Pre-Existing Immunity Predicts Response to First-Line Immunotherapy in Non-Small Cell Lung Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Blood Collection
2.2. Lymphocyte Isolation
2.3. Pre-Existing Immunity Detection and Analysis
2.4. Flow Cytometry Analysis
2.5. Statisical Analysis
3. Results
3.1. Pre-Existing TAA-Specific T Cells in the Circulation of NSCLC Patients
3.2. Pre-Existing TAA-Specific T Cells and Clinical Response
3.3. Immune Effectors in the Circulation of Pre-Existing Immunity Patients
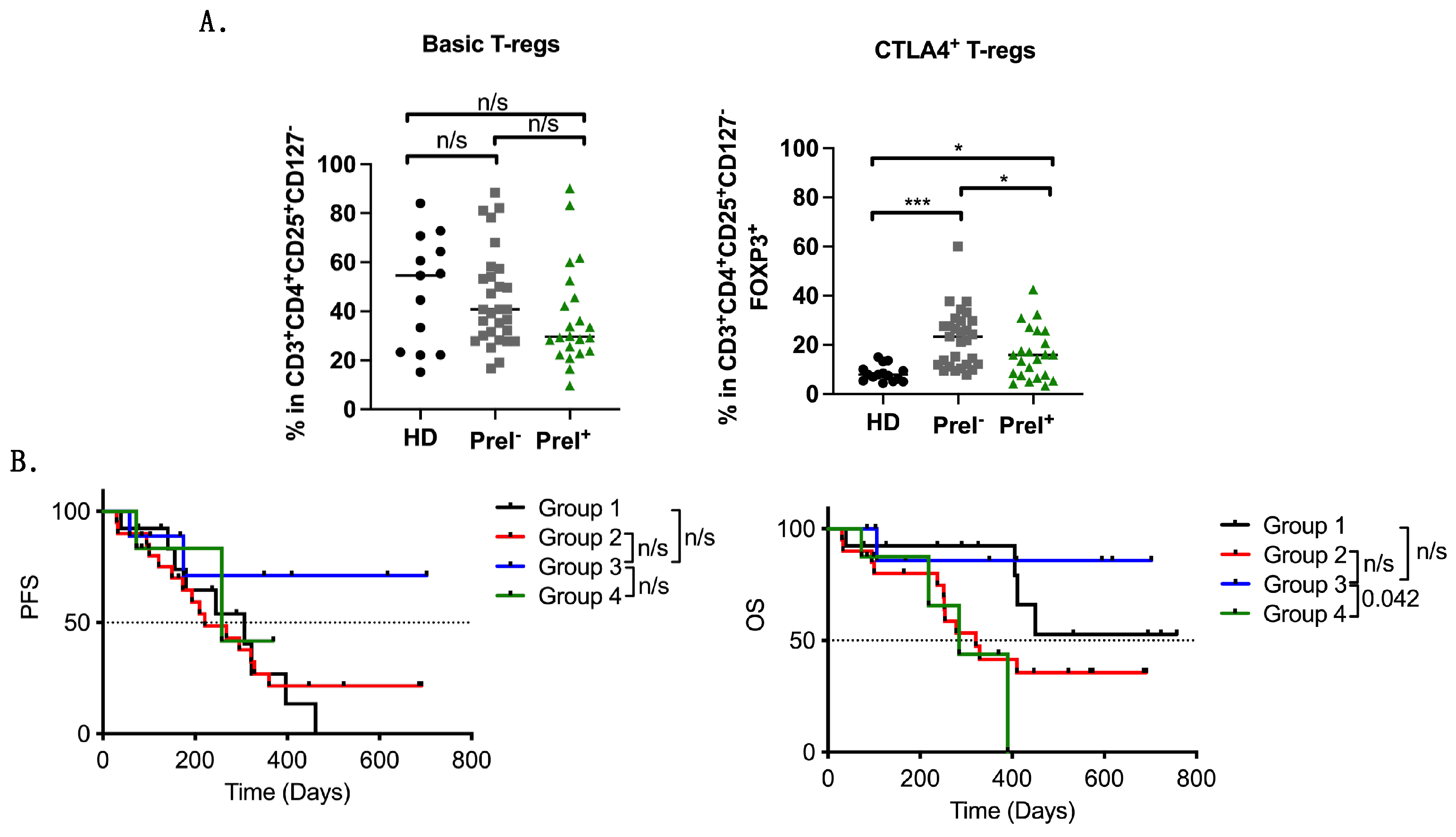
3.4. Peripheral Blood Immune Suppressor Cells in PreI+ Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Valerio, T.S.; Emmerick, I.C.M.; Sobreira-da-Silva, M.J. Factors associated with late-stage diagnosis and overall survival for lung cancer: An analysis of patients treated in a Brazilian hospital and a US-hospital from 2009 to 2019. Cancer Epidemiol. 2023, 86, 102443. [Google Scholar] [CrossRef] [PubMed]
- Sidaway, P. From ESMO 2023: Advances in lung cancer. Nat. Rev. Clin. Oncol. 2024, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non-Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Thienpont, B.; Zhao, L.; Wolf, D.; Pircher, A. Overcoming immunotherapy resistance in non-small cell lung cancer (NSCLC)-novel approaches and future outlook. Mol. Cancer 2020, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.; Wu, C.J. Dynamics and specificities of T cells in cancer immunotherapy. Nat. Rev. Cancer 2023, 23, 295–316. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.L.M.; Neefjes, J.; Spaapen, R.M. Playing hide and seek: Tumor cells in control of MHC class I antigen presentation. Mol. Immunol. 2021, 136, 36–44. [Google Scholar] [CrossRef]
- Gonzalez, H.; Hagerling, C.; Werb, Z. Roles of the immune system in cancer: From tumor initiation to metastatic progression. Genes Dev. 2018, 32, 1267–1284. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Bolonakis, I.; Cornet, S.; Myllaki, G.; Kanellou, P.; Kotsakis, A.; Galanis, A.; Nikoloudi, I.; Spyropoulou, M.; Menez, J.; et al. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology 2006, 70, 306–314. [Google Scholar] [CrossRef]
- Kotsakis, A.; Vetsika, E.K.; Christou, S.; Hatzidaki, D.; Vardakis, N.; Aggouraki, D.; Konsolakis, G.; Georgoulias, V.; Christophyllakis, C.; Cordopatis, P.; et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: Results of an expanded phase II study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2012, 23, 442–449. [Google Scholar] [CrossRef]
- Gridelli, C.; Ciuleanu, T.; Domine, M.; Szczesna, A.; Bover, I.; Cobo, M.; Kentepozidis, N.; Zarogoulidis, K.; Kalofonos, C.; Kazarnowisz, A.; et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell lung cancer: Final results of a randomised phase 2 clinical trial. Br. J. Cancer 2020, 122, 1461–1466. [Google Scholar] [CrossRef]
- Pateras, I.S.; Kotsakis, A.; Avgeris, M.; Baliou, E.; Kouroupakis, P.; Patsea, E.; Georgoulias, V.; Menez-Jamet, J.; Kinet, J.P.; Kosmatopoulos, K. Clinical Activity of an hTERT-Specific Cancer Vaccine (Vx-001) in “Immune Desert” NSCLC. Cancers 2021, 13, 1658. [Google Scholar] [CrossRef] [PubMed]
- Xagara, A.; Fortis, S.P.; Goulielmaki, M.; Koinis, F.; Chantzara, E.; Kokkalis, A.; Samaras, I.; Papadopoulos, V.; Georgoulias, V.; Baxevanis, C.N.; et al. Peripheral pre-existing cancer-antigen specific T-cell immunity as predictive biomarker for immunotherapy in NSCLC patients. J. Clin. Oncol. 2023, 41, e20504. [Google Scholar] [CrossRef]
- Xagara, A.; Roumeliotou, A.; Kokkalis, A.; Tsapakidis, K.; Papakonstantinou, D.; Papadopoulos, V.; Samaras, I.; Chantzara, E.; Kallergi, G.; Kotsakis, A. ES-SCLC Patients with PD-L1+ CTCs and High Percentages of CD8+PD-1+T Cells in Circulation Benefit from Front-Line Immunotherapy Treatment. Biomedicines 2024, 12, 146. [Google Scholar] [CrossRef] [PubMed]
- Amir, E.; Seruga, B.; Kwong, R.; Tannock, I.F.; Ocaña, A. Poor correlation between progression-free and overall survival in modern clinical trials: Are composite endpoints the answer? Eur. J. Cancer 2012, 48, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Pasalic, D.; McGinnis, G.J.; Fuller, C.D.; Grossberg, A.J.; Verma, V.; Mainwaring, W.; Miller, A.B.; Lin, T.A.; Jethanandani, A.; Espinoza, A.F.; et al. Progression-free survival is a suboptimal predictor for overall survival among metastatic solid tumour clinical trials. Eur. J. Cancer 2020, 136, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Weide, B.; Zelba, H.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Di Giacomo, A.M.; Maio, M.; Aarntzen, E.H.; de Vries, I.J.; Sucker, A.; et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Bolonaki, I.; Kotsakis, A.; Papadimitraki, E.; Aggouraki, D.; Konsolakis, G.; Vagia, A.; Christophylakis, C.; Nikoloudi, I.; Magganas, E.; Galanis, A.; et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2007, 25, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Vetsika, E.K.; Konsolakis, G.; Aggouraki, D.; Kotsakis, A.; Papadimitraki, E.; Christou, S.; Menez-Jamet, J.; Kosmatopoulos, K.; Georgoulias, V.; Mavroudis, D. Immunological responses in cancer patients after vaccination with the therapeutic telomerase-specific vaccine Vx-001. Cancer Immunol. Immunother. CII 2012, 61, 157–168. [Google Scholar] [CrossRef]
- Kotsakis, A.; Papadimitraki, E.; Vetsika, E.K.; Aggouraki, D.; Dermitzaki, E.K.; Hatzidaki, D.; Kentepozidis, N.; Mavroudis, D.; Georgoulias, V. A phase II trial evaluating the clinical and immunologic response of HLA-A2(+) non-small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer 2014, 86, 59–66. [Google Scholar] [CrossRef]
- Rooney, M.S.; Shukla, S.A.; Wu, C.J.; Getz, G.; Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015, 160, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Fratta, E.; Coral, S.; Covre, A.; Parisi, G.; Colizzi, F.; Danielli, R.; Nicolay, H.J.; Sigalotti, L.; Maio, M. The biology of cancer testis antigens: Putative function, regulation and therapeutic potential. Mol. Oncol. 2011, 5, 164–182. [Google Scholar] [CrossRef] [PubMed]
- Zelba, H.; Weide, B.; Martens, A.; Derhovanessian, E.; Bailur, J.K.; Kyzirakos, C.; Pflugfelder, A.; Eigentler, T.K.; Di Giacomo, A.M.; Maio, M.; et al. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by melan-A but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 4390–4399. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Tran, E.; Parkhurst, M.R.; Anna, P.; Ilyas, S.; Prickett, T.D.; Gartner, J.J.; Robbins, P.F.; Crystal, J.S.; Trebska-Mcgowan, K.; et al. Selection of circulating PD-1+ lymphocytes from cancer patients enriches for tumor-reactive and mutation-specific lymphocytes. J. ImmunoTherapy Cancer 2015, 3, O2. [Google Scholar] [CrossRef][Green Version]
- Pawelec, G. Immune correlates of clinical outcome in melanoma. Immunology 2018, 153, 415–422. [Google Scholar] [CrossRef] [PubMed]
- González-Navajas, J.M.; Fan, D.D.; Yang, S.; Yang, F.M.; Lozano-Ruiz, B.; Shen, L.; Lee, J. The Impact of Tregs on the Anticancer Immunity and the Efficacy of Immune Checkpoint Inhibitor Therapies. Front. Immunol. 2021, 12, 625783. [Google Scholar] [CrossRef] [PubMed]
- Kotsakis, A.; Koinis, F.; Katsarou, A.; Gioulbasani, M.; Aggouraki, D.; Kentepozidis, N.; Georgoulias, V.; Vetsika, E.K. Prognostic value of circulating regulatory T cell subsets in untreated non-small cell lung cancer patients. Sci. Rep. 2016, 6, 39247. [Google Scholar] [CrossRef] [PubMed]
- Principe, D.R.; Chiec, L.; Mohindra, N.A.; Munshi, H.G. Regulatory T-Cells as an Emerging Barrier to Immune Checkpoint Inhibition in Lung Cancer. Front. Oncol. 2021, 11, 684098. [Google Scholar] [CrossRef] [PubMed]
- Gallina, G.; Dolcetti, L.; Serafini, P.; De Santo, C.; Marigo, I.; Colombo, M.P.; Basso, G.; Brombacher, F.; Borrello, I.; Zanovello, P.; et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Investig. 2006, 116, 2777–2790. [Google Scholar] [CrossRef]
- Lindau, D.; Gielen, P.; Kroesen, M.; Wesseling, P.; Adema, G.J. The immunosuppressive tumour network: Myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013, 138, 105–115. [Google Scholar] [CrossRef]
- Peranzoni, E.; Zilio, S.; Marigo, I.; Dolcetti, L.; Zanovello, P.; Mandruzzato, S.; Bronte, V. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr. Opin. Immunol. 2010, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Guo, J.; Weng, L.; Tang, W.; Jin, S.; Ma, W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J. Hematol. Oncol. 2020, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Vetsika, E.K.; Koinis, F.; Gioulbasani, M.; Aggouraki, D.; Koutoulaki, A.; Skalidaki, E.; Mavroudis, D.; Georgoulias, V.; Kotsakis, A. A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients. J. Immunol. Res. 2014, 2014, 659294. [Google Scholar] [CrossRef] [PubMed]
- Koinis, F.; Vetsika, E.K.; Aggouraki, D.; Skalidaki, E.; Koutoulaki, A.; Gkioulmpasani, M.; Georgoulias, V.; Kotsakis, A. Effect of First-Line Treatment on Myeloid-Derived Suppressor Cells’ Subpopulations in the Peripheral Blood of Patients with Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 1263–1272. [Google Scholar] [CrossRef] [PubMed]
- Bronte, G.; Petracci, E.; De Matteis, S.; Canale, M.; Zampiva, I.; Priano, I.; Cravero, P.; Andrikou, K.; Burgio, M.A.; Ulivi, P.; et al. High Levels of Circulating Monocytic Myeloid-Derived Suppressive-Like Cells Are Associated with the Primary Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: An Exploratory Analysis. Front. Immunol. 2022, 13, 866561. [Google Scholar] [CrossRef]
- Bailur, J.K.; Gueckel, B.; Derhovanessian, E.; Pawelec, G. Presence of circulating Her2-reactive CD8+T-cells is associated with lower frequencies of myeloid-derived suppressor cells and regulatory T cells, and better survival in older breast cancer patients. Breast Cancer Res. BCR 2015, 17, 34. [Google Scholar] [CrossRef]



| Stage IIIb (n = 35) | Stage III & IV (n = 17) | All Patients (n = 52) | ||
|---|---|---|---|---|
| Characteristics | Sub-Categories | Values | Values | Values |
| Median age | 70 years (range 48–86 years) | 70 years (range 52–82 years) | 70 years (range 48–86 years) | |
| Gender | Male | 28 (80%) | 12 (70.5%) | 40 (77%) |
| Female | 7 (20%) | 5 (29.5%) | 12 (23%) | |
| Stage | IIIb | 35 (100%) | 0 (0%) | 35 (67%) |
| III (other than IIIb) | 0 (0%) | 10 (59%) | 10 (19%) | |
| IV | 0 (0%) | 6 (35%) | 6 (12%) | |
| Unknown | 0 (0%) | 1 (6%) | 1 (2%) | |
| Location of primary tumor | Left lung | 12 (34%) | 4 (23.5%) | 16 (31%) |
| Right lung | 22 (63%) | 10 (59%) | 32 (61.5%) | |
| Both lungs | 1 (3%) | 0 (0%) | 1 (2%) | |
| Unknown | 0 (0%) | 3 (17.5%) | 3 (5.5%) | |
| Histological Type | Adenocarcinoma | 17 (49%) | 9 (53%) | 26 (50%) |
| Squamous | 18 (51%) | 6 (35%) | 24 (46%) | |
| Unknown | 0 (0%) | 2 (12%) | 2 (4%) | |
| Smoking Status | Never | 4 (11.5%) | 0 (0%) | 4 (8%) |
| Former | 20 (57%) | 3 (17.5%) | 23 (44%) | |
| Curent | 11 (31.5%) | 10 (59%) | 21 (40%) | |
| Unknown | 0 (0%) | 4 (23.5%) | 4 (8%) | |
| <40 pack year | 4 (11%) | 5 (30%) | 9 (17%) | |
| 40–80 pack year | 13 (37%) | 2 (11.5%) | 15 (29%) | |
| >80 pack year | 8 (23%) | 2 (11.5%) | 10 (19%) | |
| Unknown | 10 (29%) | 8 (47%) | 18 (35%) |
| PFS | OS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| T-Cell Populations | ROC Cut Off | n | Median | 95% HR CI | p Value | Median | 95% HR CI | p Value | ||
| % in CD3+CD8+ | PD1 | 25 | High | 40 | 268 | 0.437 to 2.173 | 0.949 | 411 | 0.445 to 3.100 | 0.744 |
| Low | 12 | 296 | 390 | |||||||
| % in CD3+CD8+ CD45RA+CD45RO- | Tnaive (RA+RO-CCR7+) | 55 | High | 17 | 397 | 0.250 to 1.477 | 0.271 | und | 0.303 to 2.575 | 0.821 |
| Low | 35 | 258 | 411 | |||||||
| Teff (RA+RO-CCR7−) | 40 | High | 36 | 268 | 0.273 to 2.295 | 0.668 | 450 | 0.150 to 1.718 | 0.276 | |
| Low | 16 | 329 | 284 | |||||||
| % in CD3+CD8+ CD45RA-CD45RO+ | Tcm (RA-RO+CCR7+) | 70 | High | 16 | 245 | 0.390 to 4.029 | 0.704 | und | 0.283 to 3.310 | 0.958 |
| Low | 36 | 296 | 411 | |||||||
| Tem (RA-RO+CCR7−) | 26 | High | 35 | 307 | 0.141 to 1.380 | 0.159 | 450 | 0.126 to 1.152 | 0.087 | |
| Low | 17 | 258 | 284 | |||||||
| % in CD3+CD4+ | PD1 | 2.2 | High | 35 | 321 | 0.202 to 1.187 | 0.114 | und | 0.093 to 0.712 | 0.0089 |
| Low | 17 | 221 | 278 | |||||||
| % in CD3+CD4+ CD45RA+CD45RO- | Tnaive (RA+RO-CCR7+) | 87 | High | 17 | 268 | 0.437 to 1.373 | 0.273 | 329 | 0.314 to 1.147 | 0.271 |
| Low | 35 | 296 | 450 | |||||||
| Teff (RA+RO-CCR7−) | 13 | High | 37 | 321 | 0.249 to 1.332 | 0.197 | und | 0.237 to 1.552 | 0.297 | |
| Low | 15 | 268 | 329 | |||||||
| % in CD3+CD4+ CD45RA-CD45RO+ | Tcm (RA-RO+CCR7+) | 55 | High | 27 | 221 | 0.485 to 2.214 | 0.926 | 411 | 0.328 to 1.943 | 0.620 |
| Low | 25 | 307 | 390 | |||||||
| Tem (RA-RO+CCR7−) | 40 | High | 26 | 296 | 0.463 to 2.127 | 0.984 | 450 | 0.509 to 3.00 | 0.637 | |
| Low | 26 | 307 | 411 | |||||||
| T Reg Cells | |||
|---|---|---|---|
| CD3CD4FOXP3 | PreI− | PreI+ | |
| Mean | 8.39 | 7.76 | |
| Std.Error | 1.106 | 1.163 | |
| p-value | 0.678 | ||
| CD25+CD127− | Mean | 5.55 | 5.91 |
| Std.Error | 0.808 | 0.790 | |
| p-value | 0.397 | ||
| Basic Tregs CD25+CD127-FOXP3+ | Mean | 44.97 | 37.57 |
| Std.Error | 3.622 | 4.392 | |
| p-value | 0.118 | ||
| CTLA4+ Tregs CD25+CD127-FOXP3+CTLA4+ | Mean | 22.55 | 16.29 |
| Std.Error | 2.19 | 2.16 | |
| p-value | 0.049 | ||
| MDSCs | |||
| CD14+CD15− M-MDSCs (%in CD33+CD11b+HLA-DR-Lin-) | PreI− | PreI+ | |
| Mean | 3.38 | 3.18 | |
| Std.Error | 0.34 | 0.39 | |
| p-value | 0.713 | ||
| CD14+CD15- iNOS+ M-MDSCs (%in CD33+CD11b+HLA-DR-Lin- CD14+CD15-) | Mean | 24.60 | 22.29 |
| Std.Error | 3.085 | 3.038 | |
| p-value | 0.551 | ||
| CD14+CD15+ M-MDSCs (%in CD33+CD11b+HLA-DR-Lin-) | Mean | 1.069 | 1.327 |
| Std.Error | 0.141 | 0.207 | |
| p-value | 0.294 | ||
| CD14+CD15+ iNOS+ M-MDSCs (%in CD33+CD11b+HLA-DR-Lin- CD14+CD15+) | Mean | 35.08 | 34.54 |
| Std.Error | 2.59 | 3.59 | |
| p-value | 0.653 | ||
| Cell Populations | ROC Cut Off | n | Median | 95% HR CI | p Value | Median | 95% HR CI | p Value | |
|---|---|---|---|---|---|---|---|---|---|
| CD3CD4FOXP3 | 5 | High | 31 | 321 | 0.254 to 1.198 | 0.133 | 450 | 0.173 to 1.066 | 0.068 |
| Low | 21 | 210 | 390 | ||||||
| CD25+CD127- | 2.7 | High | 38 | 321 | 0.384 to 1.977 | 0.742 | und | 0.381 to 2.288 | 0.882 |
| Low | 14 | 201 | 411 | ||||||
| Basic Tregs | 43 | High | 18 | 268 | 0.572 to 2.625 | 0.600 | 411 | 0.392 to 2.209 | 0.871 |
| Low | 34 | 296 | und | ||||||
| CTLA4+ Tregs | 11 | High | 34 | 268 | 0.908 to 4.680 | 0.083 | 411 | 0.476 to 3.229 | 0.658 |
| Low | 18 | und | und | ||||||
| CD14+CD15- M-MDSCs | 2.3 | High | 35 | 268 | 0.444 to 2.18 | 0.967 | 411 | 0.387 to 2.404 | 0.938 |
| Low | 17 | 296 | und | ||||||
| CD14+CD15- iNOS+ M-MDSCs | 19 | High | 24 | 268 | 0.707 to 3.163 | 0.291 | 405 | 0.665 to 3.713 | 0.302 |
| Low | 28 | 321 | und | ||||||
| CD14+CD15+ M-MDSCs | 1.2 | High | 18 | 173 | 1.064 to 6.983 | 0.0047 | 321 | 1.118 to 7.617 | 0.0094 |
| Low | 34 | 329 | und | ||||||
| CD14+CD15+ iNOS+ M-MDSCs | 39 | High | 21 | 307 | 0.386 to 1.745 | 0.610 | und | 0.339 to 1.902 | 0.619 |
| Low | 31 | 258 | 405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xagara, A.; Goulielmaki, M.; Fortis, S.P.; Kokkalis, A.; Chantzara, E.; Christodoulopoulos, G.; Samaras, I.; Saloustros, E.; Tsapakidis, K.; Papadopoulos, V.; et al. Pre-Existing Immunity Predicts Response to First-Line Immunotherapy in Non-Small Cell Lung Cancer Patients. Cancers 2024, 16, 2393. https://doi.org/10.3390/cancers16132393
Xagara A, Goulielmaki M, Fortis SP, Kokkalis A, Chantzara E, Christodoulopoulos G, Samaras I, Saloustros E, Tsapakidis K, Papadopoulos V, et al. Pre-Existing Immunity Predicts Response to First-Line Immunotherapy in Non-Small Cell Lung Cancer Patients. Cancers. 2024; 16(13):2393. https://doi.org/10.3390/cancers16132393
Chicago/Turabian StyleXagara, Anastasia, Maria Goulielmaki, Sotirios P. Fortis, Alexandros Kokkalis, Evangelia Chantzara, George Christodoulopoulos, Ioannis Samaras, Emmanouil Saloustros, Konstantinos Tsapakidis, Vasileios Papadopoulos, and et al. 2024. "Pre-Existing Immunity Predicts Response to First-Line Immunotherapy in Non-Small Cell Lung Cancer Patients" Cancers 16, no. 13: 2393. https://doi.org/10.3390/cancers16132393
APA StyleXagara, A., Goulielmaki, M., Fortis, S. P., Kokkalis, A., Chantzara, E., Christodoulopoulos, G., Samaras, I., Saloustros, E., Tsapakidis, K., Papadopoulos, V., Pateras, I. S., Georgoulias, V., Baxevanis, C. N., & Kotsakis, A. (2024). Pre-Existing Immunity Predicts Response to First-Line Immunotherapy in Non-Small Cell Lung Cancer Patients. Cancers, 16(13), 2393. https://doi.org/10.3390/cancers16132393








