Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patient Characteristics
3.2. Toxicity Outcomes
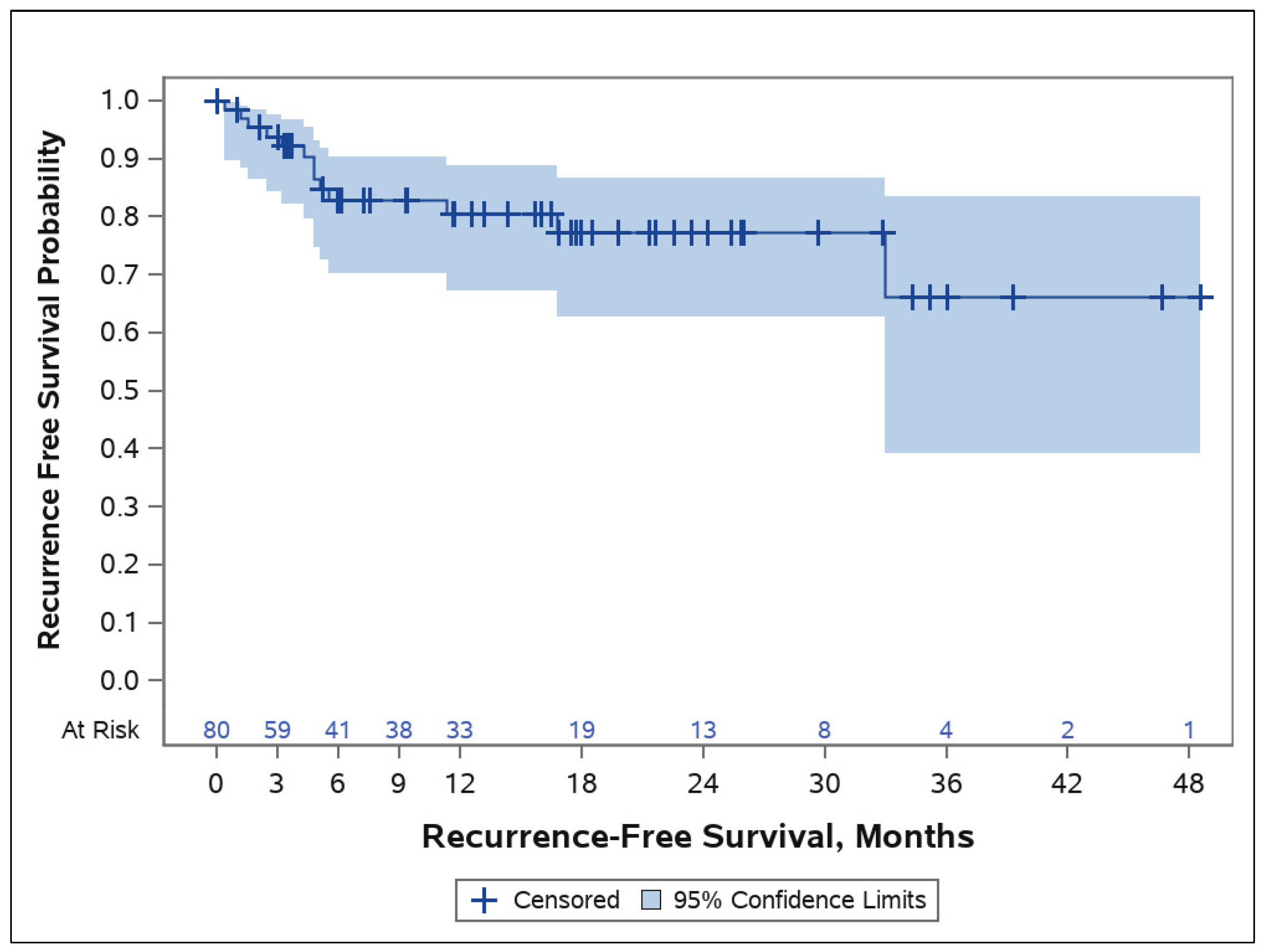
3.3. Tumor Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NCCN. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Squamous Cell Skin Cancer, Version 2; NCCN: Plymouth Meeting, PA, USA, 2018. [Google Scholar]
- Kwon, S.; Dong, Z.M.; Wu, P.C. Sentinel lymph node biopsy for high-risk cutaneous squamous cell carcinoma: Clinical experience and review of literature. World J. Surg. Oncol. 2011, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Rowe, D.E.; Carroll, R.J.; Day, C.L., Jr. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J. Am. Acad. Dermatol. 1992, 26, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.C.; Koyfman, S.A.; Bakst, R.L.; Margalit, D.N.; Beadle, B.M.; Beitler, J.J.; Chang, S.S.W.; Cooper, J.S.; Galloway, T.J.; Ridge, J.A.; et al. Retreatment of Recurrent or Second Primary Head and Neck Cancer after Prior Radiation: Executive Summary of the American Radium Society Appropriate Use Criteria. Int. J. Radiat. Oncol. Biol. Phys. 2022, 113, 759–786. [Google Scholar] [CrossRef] [PubMed]
- Likhacheva, A.; Awan, M.; Barker, C.A.; Bhatnagar, A.; Bradfield, L.; Brady, M.S.; Buzurovic, I.; Geiger, J.L.; Parvathaneni, U.; Zaky, S.; et al. Definitive and Postoperative Radiation Therapy for Basal and Squamous Cell Cancers of the Skin: Executive Summary of an American Society for Radiation Oncology Clinical Practice Guideline. Pract. Radiat. Oncol. 2020, 10, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. Treatment of solid tumors by interstitial release of recoiling short-lived alpha emitters. Phys. Med. Biol. 2007, 52, 5025–5042. [Google Scholar] [CrossRef] [PubMed]
- Arazi, L.; Cooks, T.; Schmidt, M.; Keisari, Y.; Kelson, I. The treatment of solid tumors by alpha emitters released from (224)Ra-loaded sources-internal dosimetry analysis. Phys. Med. Biol. 2010, 55, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Cooks, T.; Arazi, L.; Schmidt, M.; Marshak, G.; Kelson, I.; Keisari, Y. Growth retardation and destruction of experimental squamous cell carcinoma by interstitial radioactive wires releasing diffusing alpha-emitting atoms. Int. J. Cancer 2008, 122, 1657–1664. [Google Scholar] [CrossRef] [PubMed]
- Kelson, I.; Levy, Y.; Redmard, E. Recoil implantation of alpha sources for thickness measurement of thin films. J. Phys. D Appl. Phys. 1995, 28, 100. [Google Scholar] [CrossRef]
- Popovtzer, A.; Rosenfeld, E.; Mizrachi, A.; Bellia, S.R.; Ben-Hur, R.; Feliciani, G.; Sarnelli, A.; Arazi, L.; Deutsch, L.; Kelson, I.; et al. Initial Safety and Tumor Control Results from a “First-in-Human” Multicenter Prospective Trial Evaluating a Novel Alpha-Emitting Radionuclide for the Treatment of Locally Advanced Recurrent Squamous Cell Carcinomas of the Skin and Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 571–578. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, M.A.; VanderWalde, N.A.; Ballo, M.T.; Patra, P.; Cohen, G.N.; Damato, A.L.; Barker, C.A. Feasibility and Safety of Diffusing Alpha-Emitter Radiation Therapy for Recurrent or Unresectable Skin Cancers. JAMA Netw. Open 2023, 6, e2312824. [Google Scholar] [CrossRef] [PubMed]
- Feliciani, G.; Bellia, S.R.; Del Duca, M.; Mazzotti, G.; Monti, M.; Stanganelli, I.; Keisari, Y.; Kelson, I.; Popovtzer, A.; Romeo, A.; et al. A New Approach for a Safe and Reproducible Seeds Positioning for Diffusing Alpha-Emitters Radiation Therapy of Squamous Cell Skin Cancer: A Feasibility Study. Cancers 2022, 14, 240. [Google Scholar] [CrossRef] [PubMed]
- Heger, G.; Roy, A.; Dumančić, M.; Arazi, L. Alpha dose modeling in diffusing alpha-emitters radiation therapy-Part I: Single-seed calculations in one and two dimensions. Med. Phys. 2023, 50, 1793–1811. [Google Scholar] [CrossRef] [PubMed]
- Heger, G.; Dumančić, M.; Roy, A.; Arazi, L. Alpha dose modeling in diffusing alpha-emitters radiation therapy. Part II: Lattice studies. Med. Phys. 2023, 50, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Rudžianskas, V.; Inčiūra, A.; Vaitkus, S.; Padervinskis, E.; Rudžianskienė, M.; Kupčinskaitė-Noreikienė, R.; Saltonaitė, L.; Noreika, A.; Statnickaitė, A.; Juozaitytė, E. Reirradiation for patients with recurrence head and neck squamous cell carcinoma: A single-institution comparative study. Medicina 2014, 50, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Miszczyk, M.; Suleja, A.; Sobel, S.; Stec, M.; Chyrek, A.J.; Kolbusz, M.; Spałek, M.; Nasiek, A.; Stankiewicz, M.; Lelek, P.; et al. Salvage re-irradiation in non-melanoma skin cancers: A multicenter analysis. Radiother. Oncol. 2023, 189, 109945. [Google Scholar] [CrossRef] [PubMed]
- Mare, S.D.; Nishri, Y.; Shai, A.; Efrati, M.; Deutsch, L.; Den, R.B.; Kelson, I.; Keisari, Y.; Domankevich, V. Diffusing Alpha-Emitters Radiation Therapy Promotes a Proimmunogenic Tumor Microenvironment and Synergizes with Programmed Cell Death Protein 1 Blockade. Int. J. Radiat. Oncol. Biol. Phys. 2023, 115, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Domankevich, V.; Cohen, A.; Efrati, M.; Schmidt, M.; Rammensee, H.G.; Nair, S.S.; Tewari, A.; Kelson, I.; Keisari, Y. Combining alpha radiation-based brachytherapy with immunomodulators promotes complete tumor regression in mice via tumor-specific long-term immune response. Cancer Immunol. Immunother. 2019, 68, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Bellia, S.R.; Feliciani, G.; Duca, M.D.; Monti, M.; Turri, V.; Sarnelli, A.; Romeo, A.; Kelson, I.; Keisari, Y.; Popovtzer, A.; et al. Clinical evidence of abscopal effect in cutaneous squamous cell carcinoma treated with diffusing alpha emitters radiation therapy: A case report. J. Contemp. Brachyther. 2019, 11, 449–457. [Google Scholar] [CrossRef] [PubMed]
| Protocol Number | Clinical Site | No. of Patients | No. of Lesions |
|---|---|---|---|
| CTP-CMN-02 | Hadassah Medical Center | 28 | 33 |
| CTP-CMN-03 | Rambam Healthcare Campus | 10 | 10 |
| CTP-SCC-00 | Rabin Medical Center | 18 | 22 |
| CTP-SCC-00 | IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori” | 5 | 6 |
| CTP-SCC-MSK-00 | University Cancer Centers | 8 | 8 |
| CTP-SCC-MSK-00 | West Cancer Center | 2 | 2 |
| Total | 71 | 81 |
| Patient Characteristics, Statistics | Results | N |
|---|---|---|
| Age (years), mean ± SD (range) | 76.3 ± 11.7 (57.8–92.0) | 71 |
| Gender, n (%) | 71 | |
| Female | 25 (35%) | |
| Male | 46 (65%) | |
| BMI, mean ± SD (range) | 26.9 ± 5.8 (17.0–42.0) | 66 |
| Tumor Characteristic, Statistics | Results | N |
|---|---|---|
| Histopathology, n (%) | 81 | |
| BCC | 36 (44%) | |
| SCC | 45 (56%) | |
| Recurrent tumor, n (%) | 80 | |
| Recurrent | 38 (47%) | |
| Newly diagnosed | 42 (52%) | |
| Tumor location, n (%) | 81 | |
| Ear | 11 (14%) | |
| Extremity | 8 (10%) | |
| Eyelid | 2 (2%) | |
| Face | 13 (16%) | |
| Lip | 4 (5%) | |
| Neck | 1 (1%) | |
| Nose | 22 (27%) | |
| Oral cavity | 5 (6%) | |
| Scalp | 10 (12%) | |
| Tongue | 4 (5%) | |
| Torso | 1 (1%) | |
| Duration of disease for recurrent tumors (months), mean ± SD (range) | 70.3 ± 69.5 (1.3–285) | 38 |
| Baseline Gross Tumor Volume (cm3), mean ± SD (range) | 2.23 ± 4.38 (0.03–33.90) | 79 |
| Toxicity | Grade 1 (Mild) | Grade 2 (Moderate) | Grade 3 (Severe) | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Dermatitis Radiation | 23 | 32.4% | 4 | 5.6% | 0 | 0% | 26 | 36.6% |
| Pain at the Implanted Site | 9 | 12.7% | 15 | 21.1% | 0 | 0% | 17 | 23.9% |
| Pruritis | 11 | 15.5% | 0 | 0% | 0 | 0% | 11 | 15.5% |
| Localized Edema | 3 | 4.2% | 0 | 0% | 0 | 0% | 3 | 4.2% |
| Bruising at Implanted Site | 3 | 4.2% | 0 | 0% | 0 | 0% | 2 | 2.8% |
| Superficial Soft Tissue Fibrosis | 1 | 1.4% | 1 | 1.4% | 0 | 0% | 2 | 2.8% |
| Wound Infection | 0 | 0% | 9 | 12.7% | 0 | 0% | 2 | 2.8% |
| Mucositis Oral | 1 | 1.4% | 0 | 0% | 0 | 0% | 1 | 1.4% |
| Hypertension | 0 | 0% | 0 | 0% | 1 | 1.4% | 1 | 1.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popovtzer, A.; Mizrachi, A.; D’Andrea, M.A.; VanderWalde, N.A.; Kurman, N.; Rosenfeld, E.; Ben-Hur, R.; Bellia, S.R.; Feliciani, G.; Silvern, D.; et al. Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers. Cancers 2024, 16, 2312. https://doi.org/10.3390/cancers16132312
Popovtzer A, Mizrachi A, D’Andrea MA, VanderWalde NA, Kurman N, Rosenfeld E, Ben-Hur R, Bellia SR, Feliciani G, Silvern D, et al. Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers. Cancers. 2024; 16(13):2312. https://doi.org/10.3390/cancers16132312
Chicago/Turabian StylePopovtzer, Aron, Aviram Mizrachi, Mark A. D’Andrea, Noam A. VanderWalde, Noga Kurman, Eli Rosenfeld, Ran Ben-Hur, Salvatore Roberto Bellia, Giacomo Feliciani, David Silvern, and et al. 2024. "Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers" Cancers 16, no. 13: 2312. https://doi.org/10.3390/cancers16132312
APA StylePopovtzer, A., Mizrachi, A., D’Andrea, M. A., VanderWalde, N. A., Kurman, N., Rosenfeld, E., Ben-Hur, R., Bellia, S. R., Feliciani, G., Silvern, D., Sarnelli, A., Ballo, M. T., Patra, P., Cohen, G. N., Damato, A. L., Shkedy, Y., Den, R. B., Barker, C. A., Charas, T., & Hirshoren, N. (2024). Extended Follow-Up Outcomes from Pooled Prospective Studies Evaluating Efficacy of Interstitial Alpha Radionuclide Treatment for Skin and Head and Neck Cancers. Cancers, 16(13), 2312. https://doi.org/10.3390/cancers16132312








