewart, C.J.R.; Lee, C.H. Undifferentiated Endometrial Carcinomas Show Frequent Loss of Core Switch/Sucrose Nonfermentable Complex Proteins. Am. J. Surg. Pathol. 2018, 42, 76–83. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Liu, X.; Du, J.; Wang, Y.; Yang, J.; Li, Y.; Liu, C. Clinicopathological significance of multiple molecular features in undifferentiated and dedifferentiated endometrial carcinomas. Pathology 2021, 53, 179–186. [Google Scholar] [CrossRef]
- Köbel, M.; Meng, B.; Hoang, L.N.; Almadani, N.; Li, X.; Soslow, R.A.; Gilks, C.B.; Lee, C.H. Molecular Analysis of Mixed Endometrial Carcinomas Shows Clonality in Most Cases. Am. J. Surg. Pathol. 2016, 40, 166–180. [Google Scholar] [CrossRef]
- Espinosa, I.; D’Angelo, E.; Palacios, J.; Prat, J. Mixed and Ambiguous Endometrial Carcinomas: A Heterogenous Group of Tumors with Different Clinicopathologic and Molecular Genetic Features. Am. J. Surg. Pathol. 2016, 40, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Conlon, N.; Da Cruz Paula, A.; Ashley, C.W.; Segura, S.; De Brot, L.; da Silva, E.M.; Soslow, R.A.; Weigelt, B.; DeLair, D.F. Endometrial Carcinomas with a “Serous” Component in Young Women Are Enriched for DNA Mismatch Repair Deficiency, Lynch Syndrome, and POLE Exonuclease Domain Mutations. Am. J. Surg. Pathol. 2020, 44, 641–648. [Google Scholar] [CrossRef]
- Hopkins, M.R.; Palsgrove, D.N.; Ronnett, B.M.; Vang, R.; Lin, J.; Murdock, T.A. Molecular Analysis of HPV-independent Primary Endometrial Squamous Cell Carcinoma Reveals TP53 and CDKN2A Comutations: A Clinicopathologic Analysis with Re-evaluation of Diagnostic Criteria. Am. J. Surg. Pathol. 2022, 46, 1611–1622. [Google Scholar] [CrossRef]
- Lin, D.I.; Shah, N.; Tse, J.Y.; Killian, J.K.; Hemmerich, A.; Edgerly, C.; Haberberger, J.; Severson, E.A.; Huang, R.S.P.; Ramkissoon, S.H.; et al. Molecular profiling of mesonephric and mesonephric-like carcinomas of cervical, endometrial and ovarian origin. Gynecol. Oncol. Rep. 2020, 34, 100652. [Google Scholar] [CrossRef] [PubMed]
- Euscher, E.D.; Bassett, R.; Duose, D.Y.; Lan, C.; Wistuba, I.; Ramondetta, L.; Ramalingam, P.; Malpica, A. Mesonephric-like Carcinoma of the Endometrium: A Subset of Endometrial Carcinoma with an Aggressive Behavior. Am. J. Surg. Pathol. 2020, 44, 429–443. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Xu, C.; Deng, M.; Wu, D.; Tang, F.; Liu, X.; Han, Y.; Zhan, Y.; Miao, J. Risk stratification and molecular heterogeneity of endometrial cancer and expression profile of TIM-3: A retrospective cohort study. Gynecol. Oncol. 2023, 170, 210–220. [Google Scholar] [CrossRef]
- Momeni-Boroujeni, A.; Nguyen, B.; Vanderbilt, C.M.; Ladanyi, M.; Abu-Rustum, N.R.; Aghajanian, C.; Ellenson, L.H.; Weigelt, B.; Soslow, R.A. Genomic landscape of endometrial carcinomas of no specific molecular profile. Mod. Pathol. 2022, 35, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Mao, T.L.; Panuganti, P.K.; Kuhn, E.; Kurman, R.J.; Maeda, D.; Chen, E.; Jeng, Y.M.; Wang, T.L.; Shih, I.M. Mutation and loss of expression of ARID1A in uterine low-grade endometrioid carcinoma. Am. J. Surg. Pathol. 2011, 35, 625–632. [Google Scholar] [CrossRef]
- De Leo, A.; de Biase, D.; Lenzi, J.; Barbero, G.; Turchetti, D.; Grillini, M.; Ravegnini, G.; Angelini, S.; Zamagni, C.; Coluccelli, S.; et al. ARID1A and CTNNB1/β-Catenin Molecular Status Affects the Clinicopathologic Features and Prognosis of Endometrial Carcinoma: Implications for an Improved Surrogate Molecular Classification. Cancers 2021, 13, 950. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Kim, G.N.; Fellman, B.M.; Urbauer, D.L.; Mills, G.B.; Zhang, W.; Broaddus, R.R. CTNNB1 (beta-catenin) mutation identifies low grade, early stage endometrial cancer patients at increased risk of recurrence. Mod. Pathol. 2017, 30, 1032–1041. [Google Scholar] [CrossRef]
- Depreeuw, J.; Stelloo, E.; Osse, E.M.; Creutzberg, C.L.; Nout, R.A.; Moisse, M.; Garcia-Dios, D.A.; Dewaele, M.; Willekens, K.; Marine, J.C.; et al. Amplification of 1q32.1 Refines the Molecular Classification of Endometrial Carcinoma. Clin. Cancer Res. 2017, 23, 7232–7241. [Google Scholar] [CrossRef]
- Sengal, A.T.; Patch, A.M.; Snell, C.E.; Smith, D.S.; Leung, S.C.Y.; Talhouk, A.; Williams, E.D.; McAlpine, J.N.; Pollock, P.M. FGFR2c Mesenchymal Isoform Expression Is Associated with Poor Prognosis and Further Refines Risk Stratification within Endometrial Cancer Molecular Subtypes. Clin. Cancer Res. 2020, 26, 4569–4580. [Google Scholar] [CrossRef] [PubMed]
- Kommoss, F.K.; Karnezis, A.N.; Kommoss, F.; Talhouk, A.; Taran, F.A.; Staebler, A.; Gilks, C.B.; Huntsman, D.G.; Krämer, B.; Brucker, S.Y.; et al. L1CAM further stratifies endometrial carcinoma patients with no specific molecular risk profile. Br. J. Cancer 2018, 119, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Karnezis, A.N.; Leung, S.; Magrill, J.; McConechy, M.K.; Yang, W.; Chow, C.; Kobel, M.; Lee, C.H.; Huntsman, D.G.; Talhouk, A.; et al. Evaluation of endometrial carcinoma prognostic immunohistochemistry markers in the context of molecular classification. J. Pathol. Clin. Res. 2017, 3, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Bosquet, J.; Weroha, S.J.; Bakkum-Gamez, J.N.; Weaver, A.L.; McGree, M.E.; Dowdy, S.C.; Famuyide, A.O.; Kipp, B.R.; Halling, K.C.; Yadav, S.; et al. Prognostic stratification of endometrial cancers with high microsatellite instability or no specific molecular profile. Front. Oncol. 2023, 13, 1105504. [Google Scholar] [CrossRef]
- Ravegnini, G.; De Leo, A.; Coada, C.; Gorini, F.; de Biase, D.; Ceccarelli, C.; Dondi, G.; Tesei, M.; De Crescenzo, E.; Santini, D.; et al. Identification of miR-499a-5p as a Potential Novel Biomarker for Risk Stratification in Endometrial Cancer. Front. Oncol. 2021, 11, 757678. [Google Scholar] [CrossRef]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef]
- Perrone, E.; Capasso, I.; De Felice, F.; Giannarelli, D.; Dinoi, G.; Petrecca, A.; Palmieri, L.; Foresta, A.; Nero, C.; Arciuolo, D.; et al. Back to the future: The impact of oestrogen receptor profile in the era of molecular endometrial cancer classification. Eur. J. Cancer 2023, 186, 98–112. [Google Scholar] [CrossRef]
- McMeekin, D.S.; Tritchler, D.L.; Cohn, D.E.; Mutch, D.G.; Lankes, H.A.; Geller, M.A.; Powell, M.A.; Backes, F.J.; Landrum, L.M.; Zaino, R.; et al. Clinicopathologic Significance of Mismatch Repair Defects in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2016, 34, 3062–3068. [Google Scholar] [CrossRef]
- Carr, C.; Son, J.; Yao, M.; Priyadarshini, A.; Marquard, J.; Vargas, R.; Michener, C.; AlHilli, M.M. Clinicopathologic characteristics and outcomes of endometrial Cancer patients with mismatch repair deficiency in the era of universal Lynch syndrome screening. Gynecol. Oncol. 2020, 159, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Gambini, D.; Ferrero, S.; Kuhn, E. Lynch Syndrome: From Carcinogenesis to Prevention Interventions. Cancers 2022, 14, 4102. [Google Scholar] [CrossRef]
- Brett, M.A.; Atenafu, E.G.; Singh, N.; Ghatage, P.; Clarke, B.A.; Nelson, G.S.; Bernardini, M.Q.; Köbel, M. Equivalent Survival of p53 Mutated Endometrial Endometrioid Carcinoma Grade 3 and Endometrial Serous Carcinoma. Int. J. Gynecol. Pathol. 2021, 40, 116–123. [Google Scholar] [CrossRef]
- Ruscelli, M.; Maloberti, T.; Corradini, A.G.; Rosini, F.; Querzoli, G.; Grillini, M.; Altimari, A.; Gruppioni, E.; Sanza, V.; Costantino, A.; et al. Prognostic Impact of Pathologic Features in Molecular Subgroups of Endometrial Carcinoma. J. Pers. Med. 2023, 13, 723. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Angelico, G.; Travaglino, A.; Inzani, F.; Arciuolo, D.; Valente, M.; D’Alessandris, N.; Scaglione, G.; Fiorentino, V.; Raffone, A.; et al. New Pathological and Clinical Insights in Endometrial Cancer in View of the Updated ESGO/ESTRO/ESP Guidelines. Cancers 2021, 13, 2623. [Google Scholar] [CrossRef]
- Van Gool, I.C.; Rayner, E.; Osse, E.M.; Nout, R.A.; Creutzberg, C.L.; Tomlinson, I.P.M.; Church, D.N.; Smit, V.T.H.B.; de Wind, N.; Bosse, T.; et al. Adjuvant Treatment for POLE Proofreading Domain–Mutant Cancers: Sensitivity to Radiotherapy, Chemotherapy, and Nucleoside Analogues. Clin. Cancer Res. 2018, 24, 3197–3203. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, N.; Xie, X. The clinicopathological characteristics of POLE-mutated/ultramutated endometrial carcinoma and prognostic value of POLE status: A meta-analysis based on 49 articles incorporating 12,120 patients. BMC Cancer 2022, 22, 1157. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, T.; Li, N.; Yang, B.; Hu, Y. Clinicopathological characteristics and prognostic value of POLE mutations in endometrial cancer: A systematic review and meta-analysis. Medicine 2020, 99, e19281. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.S.; Al-Haddad, H.S.; McAllister, K.A.; Yasseen, A.A. The clinicopathology and survival characteristics of patients with POLE proofreading mutations in endometrial carcinoma: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263585. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- van den Heerik, A.S.V.M.; Horeweg, N.; Nout, R.A.; Lutgens, L.C.H.W.; van der Steen-Banasik, E.M.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe, F.L.A.; Kommoss, S.; et al. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef] [PubMed]
- van Weelden, W.J.; Massuger, L.F.A.G.; Pijnenborg, J.M.A.; Romano, A. Enitec EP645 Anti-estrogen treatment in endometrial cancer: A systematic review. Front. Oncol. 2019, 9, 359. [Google Scholar] [CrossRef] [PubMed]
- Decruze, S.B.; Green, J.A. Hormone therapy in advanced and recurrent endometrial cancer: A systematic review. Int. J. Gynecol. Cancer 2007, 17, 964–978. [Google Scholar] [CrossRef]
- Sorbe, B.; Andersson, H.; Boman, K.; Rosenberg, P.; Kalling, M. Treatment of primary advanced and recurrent endometrial carcinoma with a combination of carboplatin and paclitaxel-long-term follow-up. Int. J. Gynecol. Cancer 2008, 18, 803–808. [Google Scholar] [CrossRef]
- Halla, K. Emerging Treatment Options for Advanced or Recurrent Endometrial Cancer. J. Adv. Pract. Oncol. 2022, 13, 45–59. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular subtypes of endometrial cancer: Implications for adjuvant treatment strategies. Int. J. Gynaecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef]
- Karpel, H.C.; Powell, S.S.; Pothuri, B. Antibody-Drug Conjugates in Gynecologic Cancer. Am. Soc. Clin. Oncol. Educ. Book. 2023, 43, e390772. [Google Scholar] [CrossRef]
- Tinker, A.V.; Dhani, N.C.; Ghatage, P.; McLeod, D.; Samouëlian, V.; Welch, S.A.; Altman, A.D. A rapidly evolving landscape: Immune checkpoint inhibitors in pretreated metastatic endometrial cancer. Ther. Adv. Med. Oncol. 2023, 15, 17588359231157633. [Google Scholar] [CrossRef]
- Rubinstein, M.M.; Dickinson, S.; Narayan, P.; Zhou, Q.; Iasonos, A.; Ma, W.; Lakhman, Y.; Makker, V. Bevacizumab in advanced endometrial cancer. Gynecol. Oncol. 2021, 161, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Wu, R.C.; Guan, B.; Wu, G.; Zhang, J.; Wang, Y.; Song, L.; Yuan, X.; Wei, L.; Roden, R.B.; et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J. Natl. Cancer Inst. 2012, 104, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Choi, M.; Overton, J.D.; Bellone, S.; Roque, D.M.; Cocco, E.; Guzzo, F.; English, D.P.; Varughese, J.; Gasparrini, S.; et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl. Acad. Sci. USA 2013, 110, 2916–2921. [Google Scholar] [CrossRef] [PubMed]
- Rottmann, D.; Snir, O.L.; Wu, X.; Wong, S.; Hui, P.; Santin, A.D.; Buza, N. HER2 testing of gynecologic carcinosarcomas: Tumor stratification for potential targeted therapy. Mod. Pathol. 2020, 33, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Roque, D.M.; Siegel, E.; Buza, N.; Hui, P.; Abdelghany, O.; Chambers, S.; Secord, A.A.; Havrilesky, L.; O’Malley, D.M.; et al. Randomized Phase II Trial of Carboplatin-Paclitaxel Compared with Carboplatin-Paclitaxel-Trastuzumab in Advanced (Stage III-IV) or Recurrent Uterine Serous Carcinomas that Overexpress Her2/Neu (NCT01367002): Updated Overall Survival Analysis. Clin. Cancer Res. 2020, 26, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Pignata, S.; Califano, D.; Lorusso, D.; Arenare, L.; Bartoletti, M.; De Giorgi, U.; Andreetta, C.; Pisano, C.; Scambia, G.; Lombardi, D.; et al. MITO END-3: Efficacy of Avelumab immunotherapy according to molecular profiling in first-line endometrial cancer therapy. Ann. Oncol. 2024, 35, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Hasegawa, K.; Matsumoto, K.; Mori, M.; Hirashima, Y.; Takehara, K.; Ariyoshi, K.; Kato, T.; Yagishita, S.; Hamada, A.; et al. Trastuzumab Deruxtecan for Human Epidermal Growth Factor Receptor 2-Expressing Advanced or Recurrent Uterine Carcinosarcoma (NCCH1615): The STATICE Trial. J. Clin. Oncol. 2023, 41, 2789–2799. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef]
- Assaraf, Y.G.; Leamon, C.P.; Reddy, J.A. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist. Updat. 2014, 17, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Altwerger, G.; Bonazzoli, E.; Bellone, S.; Egawa-Takata, T.; Menderes, G.; Pettinella, F.; Bianchi, A.; Riccio, F.; Feinberg, J.; Zammataro, L.; et al. In vitro and in vivo activity of IMGN853, an antibody-drug conjugate targeting folate receptor alpha linked to DM4, in biologically aggressive endometrial cancers. Mol. Cancer Ther. 2018, 17, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Borghaei, H.; O’Malley, D.M.; Jeong, W.; Seward, S.M.; Bauer, T.M.; Perez, R.P.; Matulonis, U.A.; Running, K.L.; Zhang, X.; et al. Phase 1 dose-escalation study of mirvetuximab soravtansine (IMGN853), a folate receptor α-targeting antibody-drug conjugate, in patients with solid tumors. Cancer 2017, 123, 3080–3087. [Google Scholar] [CrossRef] [PubMed]
- Raji, R.; Guzzo, F.; Carrara, L.; Varughese, J.; Cocco, E.; Bellone, S.; Betti, M.; Todeschini, P.; Gasparrini, S.; Ratner, E.; et al. Uterine and ovarian carcinosarcomas overexpressing Trop-2 are sensitive to hRS7, a humanized anti-Trop-2 antibody. J. Exp. Clin. Cancer Res. 2011, 30, 106. [Google Scholar] [CrossRef] [PubMed]
- Bignotti, E.; Ravaggi, A.; Romani, C.; Falchetti, M.; Lonardi, S.; Facchetti, F.; Pecorelli, S.; Varughese, J.; Cocco, E.; Bellone, S.; et al. Trop-2 overexpression in poorly differentiated endometrial endometrioid carcinoma: Implications for immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Int. J. Gynecol. Cancer 2011, 21, 1613–1621. [Google Scholar] [CrossRef]
- Santin, A.; Komiya, T.; Goldenberg, D.M.; Sharkey, R.M.; Hong, Q.; Wegener, W.A.; Goswami, T.; Bardia, A. Sacituzumab govitecan (SG) in patients (pts) with previously treated metastatic endometrial cancer (mEC): Results from a phase I/II study. J. Clin. Oncol. 2020, 38, 6081. [Google Scholar] [CrossRef]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih, I.M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef]
- Suryo Rahmanto, Y.; Shen, W.; Shi, X.; Chen, X.; Yu, Y.; Yu, Z.C.; Miyamoto, T.; Lee, M.H.; Singh, V.; Asaka, R.; et al. Inactivation of Arid1a in the endometrium is associated with endometrioid tumorigenesis through transcriptional reprogramming. Nat. Commun. 2020, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.J.; Cantley, L.C. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature 2006, 441, 424–430. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Coleman, R.L. The PI3K/AKT/mTOR pathway as a therapeutic target in endometrial cancer. Clin. Cancer Res. 2012, 18, 5856–5864. [Google Scholar] [CrossRef]
- Colon-Otero, G.; Zanfagnin, V.; Hou, X.; Foster, N.R.; Asmus, E.J.; Wahner Hendrickson, A.; Jatoi, A.; Block, M.S.; Langstraat, C.L.; Glaser, G.E.; et al. Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers. ESMO Open 2020, 5, e000926. [Google Scholar] [CrossRef]
- Slomovitz, B.M.; Jiang, Y.; Yates, M.S.; Soliman, P.T.; Johnston, T.; Nowakowski, M.; Levenback, C.; Zhang, Q.; Ring, K.; Munsell, M.F.; et al. Phase II study of everolimus and letrozole in patients with recurrent endometrial carcinoma. J. Clin. Oncol. 2015, 33, 930–936. [Google Scholar] [CrossRef]
- Roncolato, F.; Lindemann, K.; Willson, M.L.; Martyn, J.; Mileshkin, L. PI3K/AKT/mTOR inhibitors for advanced or recurrent endometrial cancer. Cochrane Database Syst. Rev. 2019, 10, CD012160. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Lee, E.K.; Xiong, N.; Krasner, C.; Campos, S.; Kolin, D.L.; Liu, J.F.; Horowitz, N.; Wright, A.A.; Bouberhan, S.; et al. A Phase II, Two-Stage Study of Letrozole and Abemaciclib in Estrogen Receptor-Positive Recurrent Endometrial Cancer. J. Clin. Oncol. 2023, 41, 599–608. [Google Scholar] [CrossRef]
- Vergote, I.; Pérez-Fidalgo, J.A.; Hamilton, E.P.; Valabrega, G.; Van Gorp, T.; Sehouli, J.; Cibula, D.; Levy, T.; Welch, S.; Richardson, D.L.; et al. Oral Selinexor as Maintenance Therapy After First-Line Chemotherapy for Advanced or Recurrent Endometrial Cancer. J. Clin. Oncol. 2023, 41, 5400–5410. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Xiong, N.; Campos, S.M.; Wright, A.A.; Krasner, C.; Schumer, S.; Horowitz, N.; Veneris, J.; Tayob, N.; Morrissey, S.; et al. Phase II Study of the WEE1 Inhibitor Adavosertib in Recurrent Uterine Serous Carcinoma. J. Clin. Oncol. 2021, 39, 1531–1539. [Google Scholar] [CrossRef]
- Loukovaara, M.; Bützow, R.; Staff, S.; Mäenpää, M.; Faltinová, M.; Lassus, H.; Veijalainen, O.; Grönvall, M.; Vaalavirta, L.; Kuikka, E.; et al. PErsonalized TReatment for Endometrial Carcinoma (PETREC): Study design and methods of a prospective Finnish multicenter trial. Int. J. Gynecol. Cancer 2023, 33, 1807–1811. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, C.; Xie, H.; Chen, Y.; Lv, W.; Xie, X.; Wang, X. Molecular profile-based recommendations for postoperative adjuvant therapy in early endometrial cancer with high-intermediate or intermediate risk: A Chinese randomized phase III trial (PROBEAT). J. Gynecol. Oncol. 2023, 34, e37. [Google Scholar] [CrossRef] [PubMed]
- Consortium, R.R. Refining adjuvant treatment in endometrial cancer based on molecular features: The RAINBO clinical trial program. Int. J. Gynecol. Cancer 2022, 33, 109–117. [Google Scholar] [CrossRef]
- Guan, J.; Xue, Y.; Zang, R.Y.; Liu, J.H.; Zhu, J.Q.; Zheng, Y.; Wang, B.; Wang, H.Y.; Chen, X.J. Sentinel lymph Node mapping versus systematic pelvic lymphadenectomy on the prognosis for patients with intermediate-high-risk Endometrial Cancer confined to the uterus before surgery: Trial protocol for a non-inferiority randomized controlled trial (SNEC trial). J. Gynecol. Oncol. 2021, 32, e60. [Google Scholar] [CrossRef]
- Rodolakis, A.; Scambia, G.; Planchamp, F.; Acien, M.; Di Spiezio Sardo, A.; Farrugia, M.; Grynberg, M.; Pakiz, M.; Pavlakis, K.; Vermeulen, N.; et al. ESGO/ESHRE/ESGE Guidelines for the fertility-sparing treatment of patients with endometrial carcinoma. Hum. Reprod. Open 2023, 2023, hoac057. [Google Scholar] [CrossRef]
- Park, J.Y.; Nam, J.H. Progestins in the fertility-sparing treatment and retreatment of patients with primary and recurrent endometrial cancer. Oncologist 2015, 20, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, H.; Hu, R.; Liu, Y.; Liu, X.; Gu, L. Fertility-Preserving Treatment in Young Women with Grade 1 Presumed Stage IA Endometrial Adenocarcinoma: A Meta-Analysis. Int. J. Gynecol. Cancer 2018, 28, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Giampaolino, P.; Cafasso, V.; Boccia, D.; Ascione, M.; Mercorio, A.; Viciglione, F.; Palumbo, M.; Serafino, P.; Buonfantino, C.; De Angelis, M.C.; et al. Fertility-Sparing Approach in Patients with Endometrioid Endometrial Cancer Grade 2 Stage IA (FIGO): A Qualitative Systematic Review. BioMed Res. Int. 2022, 2022, 4070368. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, D.; Zhao, X.; Wang, C.; He, Y.; Chen, Y.; Wang, J.; Shen, D. Application of molecular classification to guiding fertility-sparing therapy for patients with endometrial cancer or endometrial intraepithelial neoplasia. Pathol. Res. Pract. 2023, 241, 154278. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.S.; Woo, H.Y.; Lee, J.Y.; Park, E.; Nam, E.J.; Kim, S.; Kim, S.W.; Kim, Y.T. Mismatch repair status influences response to fertility-sparing treatment of endometrial cancer. Am. J. Obstet. Gynecol. 2021, 224, 370.e1–370.e13. [Google Scholar] [CrossRef] [PubMed]
- Zakhour, M.; Cohen, J.G.; Gibson, A.; Walts, A.E.; Karimian, B.; Baltayan, A.; Aoyama, C.; Garcia, L.; Dhaliwal, S.K.; Elashoff, D.; et al. Abnormal mismatch repair and other clinicopathologic predictors of poor response to progestin treatment in young women with endometrial complex atypical hyperplasia and well-differentiated endometrial adenocarcinoma: A consecutive case series. BJOG 2017, 124, 1576–1583. [Google Scholar] [CrossRef]
- Agusti, N.; Kanbergs, A.; Nitecki, R. Potential of molecular classification to guide fertility-sparing management among young patients with endometrial cancer. Gynecol. Oncol. 2024, 185, 121–127. [Google Scholar] [CrossRef]

| 1975 WHO Classification (1st Edition) | 1994 WHO Classification (2nd Edition) | 2003 WHO Classification (3rd Edition) | 2014 WHO Classification (4th Edition) | 2020 WHO Classification (5th Edition) |
|---|---|---|---|---|
| Endometrial Carcinoma | ||||
| Adenocarcinoma Clear cell (mesonephroid) adenocarcinoma Squamous cell carcinoma Adenosquamous [mucoepidermoid carcinoma] Undifferentiated carcinoma | Endometrioid adenocarcinoma Secretory (variant) Ciliated cell (variant) Adenocarcinoma with squamous differentiation (adenoacanthoma; adenosquamous carcinoma) Serous adenocarcinoma Clear cell adenocarcinoma Mucinous adenocarcinoma Squamous cell carcinoma Mixed carcinoma Undifferentiated carcinoma | Endometrioid adenocarcinoma Variant with squamous differentiation Villoglandular variant Secretory variant Ciliated cell variant Mucinous adenocarcinoma Serous adenocarcinoma Clear cell adenocarcinoma Mixed cell adenocarcinoma Squamous cell carcinoma Transitional cell carcinoma Small cell carcinoma Undifferentiated carcinoma Others | Endometrioid carcinoma Squamous differentiation Villoglandular Secretory Mucinous carcinoma Serous endometrial intraepithelial carcinoma (SEIC) Serous carcinoma Clear cell carcinoma Neuroendocrine tumors Low-grade neuroendocrine tumor Carcinoid tumor High-grade neuroendocrine carcinoma Small cell neuroendocrine carcinoma Large cell neuroendocrine carcinoma Mixed cell adenocarcinoma Undifferentiated carcinoma Dedifferentiated carcinoma | Endometrioid adenocarcinoma POLE-ultramutated endometrioid carcinoma Mismatch repair-deficient endometrioid carcinoma p53-mutant endometrioid carcinoma No specific molecular profile (NSMP) endometrioid carcinoma Serous carcinoma NOS Clear cell adenocarcinoma NOS Carcinoma, undifferentiated, NOS Mixed cell adenocarcinoma Mesonephric adenocarcinoma Squamous cell carcinoma NOS Mucinous carcinoma, intestinal type Mesonephric-like adenocarcinoma Carcinosarcoma NOS |
| Trial | Acronym | Number | Type | Patients | Aim |
|---|---|---|---|---|---|
| Molecular Profile-based Versus Standard Recommendations for Adjuvant Radiotherapy for Women With Early Stage ECa [90] | PORTEC4a | NCT03469674 | Prospective, multicenter, randomized phase III | High-intermediate risk EC | No adjuvant therapy, VBT or EBRT vs. standard adjuvant VBT |
| PErsonalized TReatment for EC [122] | PETREC | NCT05655260 | Prospective, multicenter | Stage I–II high-intermediate or high-risk EC | Chemotherapy vs. chemoradiotherapy in p53abn and non-endometrioid ECs; VBT vs. EBRT in MMRd and NSMP |
| PROfiling-Based EC Adjuvant Therapy [123] | PROBEAT | NCT05179447 | Prospective, multicenter, randomized phase III | High-intermediate and intermediate risk endometrioid ECs | No adj therapy, VBT, EBRT, or CTRT based on molecular features vs. standard RT |
| Refining Adjuvant Treatment IN ECa Based On Molecular Features [124] a. p53abn-RED trial b. MMRd EC to the MMRd-GREEN trial c. NSMP EC to NSMP-ORANGE trial d. POLEmut EC to the POLEmut-BLUE trial | RAINBO | NCT05255653 | Umbrella program Randomized phase III Randomized phase III Randomized phase III Single arm phase II | Ecs eligible for adjuvant treatment | AdjCTRT + olaparib vs. adjCTRT adjEBRT + durvalumab vs. adjEBRT EBRT + Pg vs. CTRT Safety de-escalation |
| Tailored Adjuvant therapy in POLE-Mutated and p53-Wildtype/NSMP Early Stage EC a. EN10.A/RAINBO BLUE: POLE-mutated EC b. EN10.B/TAPER: p53 wildtype/NSMP EC | NCT05640999 | Not randomized, open label phase III | POLE-mute or p53wt/NSMP (p53 wt/NSMP) EC | Testing de-escalated adj treatment | |
| Letrozole as Maintenance Therapy for Post-surgical ECa Patients With NSMP | NCT05454358 | Phase II/III open label, multicenter, superiority randomized controlled | NSMP surgically treated EC pts | Letrozole as maintenance therapy on the prognosis of post-operative NSMP EC | |
| Neo-adjuvant Pembrolizumab as an Alternative Treatment for MMRd Uterine Cancer | PAM-II | NCT06180733 | Phase II | Confirmed primary diagnosis of G3/CC MMRd EC who are intended to be treated with hysterectomy | To establish fraction of patients acquiring a MPR after nine cycles of pembrolizumab |
| Early Stage ECa Based on Molecular Classification and Traditional Risk Stratification to Guide Adjuvant Radiotherapy Decisions | NCT05524389 | Phase III prospective, multicenter, randomized, open, non-inferiority | Stage I-II ECs | To assess 3-year LRR after adj RT based on molecular classification | |
| Radiomics and Radiogenomics Models to Predict Molecular Integrated Risk Classes and Prognostic Factors in ECa | ROMANTIC | NCT06279832 | Interventional | Stage IA/IB ECs | To develop radiogenomics models to stratify pts into three main risk categories according to the ProMisE model |
| Study and Transformation of Tumor Molecular Features Screening Model of EC Surgical Approach | NCT05894915 | Prospective randomized controlled | ECs without high mutational burden characteristics (including POLE mutations, MSI-H, homologous recombinant repair pathway mutations) | To assess the impact of surgical routes on the short-term safety and long-term prognosis of EC pts with different molecular characteristics | |
| Sentinel Lymph Node Sampling (SLN) for Patients With Middle-high Risk ECa Confined to the Uterus [125] | SNEC | NCT04276532 | Non-inferiority randomized controlled | Intermediate-high risk EC | To investigate the effect of SLN sampling on the prognosis |
| Patient-derived Tumor-like Cell Clusters Predict Progesterone Sensitivity in Patients With Early ECa | NCT05647109 | Observational | AEH/well-differentiated ECs G1 without myometrial invasion | To construct a prediction model of Pg sensitivity in pts with EC treated with fertility preservation | |
| feasibility safety Efficacy dostarLimab earLy-stage defIcient endomeTrial cancEr | SATELLITE | NCT06278857 | Phase IIb, open-label, single arm, multicenter, pilot study | Early-stage MMRd EC | Dostarlimab as a potential alternative to surgery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, E.; Gambini, D.; Runza, L.; Ferrero, S.; Scarfone, G.; Bulfamante, G.; Ayhan, A. Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications. Cancers 2024, 16, 2458. https://doi.org/10.3390/cancers16132458
Kuhn E, Gambini D, Runza L, Ferrero S, Scarfone G, Bulfamante G, Ayhan A. Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications. Cancers. 2024; 16(13):2458. https://doi.org/10.3390/cancers16132458
Chicago/Turabian StyleKuhn, Elisabetta, Donatella Gambini, Letterio Runza, Stefano Ferrero, Giovanna Scarfone, Gaetano Bulfamante, and Ayse Ayhan. 2024. "Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications" Cancers 16, no. 13: 2458. https://doi.org/10.3390/cancers16132458
APA StyleKuhn, E., Gambini, D., Runza, L., Ferrero, S., Scarfone, G., Bulfamante, G., & Ayhan, A. (2024). Unsolved Issues in the Integrated Histo-Molecular Classification of Endometrial Carcinoma and Therapeutic Implications. Cancers, 16(13), 2458. https://doi.org/10.3390/cancers16132458







