The Evolution of Therapies Targeting Bruton Tyrosine Kinase for the Treatment of Chronic Lymphocytic Leukaemia: Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
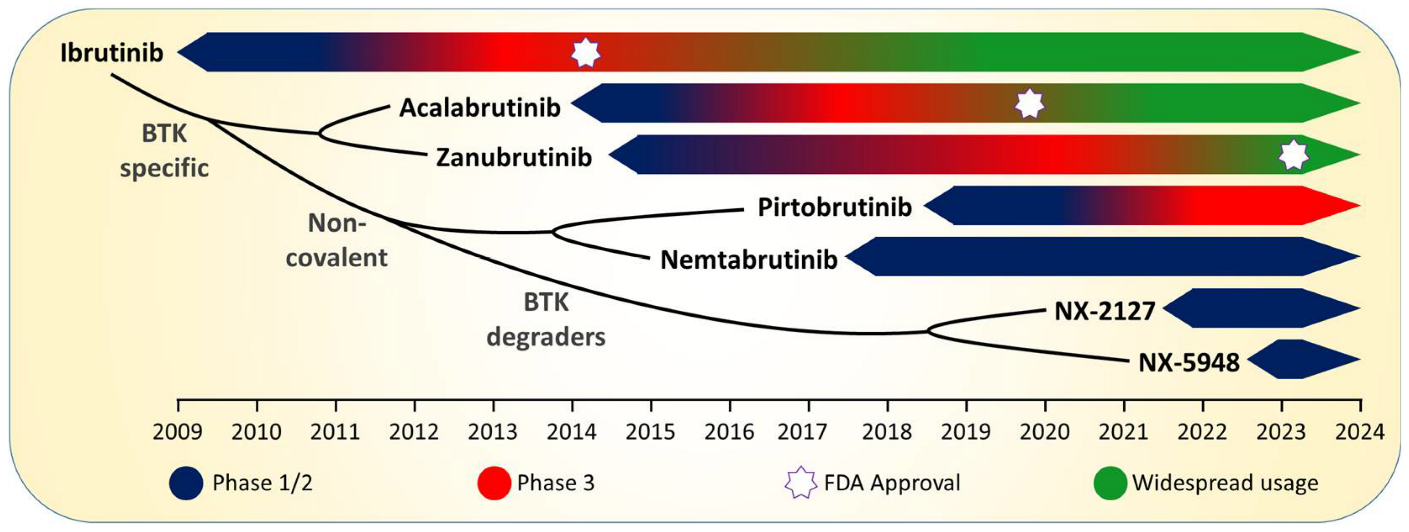
2. Ibrutinib: The “First in Class” BTKi
3. Increasing Specificity for BTK: Acalabrutinib and Zanubrutinib
4. Noncovalent BTK Inhibition: Pirtobrutinib and Nemtabrutinib
5. BTK-Degradation: NX-2127 and NX-5948
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hamblin, T.J.; Davis, Z.; Gardiner, A.; Oscier, D.G.; Stevenson, F.K. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999, 94, 1848–1854. [Google Scholar] [CrossRef] [PubMed]
- Lanham, S.; Hamblin, T.; Oscier, D.; Ibbotson, R.; Stevenson, F.; Packham, G. Differential signaling via surface IgM is associated with VH gene mutational status and CD38 expression in chronic lymphocytic leukemia. Blood 2003, 101, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Mockridge, C.I.; Potter, K.N.; Wheatley, I.; Neville, L.A.; Packham, G.; Stevenson, F.K. Reversible anergy of sIgM-mediated signaling in the two subsets of CLL defined by VH-gene mutational status. Blood 2007, 109, 4424–4431. [Google Scholar] [CrossRef] [PubMed]
- Rosen, A.; Murray, F.; Evaldsson, C.; Rosenquist, R. Antigens in chronic lymphocytic leukemia—Implications for cell origin and leukemogenesis. Semin. Cancer Biol. 2010, 20, 400–409. [Google Scholar] [CrossRef]
- Fais, F.; Ghiotto, F.; Hashimoto, S.; Sellars, B.; Valetto, A.; Allen, S.L.; Schulman, P.; Vinciguerra, V.P.; Rai, K.; Rassenti, L.Z.; et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J. Clin. Investig. 1998, 102, 1515–1525. [Google Scholar] [CrossRef]
- Ten Hacken, E.; Gounari, M.; Ghia, P.; Burger, J.A. The importance of B cell receptor isotypes and stereotypes in chronic lymphocytic leukemia. Leukemia 2019, 33, 287–298. [Google Scholar] [CrossRef]
- Burger, J.A.; Chiorazzi, N. B cell receptor signaling in chronic lymphocytic leukemia. Trends Immunol. 2013, 34, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Catera, R.; Hatzi, K.; Yan, X.J.; Zhang, L.; Wang, X.B.; Fales, H.M.; Allen, S.L.; Kolitz, J.E.; Rai, K.R.; et al. Chronic lymphocytic leukemia antibodies with a common stereotypic rearrangement recognize nonmuscle myosin heavy chain IIA. Blood 2008, 112, 5122–5129. [Google Scholar] [CrossRef]
- Herve, M.; Xu, K.; Ng, Y.S.; Wardemann, H.; Albesiano, E.; Messmer, B.T.; Chiorazzi, N.; Meffre, E. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J. Clin. Investig. 2005, 115, 1636–1643. [Google Scholar] [CrossRef]
- Duhren-von Minden, M.; Ubelhart, R.; Schneider, D.; Wossning, T.; Bach, M.P.; Buchner, M.; Hofmann, D.; Surova, E.; Follo, M.; Kohler, F.; et al. Chronic lymphocytic leukaemia is driven by antigen-independent cell-autonomous signalling. Nature 2012, 489, 309–312. [Google Scholar] [CrossRef]
- Harwood, N.E.; Batista, F.D. The cytoskeleton coordinates the early events of B-cell activation. Cold Spring Harb. Perspect Biol. 2011, 3, a002360. [Google Scholar] [CrossRef]
- Herishanu, Y.; Perez-Galan, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef]
- Woyach, J.A.; Johnson, A.J.; Byrd, J.C. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012, 120, 1175–1184. [Google Scholar] [CrossRef]
- Rolli, V.; Gallwitz, M.; Wossning, T.; Flemming, A.; Schamel, W.W.; Zurn, C.; Reth, M. Amplification of B cell antigen receptor signaling by a Syk/ITAM positive feedback loop. Mol. Cell 2002, 10, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yamanashi, Y.; Toyoshima, K. Association of Src-family kinase Lyn with B-cell antigen receptor. Immunol. Rev. 1993, 132, 187–206. [Google Scholar] [CrossRef]
- Dal Porto, J.M.; Gauld, S.B.; Merrell, K.T.; Mills, D.; Pugh-Bernard, A.E.; Cambier, J. B cell antigen receptor signaling 101. Mol. Immunol. 2004, 41, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Turck, C.W.; Kurosaki, T.; Chan, A.C. BLNK: A central linker protein in B cell activation. Immunity 1998, 9, 93–103. [Google Scholar] [CrossRef]
- Wiestner, A. BCR pathway inhibition as therapy for chronic lymphocytic leukemia and lymphoplasmacytic lymphoma. Hematology 2014, 2014, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Friedberg, J.W.; Sharman, J.; Sweetenham, J.; Johnston, P.B.; Vose, J.M.; Lacasce, A.; Schaefer-Cutillo, J.; De Vos, S.; Sinha, R.; Leonard, J.P.; et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010, 115, 2578–2585. [Google Scholar] [CrossRef]
- Jones, J.A.; Byrd, J.C. How will B-cell-receptor-targeted therapies change future CLL therapy? Blood 2014, 123, 1455–1460. [Google Scholar] [CrossRef]
- Honigberg, L.A.; Smith, A.M.; Sirisawad, M.; Verner, E.; Loury, D.; Chang, B.; Li, S.; Pan, Z.; Thamm, D.H.; Miller, R.A.; et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc. Natl. Acad. Sci. USA 2010, 107, 13075–13080. [Google Scholar] [CrossRef]
- Bruton, O.C. Agammaglobulinemia. Pediatrics 1952, 9, 722–728. [Google Scholar] [CrossRef]
- Bruton, O.C.; Apt, L.; Gitlin, D.; Janeway, C.A. Absence of serum gamma globulins. AMA Am. J. Dis. Child. 1952, 84, 632–636. [Google Scholar] [PubMed]
- Rawlings, D.J.; Saffran, D.C.; Tsukada, S.; Largaespada, D.A.; Grimaldi, J.C.; Cohen, L.; Mohr, R.N.; Bazan, J.F.; Howard, M.; Copeland, N.G.; et al. Mutation of unique region of Bruton’s tyrosine kinase in immunodeficient XID mice. Science 1993, 261, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.E.; Gordon, A.L.; Hertlein, E.; Ramanunni, A.; Zhang, X.; Jaglowski, S.; Flynn, J.; Jones, J.; Blum, K.A.; Buggy, J.J.; et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011, 117, 6287–6296. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grunhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Byrd, J.C.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Kay, N.E.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. N. Engl. J. Med. 2014, 371, 213–223. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; O’Brien, S.; Barrientos, J.C.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; Barr, P.M.; et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood 2019, 133, 2031–2042. [Google Scholar] [CrossRef]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Barr, P.M.; Owen, C.; Robak, T.; Tedeschi, A.; Bairey, O.; Burger, J.A.; Hillmen, P.; Coutre, S.E.; Dearden, C.; Grosicki, S.; et al. Up to 8-year follow-up from RESONATE-2: First-line ibrutinib treatment for patients with chronic lymphocytic leukemia. Blood Adv. 2022, 6, 3440–3450. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Jones, J.A.; Coutre, S.E.; Mato, A.R.; Hillmen, P.; Tam, C.; Osterborg, A.; Siddiqi, T.; Thirman, M.J.; Furman, R.R.; et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): A phase 2, open-label, multicentre study. Lancet Oncol. 2016, 17, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or Chemoimmunotherapy for Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Wang, X.V.; Hanson, C.A.; Paietta, E.M.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Long-term outcomes for ibrutinib–rituximab and chemoimmunotherapy in CLL: Updated results of the E1912 trial. Blood 2022, 140, 112–120. [Google Scholar] [CrossRef]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Samoilova, O.; Novak, J.; Ben-Yehuda, D.; et al. Ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab in first-line treatment of chronic lymphocytic leukaemia (iLLUMINATE): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 43–56. [Google Scholar] [CrossRef]
- Herman, S.E.; Gordon, A.L.; Wagner, A.J.; Heerema, N.A.; Zhao, W.; Flynn, J.M.; Jones, J.; Andritsos, L.; Puri, K.D.; Lannutti, B.J.; et al. Phosphatidylinositol 3-kinase-delta inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood 2010, 116, 2078–2088. [Google Scholar] [CrossRef]
- Furman, R.R.; Sharman, J.P.; Coutre, S.E.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.; et al. Idelalisib and Rituximab in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2014, 370, 997–1007. [Google Scholar] [CrossRef]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab With or Without Idelalisib Followed by Open-Label Idelalisib in Patients With Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef]
- Skanland, S.S.; Brown, J.R. PI3K inhibitors in chronic lymphocytic leukemia: Where do we go from here? Haematologica 2023, 108, 9–21. [Google Scholar] [CrossRef]
- Walewska, R.; Parry-Jones, N.; Eyre, T.A.; Follows, G.; Martinez-Calle, N.; McCarthy, H.; Parry, H.; Patten, P.E.M.; Riches, J.C.; Hillmen, P.; et al. Guideline for the treatment of chronic lymphocytic leukaemia. Br. J. Haematol. 2022, 197, 544–557. [Google Scholar] [CrossRef] [PubMed]
- Mato, A.R.; Roeker, L.E.; Jacobs, R.; Hill, B.T.; Lamanna, N.; Brander, D.; Shadman, M.; Ujjani, C.S.; Yazdy, M.S.; Perini, G.F.; et al. Assessment of the Efficacy of Therapies Following Venetoclax Discontinuation in CLL Reveals BTK Inhibition as an Effective Strategy. Clin. Cancer Res. 2020, 26, 3589–3596. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.P.S.; Lip, G.Y.H.; McCormack, T.; Lyon, A.R.; Hillmen, P.; Iyengar, S.; Martinez-Calle, N.; Parry-Jones, N.; Patten, P.E.M.; Schuh, A.; et al. Management of cardiovascular complications of bruton tyrosine kinase inhibitors. Br. J. Haematol. 2022, 196, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Munir, T.; Pitchford, A.; Bloor, A.; Broom, A.; Young, M.; Kennedy, B.; Walewska, R.; Furtado, M.; Preston, G.; Neilson, J.R.; et al. Sudden or Cardiac Deaths on Ibrutinib-Based Therapy Were Associated with a Prior History of Hypertension or Cardiac Disease and the Use of ACE-Inhibitors at Study Entry: Analysis from the Phase III NCRI FLAIR Trial. Blood 2021, 138 (Suppl. S1), 2636. [Google Scholar] [CrossRef]
- Robak, T.; Witkowska, M.; Smolewski, P. The Role of Bruton’s Kinase Inhibitors in Chronic Lymphocytic Leukemia: Current Status and Future Directions. Cancers 2022, 14, 771. [Google Scholar] [CrossRef]
- Xiao, L.; Salem, J.E.; Clauss, S.; Hanley, A.; Bapat, A.; Hulsmans, M.; Iwamoto, Y.; Wojtkiewicz, G.; Cetinbas, M.; Schloss, M.J.; et al. Ibrutinib-Mediated Atrial Fibrillation Attributable to Inhibition of C-Terminal Src Kinase. Circulation 2020, 142, 2443–2455. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Kozak, T.; Simkovic, M.; Kaplan, P.; Kraychok, I.; Illes, A.; de la Serna, J.; et al. ASCEND: Phase III, Randomized Trial of Acalabrutinib Versus Idelalisib Plus Rituximab or Bendamustine Plus Rituximab in Relapsed or Refractory Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2849–2861. [Google Scholar] [CrossRef]
- Ghia, P.; Pluta, A.; Wach, M.; Lysak, D.; Simkovic, M.; Kriachok, I.; Illes, A.; de la Serna, J.; Dolan, S.; Campbell, P.; et al. Acalabrutinib Versus Investigator’s Choice in Relapsed/Refractory Chronic Lymphocytic Leukemia: Final ASCEND Trial Results. Hemasphere 2022, 6, e801. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.; Pagel, J.M.; Flinn, I.W.; Kamdar, M.; Munir, T.; Walewska, R.; Corbett, G.; et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): A randomised, controlled, phase 3 trial. Lancet 2020, 395, 1278–1291. [Google Scholar] [CrossRef]
- Sharman, J.P.; Egyed, M.; Jurczak, W.; Skarbnik, A.P.; Kamdar, M.K.; Munir, T.; Fogliatto, L.; Herishanu, Y.; Banerji, V.; Follows, G.; et al. Acalabrutinib ± obinutuzumab versus obinutuzumab + chlorambucil in treatment-naïve chronic lymphocytic leukemia: Five-year follow-up of ELEVATE-TN. J. Clin. Oncol. 2022, 40, 7539. [Google Scholar] [CrossRef]
- Davids, M.S.; Sharman, J.P.; Eyre, T.A.; Woyach, J.A.; de Miranda, P.A.P.; Shahkarami, M.; Butturini, A.; Emeribe, U.; Byrd, J.C. Contribution of Obinutuzumab to Acalabrutinib Therapy in Patients with Treatment-Naive Chronic Lymphocytic Leukemia: Analysis of Survival Outcomes By Genomic Features. Blood 2022, 140 (Suppl. S1), 4173–4175. [Google Scholar] [CrossRef]
- Byrd, J.C.; Hillmen, P.; Ghia, P.; Kater, A.P.; Chanan-Khan, A.; Furman, R.R.; O’Brien, S.; Yenerel, M.N.; Illés, A.; Kay, N.; et al. Acalabrutinib Versus Ibrutinib in Previously Treated Chronic Lymphocytic Leukemia: Results of the First Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 3441–3452. [Google Scholar] [CrossRef] [PubMed]
- Rogers, K.A.; Thompson, P.A.; Allan, J.N.; Coleman, M.; Sharman, J.P.; Cheson, B.D.; Jones, D.; Izumi, R.; Frigault, M.M.; Quah, C.; et al. Phase II study of acalabrutinib in ibrutinib-intolerant patients with relapsed/refractory chronic lymphocytic leukemia. Haematologica 2021, 106, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Awan, F.T.; Schuh, A.; Brown, J.R.; Furman, R.R.; Pagel, J.M.; Hillmen, P.; Stephens, D.M.; Woyach, J.; Bibikova, E.; Charuworn, P.; et al. Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib. Blood Adv. 2019, 3, 1553–1562. [Google Scholar] [CrossRef]
- Brown, J.R.; Eichhorst, B.; Hillmen, P.; Jurczak, W.; Kaźmierczak, M.; Lamanna, N.; O’Brien, S.M.; Tam, C.S.; Qiu, L.; Zhou, K.; et al. Zanubrutinib or Ibrutinib in Relapsed or Refractory Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2022, 388, 319–332. [Google Scholar] [CrossRef]
- Tam, C.S.; Brown, J.R.; Kahl, B.S.; Ghia, P.; Giannopoulos, K.; Jurczak, W.; Simkovic, M.; Shadman, M.; Osterborg, A.; Laurenti, L.; et al. Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol. 2022, 23, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Trotman, J.; Opat, S.; Burger, J.A.; Cull, G.; Gottlieb, D.; Harrup, R.; Johnston, P.B.; Marlton, P.; Munoz, J.; et al. Phase 1 study of the selective BTK inhibitor zanubrutinib in B-cell malignancies and safety and efficacy evaluation in CLL. Blood 2019, 134, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M.; Flinn, I.W.; Levy, M.Y.; Porter, R.F.; Burke, J.M.; Zafar, S.F.; Misleh, J.; Kingsley, E.C.; Yimer, H.A.; Freeman, B.; et al. Zanubrutinib in patients with previously treated B-cell malignancies intolerant of previous Bruton tyrosine kinase inhibitors in the USA: A phase 2, open-label, single-arm study. Lancet Haematol. 2023, 10, e35–e45. [Google Scholar] [CrossRef]
- Woyach, J.; Huang, Y.; Rogers, K.; Bhat, S.A.; Grever, M.R.; Lozanski, A.; Doong, T.-J.; Blachly, J.S.; Lozanski, G.; Jones, D.; et al. Resistance to Acalabrutinib in CLL Is Mediated Primarily By BTK Mutations. Blood 2019, 134, 504. [Google Scholar] [CrossRef]
- Woyach, J.A.; Furman, R.R.; Liu, T.-M.; Ozer, H.G.; Zapatka, M.; Ruppert, A.S.; Xue, L.; Li, D.H.-H.; Steggerda, S.M.; Versele, M.; et al. Resistance Mechanisms for the Bruton’s Tyrosine Kinase Inhibitor Ibrutinib. N. Engl. J. Med. 2014, 370, 2286–2294. [Google Scholar] [CrossRef]
- Mato, A.R.; Shah, N.N.; Jurczak, W.; Cheah, C.Y.; Pagel, J.M.; Woyach, J.A.; Fakhri, B.; Eyre, T.A.; Lamanna, N.; Patel, M.R.; et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): A phase 1/2 study. Lancet 2021, 397, 892–901. [Google Scholar] [CrossRef]
- Mato, A.R.; Woyach, J.A.; Brown, J.R.; Ghia, P.; Patel, K.; Eyre, T.A.; Munir, T.; Lech-Marańda, E.; Lamanna, N.; Tam, C.S.; et al. Efficacy of Pirtobrutinib in Covalent BTK-Inhibitor Pre-Treated Relapsed / Refractory CLL/SLL: Additional Patients and Extended Follow-up from the Phase 1/2 BRUIN Study. Blood 2022, 140 (Suppl. S1), 2316–2320. [Google Scholar] [CrossRef]
- Bye, A.P.; Kriek, N.; Sage, T.; Rawlings, S.J.; Prodger, C.; Kesavan, M.; Lees, C.; Booth, S.; Cowen, L.G.; Shefferd, K.; et al. Pirtobrutinib results in reversible platelet dysfunction compared to ibrutinib and acalabrutinib. Haematologica 2022, 108, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.N.; Wang, M.L.; Brown, J.R.; Patel, K.; Woyach, J.A.; Wierda, W.G.; Ujjani, C.S.; Eyre, T.A.; Zinzani, P.L.; Alencar, A.J.; et al. Safety and Tolerability of Pirtobrutinib Monotherapy in Patients with B-Cell Malignancies Who Were Previously Intolerant to a Covalent BTK Inhibitor: Results from the Phase 1/2 BRUIN Study. Blood 2022, 140 (Suppl. S1), 4127–4132. [Google Scholar] [CrossRef]
- Mato, A.R.; Wierda, W.G.; Pagel, J.M.; Davids, M.S.; Zinzani, P.L.; Lu, Y.; Liu, H.; Shahda, S.; Leow, C.C.; Tam, C.S.; et al. BRUIN CLL-322: A Phase 3 Open-Label, Randomized Study of Fixed Duration Pirtobrutinib Plus Venetoclax and Rituximab Versus Venetoclax and Rituximab in Previously Treated Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma (Trial in Progress). Blood 2021, 138, 3742. [Google Scholar] [CrossRef]
- Woyach, J.A.; Flinn, I.W.; Awan, F.T.; Eradat, H.; Brander, D.; Tees, M.; Parikh, S.A.; Phillips, T.J.; Ghori, R.; Reddy, N.M.; et al. Efficacy and Safety of Nemtabrutinib, a Wild-Type and C481S-Mutated Bruton Tyrosine Kinase Inhibitor for B-Cell Malignancies: Updated Analysis of the Open-Label Phase 1/2 Dose-Expansion Bellwave-001 Study. Blood 2022, 140 (Suppl. S1), 7004–7006. [Google Scholar] [CrossRef]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef]
- Sakamoto, K.M.; Kim, K.B.; Kumagai, A.; Mercurio, F.; Crews, C.M.; Deshaies, R.J. Protacs: Chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc. Natl. Acad. Sci. USA 2001, 98, 8554–8559. [Google Scholar] [CrossRef] [PubMed]
- Riches, J.C.; Gribben, J.G. Mechanistic and Clinical Aspects of Lenalidomide Treatment for Chronic Lymphocytic Leukemia. Curr. Cancer Drug Targets 2016, 16, 689–700. [Google Scholar] [CrossRef]
- Ito, T.; Ando, H.; Suzuki, T.; Ogura, T.; Hotta, K.; Imamura, Y.; Yamaguchi, Y.; Handa, H. Identification of a Primary Target of Thalidomide Teratogenicity. Science 2010, 327, 1345–1350. [Google Scholar] [CrossRef]
- Mato, A.R.; Wierda, W.G.; Ai, W.Z.; Flinn, I.W.; Tees, M.; Patel, M.R.; Patel, K.; O’Brien, S.; Bond, D.A.; Roeker, L.E.; et al. NX-2127-001, a First-in-Human Trial of NX-2127, a Bruton’s Tyrosine Kinase-Targeted Protein Degrader, in Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia and B-Cell Malignancies. Blood 2022, 140 (Suppl. S1), 2329–2332. [Google Scholar] [CrossRef]
- Montoya, S.; Bourcier, J.; Thompson, M.C.; Noviski, M.; Tan, M.; Wang, E.; Mi, X.; Brathaban, N.; Barrientos Risso, C.; Tsai, D.; et al. Kinase Dead BTK Mutations Confer Resistance to Covalent and Noncovalent BTK Inhibitors but Are Susceptible to Clinical Stage BTK Degraders. Blood 2022, 140 (Suppl. S1), 1811–1813. [Google Scholar] [CrossRef]
- Blombery, P.; Thompson, E.R.; Lew, T.E.; Tiong, I.S.; Bennett, R.; Cheah, C.Y.; Lewis, K.L.; Handunnetti, S.M.; Tang, C.P.S.; Roberts, A.; et al. Enrichment of BTK Leu528Trp mutations in patients with CLL on zanubrutinib: Potential for pirtobrutinib cross-resistance. Blood Adv. 2022, 6, 5589–5592. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Mi, X.; Thompson, M.C.; Montoya, S.; Notti, R.Q.; Afaghani, J.; Durham, B.H.; Penson, A.; Witkowski, M.T.; Lu, S.X.; et al. Mechanisms of Resistance to Noncovalent Bruton’s Tyrosine Kinase Inhibitors. N. Engl. J. Med. 2022, 386, 735–743. [Google Scholar] [CrossRef]
- Wierda, W.G.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Opat, S.; Tedeschi, A.; Badoux, X.C.; Kuss, B.J.; Jackson, S.; Moreno, C.; et al. Ibrutinib Plus Venetoclax for First-Line Treatment of Chronic Lymphocytic Leukemia: Primary Analysis Results From the Minimal Residual Disease Cohort of the Randomized Phase II CAPTIVATE Study. J. Clin. Oncol. 2021, 39, 3853–3865. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.S.; Allan, J.N.; Siddiqi, T.; Kipps, T.J.; Jacobs, R.; Opat, S.; Barr, P.M.; Tedeschi, A.; Trentin, L.; Bannerji, R.; et al. Fixed-duration ibrutinib plus venetoclax for first-line treatment of CLL: Primary analysis of the CAPTIVATE FD cohort. Blood 2022, 139, 3278–3289. [Google Scholar] [CrossRef]
- Kater, A.P.; Owen, C.; Moreno, C.; Follows, G.; Munir, T.; Levin, M.-D.; Benjamini, O.; Janssens, A.; Osterborg, A.; Robak, T.; et al. Fixed-Duration Ibrutinib-Venetoclax in Patients with Chronic Lymphocytic Leukemia and Comorbidities. NEJM Evid. 2022, 1, EVIDoa2200006. [Google Scholar] [CrossRef]

| BTK Inhibitor | Clinical Trial | ORR | PFS | OS | Discont. Rates/AEs | Ref. |
|---|---|---|---|---|---|---|
| Ibrutinib | RESONATE | 91% (I) | Median PFS: 44.1 m (I) vs. 8.1 m (Ofa) | Median OS: not reached. Ofa group crossed over to I | Grade ≥ 3 AF: 6% (I) Grade ≥ 3 HTN: 8% (I) | [28,29] |
| RESONATE-2 | 92% (I) vs. 37% (C) | 5 y PFS: 70% (I) vs. 12% (C) | 5 y OS: 83% (I) vs. 68% (C) | AF: 16% (I) HTN: 26% (I) | [30,31,32] | |
| ECOG-ACRIN | 95.8% (IR) vs. 81.1% (FCR) | 5 y PFS: 78% (IR) vs. 51% (FCR) | 5 y OS: 95% (IR) vs. 89% (FCR) | Grade ≥ 3 AF: 4.5% (IR) vs. 0% (FCR) Grade ≥ 3 HTN: 11.4% (IR) vs. 1.9% (FCR) | [34,35] | |
| Acalabrutinib | ASCEND | 83% (A) vs. 84% (Idelalisib–R/BR) | 42 m PFS: 62% (A) vs. 19% (Idelalisib–R/BR) | 42 m OS: 78% (A) vs. 65% (Idela-R/BR) | AF: 8% (A) vs. 3% (Idela-R/BR) HTN: 8% (A) vs. 5% (Idela-R/BR) Discont: 23% (A) vs. 67% (Idela-R) vs. 17% (BR) | [47,48] |
| ELEVATE-TN | 96% (A-Obi) vs. 90% (A) vs. 83% (C-Obi) | 5 y PFS: 84% (A-Obi) vs. 72% (A) vs. 21% (C-Obi) | 5 y OS: 90% (A-Obi) vs. 84% (A) vs. 82% (C-Obi) | AF: 6.2% (A-Obi) vs. 7.3% (A) vs. 0.6% (C-Obi) HTN: 9.6% (A-Obi) vs. 8.9% (A) vs. 3.6% (C-Obi) | [49,50] | |
| ELEVATE-RR | 81% (A) vs. 77% (I) | Median PFS: 38.4 m (A) vs. 38.4 m (I) | Median OS: not reached in either treatment group | AF: 9.4% (A) vs. 16% (I) HTN: 9.4% (A) vs. 23.2% (I) Discont: 14.7% (A) vs. 21.3% (I) | [52] | |
| Zanubrutinib | SEQUOIA | 94.6% (Z) vs. 85.3% (BR) | 24 m PFS: 85.5% (Z) vs. 69.5% (BR) | 24 m OS: 94.3% (Z) vs. 94.6% (BR) | AF: 3% (Z) vs. 3% (BE) Discont: 8% (Z) vs. 14% (BR) | [56] |
| ALPINE | 83.5% (Z) vs. 74.2% (I) | 24 m PFS: 78.4% (Z) vs. 65.9% (I) | Median OS: not reached in either treatment group | C/D: 0 pts (Z) vs. 6 pts (I) AF: 5.2% (Z) vs. 13.3% (I) Discont: 62 pts (Z) vs. 92 pts (I) | [55] | |
| Pirtobrutinib | BRUIN | 74% (P) | Median PFS: 19.4 m | NE | Grade ≥ 3 AF: 1% (P) Grade ≥ 3 HTN: 3% (P) Discont: 2% (P) | [61,62] |
| Nemtabrutinib | BELLWAVE | 56% (O) | Median PFS: 24.4 m | NE | HTN: 10% (N) Discont: 13% (N) | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyre, T.A.; Riches, J.C. The Evolution of Therapies Targeting Bruton Tyrosine Kinase for the Treatment of Chronic Lymphocytic Leukaemia: Future Perspectives. Cancers 2023, 15, 2596. https://doi.org/10.3390/cancers15092596
Eyre TA, Riches JC. The Evolution of Therapies Targeting Bruton Tyrosine Kinase for the Treatment of Chronic Lymphocytic Leukaemia: Future Perspectives. Cancers. 2023; 15(9):2596. https://doi.org/10.3390/cancers15092596
Chicago/Turabian StyleEyre, Toby A., and John C. Riches. 2023. "The Evolution of Therapies Targeting Bruton Tyrosine Kinase for the Treatment of Chronic Lymphocytic Leukaemia: Future Perspectives" Cancers 15, no. 9: 2596. https://doi.org/10.3390/cancers15092596
APA StyleEyre, T. A., & Riches, J. C. (2023). The Evolution of Therapies Targeting Bruton Tyrosine Kinase for the Treatment of Chronic Lymphocytic Leukaemia: Future Perspectives. Cancers, 15(9), 2596. https://doi.org/10.3390/cancers15092596





