Simple Summary
The availability of multiple gene panel testing allows us to identify germline pathogenic variants of validated cancer genes. We evaluated the prevalence and clinical/prognostic impact of deleterious germline mutations in OC patients. Germline panel testing should be performed for all patients with ovarian cancer. Better prognosis was found for germline mutated ovarian cancer patients. Endometrioid and clear cell histology subtypes show more deleterious mutations in other genes.
Abstract
Data on deleterious variants in genes other than BRCA1/2 remain limited. A retrospective cohort study was performed, including primary OC cases with TruRisk® germline gene panel testing between 2011 and 2020. Patients with testing after relapse were excluded. The cohort was divided into three groups: (A) no mutations, (B) deleterious BRCA1/2 mutations, and (C) deleterious mutations in other genes. A total of 702 patients met the inclusion criteria. Of these 17.4% (n = 122) showed BRCA1/2 mutations and a further 6.0% (n = 42) in other genes. Three-year overall survival (OS) of the entire cohort was significantly longer in patients with germline mutations (85%/82.8% for cohort B/C vs. 70.2% for cohort A, p < 0.001) and 3-year progression-free survival (PFS) only for cohort B (58.1% vs. 36.9%/41.6% in cohort A/C, p = 0.002). In multivariate analysis for the subgroup of advanced-stages of high-grade serous OC, both cohorts B/C were found to be independent factors for significantly better outcome, cohort C for OS (HR 0.46; 95% CI 0.25–0.84), and cohort B for both OS and PFS (HR 0.40; 95% CI 0.27–0.61 and HR 0.49; 95% CI 0.37–0.66, respectively). Germline mutations were detected in a quarter of OC patients, and a quarter of those in genes other than BRCA1/2. Germline mutations demonstrate in our cohort a prognostic factor and predict better prognosis for OC patients.
1. Introduction
Ovarian carcinoma (OC) is one of the most aggressive women’s cancers, with decreasing incidence and mortality rate over the past decade [1]. In 2020, the Global Cancer Observatory reported a worldwide incidence of 313.959 (6.6 per 100,000) new cases and a total of 207,252 deaths (4.2 per 100,000) [2]. The epidemiology of this cancer shows relevant differences between ethnicities and countries due to multiple factors, including genetic and environmental [3]. Unfortunately, detection and primary diagnosis occur mostly in advanced-stages due to nonspecific symptoms and lack of sufficient predictive screening modalities.
The prevalence of genetic predisposition and hereditary mutations for cancers are various among populations. The identification of high-risk gene carriers allows crucial decisions for focused monitoring. This leads to early detection and allows interventions for prevention such as risk-reducing surgeries [4,5].
Germline mutations have been reported in up to a quarter of OC cases [6,7]. Furthermore, knowledge about deleterious mutations has been recognized as mandatory and essential for therapeutic and prognostic implications [8]. However, the heterogeneous indication for germline testing may depend on the national prevalence of deleterious BRCA1/2 mutations in each population [6]. The European Society of Gynaecological Oncology (ESGO) and the European Society of Medical Oncology (ESMO) guidelines suggest BRCA1/2 testing for all OC patients, except those with mucinous histology [8]. The Society of Gynecologic Oncology (SGO) recommends generally BRCA1/2 testing [9]. On the other hand, the American Society for Clinical Oncology (ASCO) recommends germline multiple gene testing of further other clinically validated mutations for all OC patients [10].
In the last decade, the role of targeted maintenance therapy became a main issue, and several phase III studies have shown a prognostic benefit of poly (ADP-ribose) polymerase inhibitors (PARP-i) in selected cohorts with genomic mutations/instability (germline or somatic) [11,12,13]. Thus, multiple gene panel testing has been performed more often to identify genomic and molecular alterations, which could be optional disease drivers and markers for benefit from targeted maintenance therapies.
Germline BRCA1/2 mutations, the most common mutation in OC patients, demonstrate a well-known prognostic factor, likely because of the good response to platin-based chemotherapy and maintenance therapy with PARP-i, which nowadays is approved for this patient group. A previous study showed a significantly better outcome (overall survival (OS) and progression-free survival (PFS)) for germline BRCA1/2-mutated OC patients in comparison with non-mutated patients [14]. Moreover, gene panel testing enables an extended examination of numerous high-risk hereditary mutations, such as RAD51C/D, BRIP1, PALB2, and MSH6 [4,15,16,17]. Deleterious mutations of genes other than BRCA1/2 were reported in 4–6% of OC patients. However, the role and impact of those mutations on the prognosis remains unclear [7,15].
The purpose of this current study was to evaluate the prevalence and clinical impact of deleterious germline mutations, in BRCA1/2 and other genes, on survival of OC patients.
2. Methods
We conducted a retrospective cohort study of patients with primary diagnosis of epithelial OC in any stage who underwent treatment in our tertiary gynecologic oncology center (Kliniken Essen-Mitte, Essen, Germany) between 2011 and 2020. The therapy strategy consisted of primary debulking surgery if feasible, 6 cycles of platinum-based chemotherapy (usually carboplatin AUC 5 and paclitaxel 175 mg/m2, every 3 weeks), and maintenance therapy (in stage FIGO III/IV) with bevacizumab (if no contraindication), PARP-I, or both. The surgical strategy aimed at macroscopic complete resection was performed by a specialized team including at least one board-certified gynecologic oncologist.
Patient information, demographic/clinical, and tumor-related characteristics were extracted from medical records and retrieved from our prospectively maintained clinical tumor registry. Only patients with germline gene panel testing were included in the analysis. All patients were tested at primary diagnosis. Patients with testing after relapse were excluded.
All patients provided signed informed consent prior for documentation of clinical data in the tumor registry for clinical research, quality assurance analyses, and publication. In addition, all patients gave written informed consent according to the German genetic diagnostics law after detailed counseling. Genetic testing was performed according to German guidelines [7] and as gene panel analyses in cooperation with the German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC) in Cologne. Genetic testing and variant classification (at GC-HBOC) was performed as described previously [6].
From 2015 onwards, genetic analysis within the GC-HBOC was based on the TruRisk® gene panel test [18], including additionally other clinically relevant genes listed in the National Comprehensive Cancer Network (NCCN) breast and ovarian genetic/familiar cancer guidelines: RAD51C, RAD51D, PALB2, BRIP1, CHEK2, MLH1, MSH2, MSH6, PMS2, TP53, ATM, CDH1, NBN, EPCAM, PTEN, STK11, and BARD1 [19]. Indication of genetic counseling and testing were previously based on strict orientated individual and family history for breast/ovarian cancer. However, counseling and testing has been offered since 2017 for every OC patient based on the inclusion criteria of the GC-HBOC [7].
Based on the results of the gene panel testing, our cohort was divided into three groups: (A) no deleterious germline mutation, (B) BRCA1/2 mutation, and (C) other deleterious germline mutation.
3. Statistical Analysis
Statistical analysis was performed using GraphPad Prism (version 7) and SPSS (version 27.0, IBM Corporation, New York, NY, USA) software. Initial analysis compared background, and tumor characteristics between the different study groups, using the chi squared test for categorical data or based on variable characteristics and normal distribution t-test or non-parametric Mann–Whitney test, and ANOVA or Kruskal–Wallis H for continuous variables.
Patients’ medical history and tumor characteristics were analyzed and compared for the entire cohort and as a sensitivity analysis for subgroup advanced-stages: International Federation of Gynecology and Obstetrics (FIGO) III and IV and high-grade serous OC. Kaplan–Meier survival curves were constructed for this subgroup cohort—OS (defined as the duration of patient survival from the date of primary diagnosis) and PFS (defined as the time from primary diagnosis date until first disease recurrence or patient death without recurrence)—and compared using the Cox–Mantel log-rank test. In addition, uni- and multivariate Cox regression analyses for OS and PFS separately using the Wald test were performed for the latter patient subgroup of advanced-stage high-grade serous histology, with and without testing after recurrence, after adjusting for relevant confounders. Follow-up was performed according to the German guideline’s recommendation.
All analyses were regarded as hypothesis-generating, and a p-value of 0.05 was interpreted as being significant.
4. Results
- Entire cohort
- Patient characteristics
During the study period, 1584 patients with primary diagnosed OC were treated in our center. Of these, 702 patients (44.3%) fulfilled the inclusion criteria for this analysis with evaluable germline gene panel testing results. A majority (94%, n = 660) had been tested within 2 years from the primary diagnosis.
Table 1 presents baseline patients’ demographic and tumor characteristics in the different groups of the entire cohort: 76.6% (n = 538) had no deleterious mutation (cohort A); 17.4% (n = 122) a deleterious BRCA1/2 mutation (cohort B): BRCA1- 69.7% (n = 85), BRCA2- 30.3% (n = 37); and 6% (n = 42) showed another deleterious mutation (cohort C). The following gene mutations were detected in cohort C: 19.0% (n = 8) RAD51C, 14.3% (n = 6) MSH6, 14.3% (n = 6) BRIP1, 11.9% (n = 5) RAD51D, 11.9% (n = 5) PALB2, 9.5% (n = 4) CHEK2, 4.8% (n = 2) ATM, 4.8% (n = 2) PMS2, 4.8% (n = 2) TP53, 2.4% (n = 1) MLH1, and 2.4% (n = 1) BARD1.

Table 1.
Demographic and tumor characteristics of the entire cohort in the different study groups.
The median patient age was younger in cohort B (57 years) than in other cohorts (cohort A 60 years and cohort C 63 years, p = 0.005). Rate of high age-adjusted Charlson Comorbidity Index (ACCI ≥ 4) was significantly lower in cohort B (4.9%) than in other cohorts (14.9% and 21.4% in cohort A and C, p = 0.013). The rate of previous malignancy, mainly breast cancer, was significantly higher in patients with germline mutation (21.3%, n = 26 in cohort B and 33.4%, n = 14 in cohort C vs. 13%, n = 70 in cohort A, p = 0.001). Most cases consisted of high-grade serous histology (80.3%, n = 565), which was significantly more common in cohort B (97.5%, n = 119 vs. 73.8%, n = 31 in cohort C and 77%, n = 414 in cohort A, p < 0.001). Only one patient (1.5%) with low-grade OC histology had BRCA1/2 mutation, and a further five patients (7.6%) in other genes. Mucinous OC patients showed no germline mutations (n = 14, 2%). No significant differences between the study groups were detected for performance status, FIGO stage, surgery timing, or residual disease after debulking surgery.
In terms of systemic therapy, no significant differences were found in the application of first-line chemotherapy (a majority received combination chemotherapy with carboplatin and paclitaxel: 85.3%, n = 599) and bevacizumab maintenance therapy (59.5%, n = 418).
However, the use of PARP-i maintenance therapy in the first-line setting was significantly higher in patients with BRCA1/2 mutation (cohort B 32%, n = 39 vs 13.3%, n = 6 in cohort C and 9.9%, n = 53 in cohort A, p < 0.001).
- Survival outcome
Median OS/PFS was 57.5/26.0 months for cohort A, (not reached)/48.3 months for cohort B, and 70.7/26.3 for cohort C, which was significantly different between the groups: p < 0.001/p = 0.002, respectively.
- 2.
- Advanced-stage high-grade serous cohort
- Patient characteristics
Due to very heterogeneous survival outcomes of different OC stages/histology, we conducted separate analyses for the subgroup of advanced-stage (III–IV), high-grade serous histology (n = 513): 73.3% (n = 376) had no deleterious mutation (cohort A); 20.9% (n = 107) a deleterious BRCA1/2 mutation (cohort B); and 5.8% (n = 30) showed another deleterious mutation (cohort C).
Patient characteristics did not substantially differ between the study groups. However, the incidence of complete tumor resection was lower in cohort C (55.6%, n = 15) than other cohorts (cohort A 71.5% and cohort B 79%, Table 2). In addition, the rate of PARP-i maintenance therapy in the first-line setting was significantly higher in patients with germline mutation (cohort B 34.6%, n = 37 vs 20%, n = 6 in cohort C and 13%, n = 49 in cohort A, p < 0.001), as well after the first relapse (cohort B 49%, n = 25 vs 55%, n = 11 in cohort C and 28.7%, n = 62 in cohort A, p = 0.003, Table 2).

Table 2.
Selected primary and recurrence disease characteristics of the subgroup advanced-stage high-grade serous OC.
- Survival outcome
The Kaplan–Meier curve (Figure 1) showed significantly longer median OS for both B and C cohorts (91.1 and 70.7 months, respectively, vs 45.7 in cohort A, log rank < 0.001). Superior median PFS (Figure 2) could be demonstrated only for cohort B (37.6 months vs 23.5 for cohort C, and 22.4 in cohort A, log rank < 0.001).
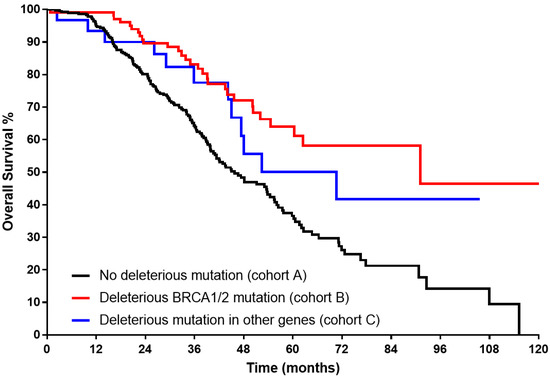
Figure 1.
Kaplan–Meier curve for overall survival of each study group in advanced-stage high-grade serous histology (log rank < 0.001).
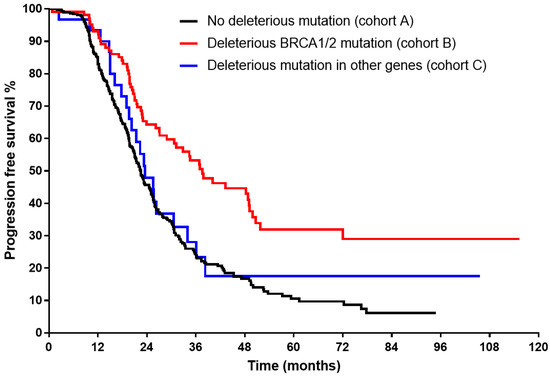
Figure 2.
Kaplan–Meier curve for progression-free survival of each study group in advanced-stage high-grade serous histology (log rank < 0.001).
- Multivariate analysis for survival
In multivariate Cox regression analysis (Table 3) for this subgroup, both cohorts B and C (vs cohort A as reference) were found to be independent factors for a significantly superior outcome: cohort C for OS (hazard ratio [HR] 0.46; 95% CI 0.25–0.84, p = 0.011), and cohort B for both OS and PFS (HR 0.40; 95% CI 0.27–0.61, p < 0.001; and HR 0.49; 95% CI 0.37–0.66, p < 0.001, respectively), while controlling for previous malignancy, ACCI, FIGO stage, surgery timing and residual tumor.

Table 3.
Uni- and multivariate Cox analysis for progression-free and overall survival of the entire advanced-stage III–IV, high-grade serous histology patients.
Further multivariate analysis for this subgroup after excluding patients that had been tested after diagnosis of recurrence showed similar findings (Table 4).

Table 4.
Uni- and multivariate Cox analysis for progression-free and overall survival of advanced-stage III–IV, high-grade serous histology patients without tests after recurrence diagnosis.
5. Discussion
Deleterious germline mutations were found in almost a quarter (23.4%) of the entire cohort included in the current study. In more than a quarter of these (25.6%) a mutation in genes other than BRCA1/2 could be identified. In our cohort of patients with advanced-stage high-grade serous OC and germline gene panel testing, deleterious mutations proved to be significantly associated with a favorable prognosis: BRCA1/2 for both OS and PFS and other genes for OS.
Survival impact of germline deleterious mutations, particularly BRCA1/2, in OC patients showed conflicting results. While some authors have shown a superior prognosis for germline BRCA1/2-mutated patients [20,21,22], others could not prove an association regarding long-term survival [23,24]. A multicenter case–control study of the Australian Ovarian Cancer Study Group found that germline BRCA1/2 mutation was an independent predictor of improved OS and PFS [25]. Pennington et al. demonstrated a similar incidence and proportion of deleterious germline mutations (26% in other genes), with better OS for both mutated patient groups—BRCA1/2 and other genes [26]. They predicted germline mutations were a significant factor for better first-line platinum-based chemotherapy response, the main hypothesis for the favorable outcome in these subgroups. Our finding of longer OS for deleterious mutation in other genes may also be addressed by adjuvant chemo- and maintenance therapy.
Following the results of the SOLO-1 study published 2018 [11], PARP-i (olaparib) was approved as primary maintenance therapy for BRCA1/2-mutated patients with advanced-stage high-grade OC. This fact supports the higher rate of PARP-i application for our BRCA1/2-mutated cohort as first-line treatment, which may be a further hypothetic explanation for the superior short-term outcome (PFS) in this subgroup.
Furthermore, recently published studies confirmed the activity of PARP-i also in breast cancer patients with other germline mutated genes such as PALB2 [27,28]. Other trials showed survival benefit from PARP-i for ATM-mutated patients with different malignancies [29,30]. The exact underlying mechanism is still unclear; however, genome instability and diminished DNA-repair pathways can increase sensitivity to PARP-i therapy. If similar effects can be expected for OC patients, further trials focusing on maintenance treatment and deleterious germline mutations other than BRCA1/2 are necessary.
Recent ESMO guidelines for risk reduction and screening of individuals with a hereditary breast and ovarian cancer syndrome suggested a broad classification of other genes according to their risk for OC [5]: high-risk genes such as RAD51C/D, moderate-risk genes such as PALB2, and no-risk mutations such as CHEK2 and TP53. A majority of deleterious other genes mutations of the present study were high-risk genes for OC.
Considering histology-related mutation rate, in agreement with our findings, high-grade serous histology stratifies the highest incidence of germline deleterious mutations, particularly in BRCA1/2 genes, while other histology shows a very low rate [31]. In our cohort, mucinous histology has never shown germline mutations, supporting the abovementioned ESGO/ESMO germline testing recommendation. The proportion of endometrioid and clear-cell histology subtypes was higher in other gene deleterious mutations, related to the frequent Lynch syndrome gene mutation [15,31,32], predominantly in MSH6.
Strengths and Limitations
The large number of participants (n = 702) in a non-selected cohort with germline gene panel testing in primary diagnosed EOC contributes to the strength of this study. In addition, the continuous follow-up allowed close evaluation of any events that occurred. Nevertheless, the study has several notable limitations, mainly due to its retrospective design. Thus, the results suggest an association only, rather than causation or underlying pathogenesis. Several tests have been performed after recurrence. Additionally, we focused on germline mutation in the mentioned genes, given the nature of our database and missed the data on somatic mutation or homologous recombination deficiency. Due to the scarcity of mutations in some genes, the small samples in the different subgroups limit the interpretation of our findings.
6. Conclusions
To the best of our knowledge, this is the largest recent single-center study to evaluate the outcome impacts of deleterious germline mutations on primary OC compared to non-mutated patients. We conclude that genetic counseling and germline panel testing provide essential information for affected OC patients and their families. Hereditary deleterious germline mutations in both BRCA1/2 or other genes were identified in a quarter of OC patients, and our data suggest a favorable prognosis in advanced-stage high-grade serous OC. Germline panel testing use to be focused on specific genes depending on histology subtype, particularly on genes other than BRCA1/2 for endometrioid and clear-cell cases. As the number of patients with mucinous OC was limited in our cohort, we could not draw any firm conclusions for this subgroup. Finally, the effect of targeted maintenance therapies for other germline-mutated OC patients necessitates further investigation.
Author Contributions
Conceptualization: B.A. and M.I.; methodology: B.A., P.H., A.d.B., K.R., R.S., A.T. and M.I.; software: B.A., M.I. and A.T.; validation: B.A. and M.I.; formal analysis: B.A., A.T. and M.I.; investigation: B.A., F.H., S.S., K.R. and R.S.; resources: B.A., P.H., A.d.B., F.H. and S.S.; data curation: B.A., M.I. and A.T.; writing—original draft preparation: B.A., P.H., A.d.B., F.H. and M.I.; writing—review and editing: all authors; visualization: B.A. and M.I.; supervision: A.d.B., P.H. and B.A.; project administration: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Landesaerztekammer Nordrhein (protocol code 51-2017, date of approval 13 March 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
No data available.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef] [PubMed]
- The Global Cancer Observatory—All Rights Reserved, December 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 1 March 2021).
- Gaona-Luviano, P.; Medina-Gaona, L.A.; Magaña-Pérez, K. Epidemiology of ovarian cancer. Chin. Clin. Oncol. 2020, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Leslie, G.; Doroszuk, A.; Schneider, S.; Allen, J.; Decker, B.; Dunning, A.M.; Redman, J.; Scarth, J.; Plaskocinska, I.; et al. Cancer Risks Associated with Germline PALB2 Pathogenic Variants: An International Study of 524 Families. J. Clin. Oncol. 2020, 38, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Sessa, C.; Balmaña, J.; Bober, S.; Cardoso, M.; Colombo, N.; Curigliano, G.; Domchek, S.; Evans, D.; Fischerova, D.; Harbeck, N.; et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2023, 34, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Hauke, J.; Heitz, F.; Reuss, A.; Kommoss, S.; Marmé, F.; Heimbach, A.; Prieske, K.; Richters, L.; Schmutzler, R.; et al. Prevalence of deleterious germline variants in risk genes including BRCA1/2 in consecutive ovarian cancer patients (AGO-TR-1). PLoS ONE 2017, 12, e0186043. [Google Scholar] [CrossRef]
- Ataseven, B.; Tripon, D.; Rhiem, K.; Harter, P.; Schneider, S.; Heitz, F.; Baert, T.; Traut, A.; Pauly, N.; Ehmann, S.; et al. Prevalence of BRCA1 and BRCA2 Mutations in Patients with Primary Ovarian Cancer - Does the German Checklist for Detecting the Risk of Hereditary Breast and Ovarian Cancer Adequately Depict the Need for Consultation? Geburtshilfe Frauenheilkd 2020, 80, 932–940. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; Du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Zeimet, A.G.; et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- SGO Clinical Practice Statement: Genetic Testing for Ovarian Cancer 2014. Available online: https://www.sgo.org/clinical-practice/guidelines/genetic-testing-for-ovarian-cancer/ (accessed on 1 July 2021).
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; DePont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Ataseven, B.; Tripon, D.; Schwameis, R.; Harter, P.; Rhiem, K.; Schneider, S.; Heikaus, S.; Baert, T.; Francesco, A.P.; du Bois, A.; et al. Clinical outcome in patients with primary epithelial ovarian cancer and germline BRCA1/2-mutation—Real life data. Gynecol. Oncol. 2021, 163, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Norquist, B.M.; Harrell, M.I.; Brady, M.F.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Yi, Q.; Burger, R.A.; et al. Inherited Mutations in Women with Ovarian Carcinoma. JAMA Oncol. 2016, 2, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Song, H.; Dicks, E.; Tyrer, J.P.; Rosenthal, A.N.; Intermaggio, M.P.; Fraser, L.; Gentry-Maharaj, A.; Hayward, J.; Philpott, S.; et al. Germline Mutations in the BRIP1, BARD1, PALB2, and NBN Genes in Women with Ovarian Cancer. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, H.; Leslie, G.; Engel, C.; Hahnen, E.; Auber, B.; Horvat, J.; Kast, K.; Jacobs, I.; Antoniou, A.C.; et al. Ovarian and Breast Cancer Risks Associated with Pathogenic Variants in RAD51C and RAD51D. J. Natl. Cancer Inst. 2020, 112, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Hauke, J.; Horvath, J.; Groß, E.; Gehrig, A.; Honisch, E.; Hackmann, K.; Schmidt, G.; Arnold, N.; Faust, U.; Hahnen, E.; et al. Gene panel testing of 5589 BRCA1/2-negative index patients with breast cancer in a routine diagnostic setting: results of the German Consortium for Hereditary Breast and Ovarian Cancer. Cancer Med. 2018, 7, 1349–1358. [Google Scholar] [CrossRef]
- Daly, M.B.; Pilarski, R.; Berry, M.; Buys, S.S.; Farmer, M.; Friedman, S.; Garber, J.E.; Kauff, N.D.; Khan, S.; Klein, C.; et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2. 2017. J. Natl. Compr. Cancer Netw. 2017, 15, 9–20. [Google Scholar] [CrossRef]
- Harter, P.; Johnson, T.; Berton-Rigaud, D.; Park, S.Y.; Friedlander, M.; Del Campo, J.M.; Shimada, M.; Forget, F.; Mirza, M.R.; Colombo, N.; et al. BRCA1/2 mutations associated with progression-free survival in ovarian cancer patients in the AGO-OVAR 16 study. Gynecol. Oncol. 2016, 140, 443–449. [Google Scholar] [CrossRef]
- Bolton, K.L.; Chenevix-Trench, G.; Goh, C.; Sadetzki, S.; Ramus, S.J.; Karlan, B.Y.; Lambrechts, D.; Despierre, E.; Barrowdale, D.; McGuffog, S.; et al. Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012, 307, 382–390. [Google Scholar] [CrossRef]
- Hjortkjær, M.; Jørgensen, M.M.A.; Waldstrøm, M.; Ørnskov, D.; Søgaard-Andersen, E.; Jakobsen, A.; Dahl-Steffensen, K. The clinical importance of BRCAness in a population-based cohort of Danish epithelial ovarian cancer. Int. J. Gynecol. Cancer 2019, 29, 166–173. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Rosen, B.; Fan, I.; Moody, J.; McLaughlin, J.R.; Risch, H.; May, T.; Sun, P.; Narod, S.A. Ten-year survival after epithelial ovarian cancer is not associated with BRCA mutation status. Gynecol. Oncol. 2016, 140, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Chen, J.; Li, Q.; Hou, J.; Wei, Y.; Yang, X.; Ma, Y.; He, H.; Zhang, Y.; Kong, B. BRCA mutation frequency and clinical features of ovarian cancer patients: A report from a Chinese study group. J. Obstet. Gynaecol. Res. 2019, 45, 2267–2274. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; DeFazio, A.; Emmanuel, C.; George, J.; .Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Mitchell, G.; et al. BRCA mutation frequency and patterns of treatment response in BRCA muta-tion-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Tung, N.M.; Robson, M.E.; Ventz, S.; Santa-Maria, C.A.; Nanda, R.; Marcom, P.K.; Shah, P.D.; Ballinger, T.J.; Yang, E.S.; Vinayak, S.; et al. TBCRC 048: Phase II Study of Olaparib for Metastatic Breast Cancer and Mutations in Homologous Recombination-Related Genes. J. Clin. Oncol. 2020, 38, 4274–4282. [Google Scholar] [CrossRef] [PubMed]
- Gruber, J.J.; Afghahi, A.; Hatton, A.; Scott, D.; McMillan, A.; Ford, J.M.; Telli, M.L. Talazoparib beyond BRCA: A phase II trial of talazoparib monotherapy in BRCA1 and BRCA2 wild-type patients with advanced HER2-negative breast cancer or other solid tumors with a mutation in ho-mologous recombination (HR) pathway genes. J. Clin. Oncol. 2019, 37, 3006. [Google Scholar] [CrossRef]
- Bang, Y.-J.; Im, S.-A.; Lee, K.-W.; Cho, J.Y.; Song, E.-K.; Lee, K.H.; Kim, Y.H.; Park, J.O.; Chun, H.G.; Zang, D.Y.; et al. Randomized, Double-Blind Phase II Trial with Prospective Classification by ATM Protein Level to Evaluate the Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients With Recurrent or Metastatic Gastric Cancer. J. Clin. Oncol. 2015, 33, 3858–3865. [Google Scholar] [CrossRef]
- Mateo, J.; Carreira, S.; Sandhu, S.; Miranda, S.; Mossop, H.; Perez-Lopez, R.; Nava Rodrigues, D.; Robinson, D.; Omlin, A.; Tunariu, N.; et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N. Engl. J. Med. 2015, 373, 1697–1708. [Google Scholar] [CrossRef]
- Carter, N.J.; Marshall, M.L.; Susswein, L.R.; Zorn, K.K.; Hiraki, S.; Arvai, K.J.; Torene, R.I.; McGill, A.K.; Yackowski, L.; Murphy, P.D.; et al. Germline pathogenic variants identified in women with ovarian tumors. Gynecol. Oncol. 2018, 151, 481–488. [Google Scholar] [CrossRef]
- Kim, R.S.; Oldfield, L.E.; Tone, A.; Pollett, A.; Van De Laar, E.; Pedersen, S.; Wellum, J.; Clarke, B.; Pugh, T.J.; Ferguson, S. Comprehensive molecular assessment of mismatch repair deficiency in Lynch-associated ovarian cancers using next-generation sequencing (NGS) panel. J. Clin. Oncol. 2020, 38, 1523. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).