Simple Summary
Lymph node (LN) metastases are a significant concern in urological malignancies. However, current imaging techniques do not reliably detect micrometastases; thus, lymph node dissection (LND) remains the gold standard. Nevertheless, there is no established ideal LND template, leading to unnecessary invasive staging and the possibility of missing LN metastases located outside the standard template. The sentinel lymph node (SLN) concept has been proposed to address this issue, but its application in uro-oncology is still mostly experimental. Nevertheless, new techniques may improve its potential. This review aims to discuss the current and future role of the SLN procedure in managing urological malignancies.
Abstract
Lymph node (LN) metastases have a significant negative impact on the prognosis of urological malignancies. Unfortunately, current imaging modalities are insufficient when it comes to detecting micrometastases; thus, surgical LN removal is commonly used. However, there is still no established ideal lymph node dissection (LND) template, leading to unnecessary invasive staging and the possibility of missing LN metastases located outside the standard template. To address this issue, the sentinel lymph node (SLN) concept has been proposed. This technique involves identifying and removing the first group of draining LNs, which can accurately stage cancer. While successful in breast cancer and melanoma, the SLN technique in urologic oncology is still considered experimental due to high false-negative rates and lack of data in prostate, bladder, and kidney cancer. Nevertheless, the development of new tracers, imaging modalities, and surgical techniques may improve the potential of the SLN procedures in urological oncology. In this review, we aim to discuss the current knowledge and future contributions of the SLN procedure in the management of urological malignancies.
1. Introduction
The basic assumption of sentinel lymph node biopsy (SLNB) is the concept of malignant cells spreading from the primary tumor through the lymphatic vessels to a selected group of lymph nodes (LNs), called sentinel LNs (SLNs), before they reach LNs further downstream. In the beginning, SLNB was used to investigate the routes of lymphatic spread taken by malignant tumors and study the anatomy of lymphatic drainage from primary site. With time, SLNB started to be used as a method to improve nodal staging, reduce morbidity, and improve survival in some types of cancer. Currently, it is known that macrometastases (metastases larger than 2 mm) worsen the prognosis of the disease, as do micrometastases (defined as clusters of cancer cells that are between 0.2 mm and 2 mm in diameter) in the sentinel node. Research to determine the significance of micrometastases in the sentinel node is ongoing [1,2]. Penile cancer (PeCa) is a type of cancer that is ideal for further investigating the SLNB concept because of its characteristic of lymphatic spread in which distant metastases without prior involvement of inguinal LNs occur extremally rarely. In some types of cancer, such as PeCa, melanoma, or breast cancer, the role of SLNB has been established and applied in clinical practice with good outcomes for many years. For instance, in breast cancer, SLNB, rather than complete axillary lymph node dissection (LND), is recommended in clinically node-negative breast cancer; this approach results in less morbidity, such as arm swelling and shorter hospital stay [3]. Guidelines for the treatment of melanoma, as well as breast cancer, underline that SLNB should be performed in experienced centers [4]. In other malignancies, such as prostate, bladder, or kidney cancer, the role of SLNB is still undetermined, and further investigations are needed. SLN techniques are also explored in genitourinary cancers in women, such as vulvar, cervical, or endometrial cancer [5,6,7,8,9]. However, for the purposes of this study, we decided to focus on neoplasms primarily in the range of urologists’ management strategies. The aim of this review is to summarize the available data on the utility of SLN techniques in current urologic oncology, as well as to point out future directions in this field.
2. Markers and Imaging Methods
With the continuous development of new imaging methods and operation techniques, the procedure of SLNB has evolved in order to improve outcomes.
In the first study on SLNB, by Cabanas et al. published in 1977, the authors did not use any marker; surgeons relied only on lymphangiograms and surgical anatomical landmarks during operation [10]. Improvements in intraoperative lymphatic drainage visualization were achieved through introducing blue dye injection into the tumor [11,12]. This method had many limitations, such as a spillage effect which could worsen the visibility of anatomical structures in the operating field, and staining of the application site, which could potentially hamper the resection of the primary tumor [13]. Nowadays, there are many different types of blue dyes in use, most commonly patent blue, methylene blue, and isosulfan blue. A meta-analysis conducted by Peek et al. on the impact of different types of blue dye on the detection rate of SLN revealed that methylene blue presented the lowest false-negative rates, but no superiority of any particular blue dye could be proven in regard to detection rate [14]. The use of blue dye is associated with the risk of allergic reactions and even anaphylaxis. A recent meta-analysis and systematic review on a group of over 60,000 SLNB procedures has shown a weighed anaphylaxis rate of 0.061%, identifying isosulfan blue as the most anaphylactogenic blue dye [15].
The next step in the development of the SLNB procedure came with two studies published in 1993 on the use of a radiotracer and gamma probe for LN detection, in which the authors showed that this method is feasible and improves detection rate [16,17]. The most commonly used radiotracer is 99mTechnetium (99mTc). The advantages of using a radioisotope include good tissue penetration, enabling the detection of deeper LNs, as well as preoperative visual information (with the use of a gamma camera) and acoustic guidance during operation (generated by a gamma probe). However, significant restrictions related to the use of these markers should be noted, such as the need to have a nuclear medicine department, a specific period of time in which the procedure should be performed (related to the radiotracer decay), and exposure to ionizing radiation of medical staff (which, while maintaining standard safety procedures, is at a low, safe level) [18]. A meta-analysis conducted on a large group of patients with breast cancer has proven the high accuracy of this method in detecting SLNs, with detection rates ranging between 85 and 100% [19]. However, the combined method using a radiotracer and blue dye simultaneously is more commonly seen in practice. The data suggest higher detection rates of SLN for the combined approach, rather than for the use of a radioisotope alone [20].
To improve intraoperative visualization of SLNs, surgeons started using indocyanine green (ICG), a fluorescent marker. ICG accumulates in tissues and, in near-infrared (NIR) imaging, allows for the visualization of LNs. In comparison with blue dye, ICG has a deeper tissue penetration (though this is still very limited compared to a radiotracer and can be significantly reduced in patients with a higher body mass index) and does not stain the injection site or surgical field during tissue preparation (ICG is invisible in white light; to visualize SLNs, an additional fluorescence camera is required) [21]. In contrast to a 99mTc-nanocolloid tracer, the use of ICG dye does not require a nuclear medicine facility, and there is no risk of additional medical staff exposure to radiation. Furthermore, the development of laparoscopic cameras and software facilitates better visualizing potential SLNs during surgery with the use of different light filters and image processing in real time (e.g., Figure 1). A meta-analysis on cervical cancer patients regarding the effectiveness of the method in detecting SLN reported a higher bilateral detection rate for ICG than for 99mTc with blue dye; 90.3% versus 73.5%, respectively [22]. Similar results were achieved in large studies comparing ICG to other methods in endometrial cancer and esophageal cancer, but at the same time, results were inconclusive for lung cancer and colorectal cancer [23,24,25,26].
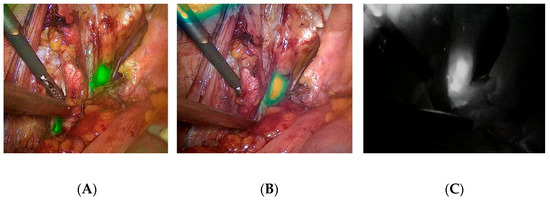
Figure 1.
Different presentation of potential SLNs during laparoscopic RP and SLNB. LNs are marked as green or white regions: (A) NIR/ICG + white light overlay image; (B) intensity map presentation; (C) monochromatic presentation. Images were taken using the IMAGE1 S™ RUBINA-KARL STORZ system.
The introduction of hybrid markers, such as 99mTc-nanocolloid with ICG, reduced some limitations in the application of both techniques while maintaining their advantages (e.g., deeper penetration of radiotracer and intraoperative visualization of SLNs without staining other tissues in the operation field via ICG) [27]. A study by KleinJan et al. revealed the superiority of a hybrid tracer over the blue dye technique in various malignancies. Blue dye-based detection rates were poor compared to that of the hybrid tracer (p < 0.001) [28]. In a recent study by Wit et al., a hybrid approach appeared to be more effective than 99mTc-nanocolloid and ICG alone, with regard to the detection of PCa. The rate of metastatic fluorescent LNs was higher in the hybrid group (7.4%) compared to the sequential group (2.6%; p = 0.002) [29].
With the development of imaging techniques, methods such as single-photon emission computed tomography (SPECT/CT) were implemented to improve the preoperative visualization of lymphatic drainage and potential SLNs. With the aid of SPECT/CT, surgeons obtain precise, three-dimensional information on the location of LNs and their relationship to other anatomical structures, which allows them to better plan the operation and facilitate the detection of SLNs [30,31]. Due to the rising importance of prostate-specific membrane antigen (PSMA) in PCa diagnostics, positron emission tomography (PET) has been adopted as an SLN techniques as well. A recent meta-analysis including 10 studies on LN the assessment of metastases using 68Ga-PSMA PET/CT revealed a per-patient pooled sensitivity of 61% and specificity of 96%. However, the authors emphasized the major heterogeneity of their findings, as the sensitivity ranged from 33.3% to 96.08% [32]. Both SPECT and PET require further prospective, large cohort studies to evaluate their efficacy with various markers and to prove their accuracy.
Currently, more papers describing a new technique using superparamagnetic iron oxide nanoparticles (SPIONs) as a tag in SLNB are being published. This new imaging technique could potentially replace radiocolloid tracers in the future, providing a nonradioactive solution. After injection around the primary tumor before surgery, SPIONs migrate through the lymphatic drainage system and accumulate in SLNs, acting as a contrast agent. LNs can be then visualized via MRI. During operation, tissues in which SPIONs are accumulated can be detected using a handheld magnetometer [33]. A recent study reported the development of a laparoscopic differential magnetometer probe, which showed promising results in terms of intraoperative SLN localization [34]. Although it is a relatively new method, the available data indicate its high efficiency in detecting SLNs. The SentiMag Pro II Study regarding the detectability of SLNs in intermediate- and high-risk prostate cancer reported a 100% identification rate, with a high sensitivity and specificity, 100% and 97%, respectively [35]. This translates into the possibility of reducing overtreatment, such as unnecessary LND. As the authors of another study demonstrated, in the case of treating patients with a 5–20% lymph node invasion risk prior to surgery, extended LND could be omitted in favor of only sentinel LND (SLND), as SPION-guided SLND is reliable in detecting almost all LN metastases in this group of cancer patients [36]. The use of SPIONs enables the more accurate identification of nodal micrometastases [37,38].
An alternative method to detect SLN first introduced in breast cancer surgery is contrast enhanced ultrasound (CEUS) using microbubbles [39]. This technique is based on the dispersion effect of microbubbles as contrast agents with sulfur hexafluoride gas, which are injected intradermally. Among the advantages of this approach is real time visualization. Moreover, this approach only requires an ultrasound machine and contrast agent—there is no need for a nuclear medicine department. Up until this point, reports of patients experiencing allergic reactions due to this approach are scarce. However, this type of imaging method is biased; hence, the visual outcome of a USG-based technique depends on the operator’s skills. Linked to this obstacle is another disadvantage of the technique, which is the long learning curve [40]. CEUS with the use of microbubbles is a new technique which still requires further investigation. A systematic review revealed that the combined sensitivity of SLN identified by CEUS in diagnosing overall sentinel nodes pathological status is 98%, specificity is 100% [41]. Further studies are necessary to standardize the method and clarify its specificity and sensitivity. As of now, there are no published studies investigating the use of this technique in the detection of SLN in urologic malignancies.
3. Urological Cancers
3.1. Penile Cancer
PeCa is a cancer that metastasizes primarily through the lymphatic pathway, first through the inguinal LNs, and then to the pelvic regions. The detection of metastatic LNs is crucial because the presence of nodal metastases is the most important prognostic factor in the survival of patients with PeCa and determines future treatment [42].
Radical inguinal lymph node dissection (ILND) is associated with significant morbidity. In a study conducted by Gopman, a wide spectrum of complications after ILND and their prevalence were described, namely the following: minor complications such as wound infection, seroma, lymphocele, wound dehiscence, cellulitis, scrotal edema, fever, or thigh numbness occurred in 65.7% of patients, while major complications occurred in 34.3% of patients and included the most severe conditions such as wound infections treated with i.v. antibiotics, skin flap necrosis, lymphocele requiring surgical intervention, nonhealing wounds, hematoma, pulmonary embolism, and sepsis [43].
The high prevalence of major complications after ILND and a high rate of overtreated patients without palpable inguinal LNs (75–80% of patients in cN0 stage did not develop metastases) initiated the need to develop a less invasive but still reliable method of nodal staging [42,44].
The first attempt to reduce the extent of lymphadenectomy without compromising oncological outcomes was the SLNB described in 1977 by Cabanas et al. on a group of patients with PeCa [10]. The promising results of this study initiated a series of studies improving the techniques of detecting SLNs and evaluating the oncological results of patients undergoing this procedure, which confirmed the benefits of its use.
This is reflected in the European Association of Urology (EAU) guidelines, which recommend performing SLNB in the case of cN0 high-risk tumors (pT1b G2 or any pT2–4), as well as individually considering SLNB in the case of intermediate-risk tumors (pT1a G2) [45]. If the SLNB shows the presence of neoplastic changes, total ILND should be performed on the same side. However, if SLNB is negative, the patient undergoes regular physician or self-examination with an optional ultrasound inspection and fine needle aspiration cytology of the inguinal LNs every 3 months (annually after 2 years). Despite the continuous development of imaging techniques, the sensitivity of noninvasive imaging methods of the affected LNs is still insufficient, which makes surgical SLNB still irreplaceable because, in up to 25% of patients in cN0 stage, micrometastases are present in inguinal LNs [45,46].
The choice of a less invasive procedure, which is SLNB compared to the classic ILND, is associated with a significantly lower complication rate (in up to 55% of patients after ILND and in about 5.7–7% of patients after SLNB) [43,47,48].
Initial studies on the reliability of SLNB showed a high false-negative rate (FNR) of 22% per patient, which means that 22% of patients with metastases in inguinal LNs were not detected with this method [49]. New research conducted in recent years aimed to develop an optimal protocol and improve the technical aspects of SLNB while maintaining the lowest possible number of complications and FNR results. Recent data showed that, along with the increase in experience of centers performing SLNB, the improvement of imaging methods, techniques of SLNB, and more detailed histopathological assessment, the FNR improved to within a range from 4.8% to 8.7% per groin (10% per patient) [27,47]. Moreover, the use of a 1-day protocol instead of a 2-day protocol can additionally decrease FNR, but with a slightly higher complication rate [50].
Simultaneously with the development of intraoperative techniques for detecting SLNs, imaging methods were improved, allowing medical professionals to plan the course of the procedure itself through the precise visualization of lymphatic drainage and more accurate determination of the SLNs. Primarily, simple lymphoscintigrams were applied to visualize SLNs. Subsequently, SPECT/CT became the new standard because of the better results in detection and more precise anatomical visualization of SLNs (due to providing 3D anatomical information) with fewer false-positive findings than planar scintigraphy [30].
Currently, the most common method of performing SLNB is by using 99mTc-nanocolloid in combination with SPECT/CT, often with additional blue dye. The first step is to administer 3–4 doses of the tracer around the penis and the primary tumor the day before or on the day of the procedure depending on the implemented 1- or 2-day protocol. Then, after 10, 20, and 120 min, lymphoscintigraphy and SPECT/CT are performed. Immediately before the procedure, the location of the marker accumulation can be verified using a portable gamma camera. Intraoperatively, SLNs are detected with the use of a gamma probe (probe generates sound when a radioactive signal is detected) and then excised. Removed tissues are submitted for histopathological examination performed via serial sectioning and immunohistochemical staining to determine SLN status [51].
Since 2012, many studies describing the beneficial effect of combining 99mTc-nanocolloid with ICG in relation to the increased detection of SLNs and lower FNR have been published. These outcomes emerge from the complementary properties of both markers. The results of a study by Dell’Oglio et al. confirmed the reliability and safety of using hybrid tracers with SPECT in the largest group of patients with PeCa who underwent SLNB [27]. The use of ICG–99mTc-nanocolloid resulted in a significantly better visualization rate of SLNs than blue dye (95% vs. 56% intraoperative visualization rate). However, the authors of this study emphasized that the use of hybrid markers for SLNB still requires multicenter validation and greater evaluation of the cost-effectiveness of this procedure (an example of the use of ICG during SLNB is shown in Figure 2).
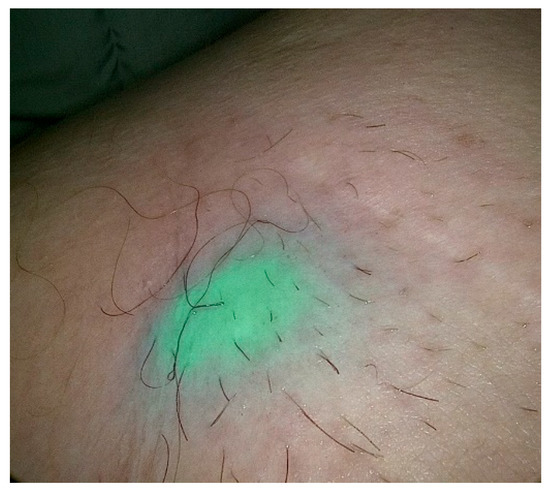
Figure 2.
Percutaneous view of ICG marked SLN during SLNB procedure in patients with PeCa.
The use of SPIONs for SLNB in patients with PeCa was first reported by Tabatabaei et al. in 2005 [52]. In the past few years, new studies on this topic have been published, suggesting that this technique is reliable and safe but needs further investigation [53,54]. A recent study by Azargoshasb et al. reported the implementation of a hybrid ICG–SPION approach in phantom measurements, ex vivo human skin explants, and porcine models, with the intention of future application in PeCa surgery [55].
A recently published systematic review by Fallara et al. evaluated the diagnostic accuracy of dynamic sentinel lymph node biopsy (DSLNB) in a total of 2893 cN0 patients. Patients were offered further radical ILND if DSLNB revealed micrometastases. DSLNB showed a pooled weighted sensitivity of 0.50 (95% CI: 0.24–0.59), specificity of 0.82 (95% CI: 0.78–0.87), and log diagnostic odds ratio of 1.18 (95% CI: 0.29–2.97) for detecting further positive LNs at radical LND. The authors concluded that, at this point, a positive DSLNB is poorly able to distinguish further metastatic involvement upon completion of radical LND; therefore, the diagnostic accuracy of DSLNB requires further improvement [56].
De Vries et al. recently published the results of their multicenter study on the clinicopathological predictors of finding additional inguinal LNM in PeCa patients after positive DSLNB [57]. Their results corresponded to the above conclusion. Among 407 inguinal basins with a positive DSLNB, additional LNMs at ILND were present in only 64 of them (16%). A clinical prediction model based on the number of positive nodes at positive DSLNB and largest metastasis size in mm was constructed. A statistical analysis of the model revealed that it did not show clinical benefit in predicting whether to omit further surgical intervention or carry out ILND. Therefore, the completion of ILND remains indispensable in all basins with a positive DSLNB [57].
At present, further scientific research in the field of SLNB in PeCa is being conducted. This is expressed in the EAU announcement of including the results of an ongoing systematic review and meta-analysis of minimally invasive procedures for inguinal nodal staging in penile carcinoma (DSLNB and videoendoscopic inguinal lymphadenectomy [VEIL]) in the updated 2024 EAU–American Society of Clinical Oncology (ASCO) Penile Cancer Guidelines [45].
3.2. Prostate Cancer
Prostate cancer (PCa), unlike PeCa, represents one of the most common malignancies. It is the second most prevalent cancer and the fifth leading cause of cancer-related death in men, and it is the most frequently diagnosed cancer in 112 countries of the world [58]. One of the most significant issues in the management of PCa patients is a process of metastasis to LNs, which negatively impacts survival, influences future therapeutic decisions, and occurs in 3–42% of patients [59,60]. Extended pelvic lymph node dissection (ePLND) conducted during radical prostatectomy (RP) remains the gold-standard nodal staging procedure due to the suboptimal accuracy of preoperative imaging methods [61,62,63,64]. Intermediate- and high-risk PCa patients have a 5–70% risk of developing nodal metastases [65]. Therefore, ePLND is recommended in these groups when the risk of nodal involvement exceeds 5% [66,67]. However, the precise extent of the PLND template is still debatable, as the wide extent of lymphadenectomy may lead to a variety of comorbidities, such as lymphoceles, obturator nerve injuries, and lymphedema [68,69,70]. The oncological outcomes of PLND remain questionable, partially because nodal metastases may occur outside of the recommended PLND template [71,72,73]. SLNB would hopefully detect additional metastatic LNs, reduce the frequency of complications, and decrease the number of PLND procedures.
At this point, the EAU guidelines point toward the insufficiency of evidence supporting SLNB [66]. This state may be due to various factors, including significant the heterogeneity and inconsistency of definitions, thresholds, types of tracers, surgical approaches, detection methods, and outcome measurements [74,75,76]. SLNB in PCa was first described by Wawroschek et al. in 1999, and then investigated by the same group, as well as Rudoni et al. [77,78,79]. Now, almost 25 years later, SLNB is still considered an experimental procedure in PCa [80].
In the SLNB technique presented by Wawroschek et al., 99mTc-nanocolloid was injected into the prostate before open retropubic RP. All preoperatively and intraoperatively identified LNs (‘hot spots’) are defined as SLNs [77]. Since then, novel variants of different radiotracers have been thoroughly investigated. In 1999, Motomura et al. introduced the use of ICG for detecting SLNs in breast cancer patients intraoperatively using a laparoscopic NIR fluorescence camera [81]. In 2011, Inoue et al. were the first to describe lymphoscintigraphy with ICG in PCa patients during open surgery [82]. Van der Poel et al. described the use of a hybrid tracer—a combination of a fluorescent ICG molecule with a 99mTc radiotracer. The tracers were fused and injected simultaneously into the prostate [83]. The authors pointed out that the use of a hybrid tracer, in addition to preoperative SLN mapping, facilitates the intraoperative detection of SLNs. Further studies have demonstrated the overall benefits of both pre- and intraoperative detection compared to preoperative detection alone [84]. Jeschke et al. described a different approach. They applied the sequential dual-tracing method using separate injections of a radiocolloid and free ICG, allowing SLN detection both pre- and intraoperatively [85]. Other radiotracers mainly include 99mTc-labeled colloids (albumin, sulfur, or phytate) and novel particles, such as SPIONs [86,87,88]. In addition to the variety of tracers, diverse navigational systems are used during SLNB in PCa, and their role is to optimize the procedure and improve detection rates of SLNs [73,84,89,90,91,92,93,94,95].
The most common method for tracer injections is the transrectal or the transperineal route. The tracer is injected into each lobe of the prostate under ultrasonographic guidance. The volume of the injected tracer depends on its type. A study by Manny et al. evaluated tracer administration routes. They concluded that robotic-guided intraoperative ICG injection was more effective than that through the cystoscope or transrectal routes [90]. A study by de Korne et al. revealed a correlation between the localization of the tracer, the location of the tumor, and SLNs. The authors concluded that tracer injection near or into the cancerous tissue is beneficial for detection [96]. Nevertheless, the most efficient method of tracer administration remains undefined, and more studies are required to optimize the procedure.
There were three major systematic reviews concerning SLN techniques [75,97,98]. Although the authors investigated slightly different aspects of SLN techniques, they observed a low specificity (except the systematic review by Wit et al.) and overall usability but indicated promising mapping qualities.
More recent studies revealed the plausible advantages of SLN techniques incorporating novel tracers, various imaging techniques, and other procedures (namely, ePLND). A study by Claps et al. showed the staging benefit of ePLND combined with ICG-guided LND over ePLND alone. The group with additional fluorescence guidance had a higher rate of LN metastases (65.9% vs. 34.1%, p = 0.01) in the overall cohort and higher rates of 5-year BCR-free survival (54.1% vs. 24.9%, p = 0.023) in the pN+ cohort [99]. Similar results were revealed by Grivas et al. [100]. Hinsenveld et al. evaluated the additive effect of the PSMA PET/CT and SLNB combination. Blending these modalities resulted in the correct nodal staging of 50 out of 53 patients (94% diagnostic accuracy). All pN+ patients were identified [101]. A study by Fumadó et al. investigated SLNB, preceded by the identification of an index lesion (IL) via MRI, which was a target for the radiotracer injection. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 94.4%, 100%, 100%, and 97.8%, respectively. Negative SLNB was a predictor of negative ePLND. The false-negative (FN) rate was 5.5%, whereby one patient had nodal metastasis, despite negative SLNs. Moreover, only 10 of 18 patients (55.6%) had metastasis to SLNs only [80]. Some studies showed similar results, indicating high accuracy rates, but many of them also revealed low detection rates of all metastatic LNs, preventing the application of SLND only [102,103,104,105,106,107]. Another study evaluating the efficacy of intratumoral (IT) radiotracer injections was conducted by Wit et al. In the IT group, metastatic SLNs were 73.7% of all positive LNs compared to 37.3% in the group receiving intraprostatic (IP) injections (p = 0.015). The authors suggested combining IT and IP routes while performing ICG–99mTc-nanocolloid injections in patients with an imaging-visible tumor [108]. Another promising direction of SLN-centered investigations is the usage of magnetometer-based techniques and SPIONs [109]. The first studies revealed very promising outcomes, although the reliability of magnetometer techniques require further, more thorough studies involving larger cohorts [35,36,110,111].
In a PCa setting, SLNB aims to improve the accuracy of positive LN detection and reduce the morbidity associated with ePLND. Additionally, this technique might allow the precise determination of individual lymphatic drainage patterns for each patient, avoiding the omission of potentially metastatic LNs outside of the traditional ePLND template.
3.3. Bladder Cancer
SLNB for bladder cancer (BCa) is a promising technique, but its application is limited due to the anatomical complexities of the bladder’s bilateral lymphatic drainage system and intricate mechanism of lymphatic metastasis [112]. The primary lymphatic drainage from the bladder flows toward multiple groups of LNs in the pelvis, serving as intermediate stations, and it may extend beyond the perivesical region, resulting in challenges for the SLNB technique. In 2001, the first study on the use of SLNB in patients with BCa was published by Sherif et al. Before the surgery, the authors performed planar lymphoscintigraphy and then intraoperatively used a gamma probe to detect SLNs. At least one SLN was detected in 11 out of 12 patients [113].
More recently, Zarifmahmoudi et al. conducted a meta-analysis on SLNB in muscle-invasive bladder cancer (MIBC), which included 336 patients [114]. In the analyzed studies, the authors performed ePLND after detecting and excising SLNs to verify the accuracy of the SLNB technique in nodal staging. The technical aspects of the SLNB procedure differed between studies. The main differences included the injection site (submucosal or into the detrusor muscle), used markers (radioisotope, ICG, or blue dye), and the method of detection. A crucial indicator of diagnostic performance in SLNB is the false-negative (FN) rate. Various factors can contribute to an increased FN rate, including technical difficulties during tracer injection, obstruction of lymph flow to SLNs due to the metastatic mass, tracer redirection to a non-SLN, and unilateral SLN detection despite bilateral involvement [115,116,117]. The pooled detection rate of the studies included was 90% (95% CI: 85.2–93.3%), and the combined sensitivity was 79% (95% CI: 0.69–0.89%). Overall, the authors showed that pT1–2 BCa patients with cN0 would be the most suitable cohort for SLNB [114]. Analyzing SLNB in relation to the primary tumor advancement stage, including pT3/4 stage patients, results in a decreased sensitivity of 70%. However, when this group of patients is excluded, the sensitivity of the technique increases to 93%. Therefore, SLNB mapping exhibits the highest accuracy in patients with MIBC at lower local advancement stages. This finding is in accordance with similar studies in other malignancies [118,119]. More recent studies have assessed SLN techniques in various clinical settings and concluded that they are feasible and safe in BCa, although their efficacy remains questionable [120,121,122]. In 2022, Rietbergen et al. initiated a prospective pilot study evaluating the usage of a hybrid ICG–99mTc-nanocolloid radiotracer. In three patients, SLNs were outside the ePLND template, and in 53% of the patients, the preoperative SLN procedure was successful [123].
Despite revealing a high sensitivity for BCa, studies on SLNB require larger prospective trials to confirm the reliability and accuracy of this nodal staging tool and to standardize the procedure.
3.4. Renal Cancer
The role of LND in renal cell carcinoma (RCCa) remains controversial. Research conducted in order to establish a potential survival benefit after LND failed to do so [124]. The cause of this may be the hematogenous route of spread in RCCa. Moreover, unpredictable lymphatic drainage may hamper the detection of suspicious LNs and, as a consequence, the completeness of LND and its outcome. The majority of patients in the pN+ stage simultaneously acquire hematogenous metastases [125]. Thus, the presence of metastatic LNs may indicate a systematic spread of disease, which significantly worsens survival prognosis in patients with RCCa.
SLNB can potentially improve the detection of metastatic LNs. The first two studies by Bex et al. on SLNB in patients with cT1–2N0M0 RCCa were conducted with the use of 99mTc-nanocolloid injected percutaneously under ultrasonography guidance into the primary tumor the day before surgery [126,127]. After the injection, lymphoscintigraphy and SPECT/CT were performed. During surgery with the use of a gamma probe and gamma camera, the surgeons localized and then removed SLNs with adherent lymphatic tissues. In the case of the first study, all nodes could be visualized with the use of SPECT/CT, whereas in the case of the second study, SPECT/CT failed to detect nodes in approximately 30% of cases. Nevertheless, it outperformed planar lymphoscintigraphy in lymphatic mapping. The authors concluded that SLNB in RCCa is feasible and safe, bringing new data on lymphatic drainage in this type of tumor, which could be useful for planning the extent of LND. Nevertheless, the non-visualization of lymph nodes, up to this day, remains one of the limiting factors for broad the clinical implementation of the SLNB procedure using SPECT/CT in RCCa, as shown by another study investigating the possible factors influencing detection rate [128].
The issue of isolated metastatic LNs in the mediastinum, frequently observed in RCCa, was the cause of further investigations on the lymphatic spread in this type of cancer. A study by Brouwer et al., in which the authors examined lymphatic drainage from RCCa using intratumorally injected 99mTc-nanocolloid followed by lymphoscintigraphy and SPECT/CT imaging, showed that, in about 18% of patients with visualized SLNs, a direct drainage throughout the thoracic duct occurred, omitting LNs [129]. In the largest prospective study investigating the pattern and location of lymphatic drainage from renal tumors using a radiotracer by Kuusk et al., the authors detected supra-diaphragmatic SLNs in 20% of patients [130]. These results may support the hypothesis of an important role of lymphovenous connections between renal tumors and the thoracic duct in the unpredictable systemic spread of RCCa.
ICG fluorescent lymphography is a technique which potentially could improve SLN detection in RCCa. The promising results of the application of ICG in other types of cancer make it worth investigating in RCCa patients [131].
Hybrid tracers such as ICG combined with 99mTc-nanocolloid and used with SPECT/CT before surgery present promising results in patients treated for PeCa. To date, there is no study investigating this very promising approach in patients with RCCa; thus, its impact here remains to be seen.
SLNB with locoregional LND in RCCa seems to be a surgically safe procedure, with no significant variations in complication rate across different surgical techniques [132]. However, due to the lack of large prospective studies, SLNB in patients with RCCa currently has no clinical implication. Conclusions from studies in which patterns of lymphatic drainage from renal tumors were investigated to apply the SLNB procedure cannot yet be implemented in clinical practice, and perhaps never will be, because of the very complex way in which RCCa metastasizes.
3.5. Testicular Cancer
SLNB in patients with testicular germ cell tumor (TGCT) was first described in 2002. The authors of two different studies on clinical stage I TGCT patients demonstrated the feasibility of this method [133,134]. After the injection of a single dose of 99mTc-nanocolloid into the funiculus or testicular parenchyma around the tumor, lymphoscintigraphy was performed. During laparoscopic SLNB, an endoscopic gamma probe was used to facilitate the detection of SLNs. In a study by Tanis et al., in two patients, patent blue dye was additionally injected intratesticularly during surgery, with no discoloration of the detected SLNs. After the initial funicular administration of the radioactive tracer, lymphoscintigraphy showed lymphatic drainage only to the inguinal region, meaning that the injection site had to be changed and the injection had to be administered intratesticularly [133]. Intratesticular injection enabled the visualization of SLNs in the para-aortic region (especially in the cases of left testis tumors), as well as the inter-aortocaval, paracaval, or common iliac region (when primary tumors were in the right testis) [134].
In a study by Satoh et al., SLNB was investigated in a group of 22 patients with clinical stage I TGTC and was the first in which all participants underwent laparoscopic retroperitoneal PLND (RPLND). The radiotracer administration protocol was similar to previous studies except for the time of tracer administration, which in this case, was always 24 h before surgery. SLNs were detected in 95% of patients, and nearly all were localized on the ventral or lateral side of the vena cava or at the aorta between the levels of the aortic bifurcation and under the levels of the gonadal arteries. Two micrometastases were found out of a group of 22 patients: 1 case of seminoma and 1 case of nonseminoma. These two patients were treated with two cycles of BEP without relapse for 29 months (seminoma) and 31 months (non-seminoma) of follow-up. All dissected non-sentinel retroperitoneal LNs were free of metastases [135].
Further studies using SPECT/CT and the portable gamma camera revealed that the use of an intraoperative gamma probe can improve the detection of additional SLNs by up to 20% [136].
Currently, in the largest study conducted by Blok et al., occult micrometastases were detected in 13% of patients diagnosed with TGCT in clinical stage I who underwent SLNB. The early detection of nodal metastases allowed for the early initiation of adjuvant systemic treatment. All patients with TGCT were without evidence of disease at a median follow-up of 63.9 months [137].
The excellent treatment results of TGCT, with 5- and 10-year overall cancer-specific survival rates close to 100%, indicate that there is no urgent need to improve the methods of treatment and diagnostics in these patients [138]. Moreover, the currently accepted scheme of surveillance after surgical treatment allows for the early detection of disease relapse or lymphatic spread. Additionally, in the case of disease recurrence, due to the high chemosensitivity of this type of tumor, it can be treated with good results using systemic therapy, but this adjuvant therapy also has potential serious short- and long-term side-effects. The use of SLNB could allow for a better selection of patients being eligible for adjuvant treatment; in patients with detected occult nodal metastases, it would allow for the commencement of treatment at an early stage, while in patients with negative biopsy, it would enable the safe activation of active surveillance. This approach could result in an overall reduction in complications associated with adjuvant therapy by lowering the risk of relapse through early treatment and of unnecessary adjuvant therapy in patients with negative biopsy. Another potential benefit of applying SLNB may be the reduction in the frequency of CT scans in biopsy negative patients, which given the young age of patients, may be beneficial due to the significant reduction in radiation exposure. Due to the limited amount of research on this issue and potential complications resulting from RPLND, further work is necessary to be able to consider changes in the currently adopted procedure and recommendations.
Continuous investigation regarding the use of the SLN technique in TGCT is in progress. A recently published paper by Zarifmahmoudi et al. described a series of nine patients with a history of non-seminomatous testicular cancer who underwent radical orchidectomy and systemic chemotherapy in the past but were qualified for RPLND because of the presence of residual retroperitoneal masses of size ≥ 1 cm. SLN mapping using 99mTc-labeled phytate enabled the identification of SLNs with a detection rate of 66% and a false-negative rate of 0% [139]. The authors emphasized the need for further studies to improve the detection rate of SLNs in these patient groups.
Table 1 constitutes an overview of SLN techniques indications provided by the current guidelines.

Table 1.
Overview of indications concerning SLNB and SLND in urological malignancies, provided by the EAU and the AUA guidelines.
4. Conclusions
The continuous development of imaging methods, surgical techniques, and new markers may change the role of SLN procedures in urologic oncology in the future. SLNB is a promising approach to nodal staging with the potential to improve diagnostic accuracy, reduce morbidity, and individualize treatment in patients with urological malignancies. Nevertheless, further research is needed to fully understand its potential benefits and optimize its use in clinical practice, especially considering the small study cohorts in previous investigations.
Author Contributions
Conceptualization, B.M. and P.K.; methodology, P.K., M.K. and J.K.; validation, P.K., M.K. and B.M.; formal analysis, B.M. and P.K.; investigation, P.K., J.K. and B.M.; resources, A.P.; W.K., M.K. and J.K.; data curation, B.M., P.K. and M.K.; writing—original draft preparation, B.M., P.K., M.K., A.P. and J.K.; writing—review and editing, P.K. and B.M.; visualization, P.K.; supervision, R.Z., T.S. and B.M.; project administration, B.M.; funding acquisition, B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research grant from the Wroclaw Medical University (SUBZ.C090.23.080).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naidoo, K.; Pinder, S.E. Micro- and Macro-Metastasis in the Axillary Lymph Node: A Review. Surgeon 2017, 15, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Miyake, H. Significance of Micrometastases in Prostate Cancer. Surg. Oncol. 2008, 17, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann. Oncol. 2019, 128, 1194–1220, Erratum in Ann. Oncol. 2019, 30, 1674. [Google Scholar] [CrossRef] [PubMed]
- Michielin, O.; Van Akkooi, A.C.J.; Ascierto, P.A.; Dummer, R.; Keilholz, U. Cutaneous Melanoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1884–1901. [Google Scholar] [CrossRef]
- Alhatem, A.; Lambert, W.C.; Karanfilian, K.; Behbahani, S.; Heller, D. Impact of Partnership Status on Clinical Outcomes of Patients with Vulvar Squamous Cell Carcinoma and Performance of Sentinel Lymph Node Biopsy. Int. J. Gynecol. Cancer 2020, 30, 583–589. [Google Scholar] [CrossRef]
- Pascoal, E.; Alyafi, M.; Pokoradi, A.; Eiriksson, L.; Helpman, L. Inguinofemoral Sentinel Lymph Node Biopsy by Scar Injection in Vulvar Cancer: An Assessment of Feasibility and Long-Term Outcomes. Int. J. Gynecol. Cancer 2022, 32, 1512–1518. [Google Scholar] [CrossRef]
- Aoki, Y.; Kanao, H.; Fusegi, A.; Omi, M.; Okamoto, S.; Tanigawa, T.; Nomura, H.; Omatsu, K.; Tonooka, A. Indocyanine Green-Guided Sentinel Lymph Node Mapping during Laparoscopic Surgery with Vaginal Cuff Closure but No Uterine Manipulator for Cervical Cancer. Int. J. Clin. Oncol. 2022, 27, 1499–1506. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Valentina, C.; Giulio, S.; Alessandra, C.; Giulia, G.; Giulia, A.; Vito, C.; Roberto, B. Sentinel Node Mapping in Endometrial Cancer: Tips and Tricks to Improve Bilateral Detection Rate. The Sentitricks Study, a Monocentric Experience. Taiwan. J. Obstet. Gynecol. 2021, 60, 31–35. [Google Scholar] [CrossRef]
- Matanes, E.; Amajoud, Z.; Kogan, L.; Mitric, C.; Ismail, S.; Raban, O.; Knigin, D.; Levin, G.; Bahoric, B.; Ferenczy, A.; et al. Is Sentinel Lymph Node Assessment Useful in Patients with a Preoperative Diagnosis of Endometrial Intraepithelial Neoplasia? Gynecol. Oncol. 2023, 168, 107–113. [Google Scholar] [CrossRef]
- Cabanas, R.M. An Approach for the Treatment of Penile Carcinoma. Cancer 1977, 39, 456–466. [Google Scholar] [CrossRef]
- Morton, D.L.; Wen, D.R.; Wong, J.H.; Economou, J.S.; Cagle, L.A.; Storm, F.K.; Foshag, L.J.; Cochran, A.J. Technical Details of Intraoperative Lymphatic Mapping for Early Stage Melanoma. Arch. Surg. 1992, 127, 392–399. [Google Scholar] [CrossRef]
- Uren, R.F.; Howman-Giles, R.B.; Shaw, H.M.; Thompson, J.F.; McCarthy, W.H. Lymphoscintigraphy in High-Risk Melanoma of the Trunk: Predicting Draining Node Groups, Defining Lymphatic Channels and Locating the Sentinel Node. J. Nucl. Med. 1993, 34, 1435–1440. [Google Scholar]
- Brouwer, O.R.; Van Den Berg, N.S.; Mathéron, H.M.; Van Der Poel, H.G.; Van Rhijn, B.W.; Bex, A.; Van Tinteren, H.; Valdés Olmos, R.A.; Van Leeuwen, F.W.B.; Horenblas, S. A Hybrid Radioactive and Fluorescent Tracer for Sentinel Node Biopsy in Penile Carcinoma as a Potential Replacement for Blue Dye. Eur. Urol. 2014, 65, 600–609. [Google Scholar] [CrossRef]
- Peek, M.C.; Charalampoudis, P.; Anninga, B.; Baker, R.; Douek, M. Blue Dye for Identification of Sentinel Nodes in Breast Cancer and Malignant Melanoma: A Systematic Review and Meta-Analysis. Future Oncol. 2017, 13, 455–467. [Google Scholar] [CrossRef]
- Perenyei, M.; Barber, Z.E.; Gibson, J.; Hemington-Gorse, S.; Dobbs, T.D. Anaphylactic Reaction Rates to Blue Dyes Used for Sentinel Lymph Node Mapping: Systematic Review and Meta-Analysis. Ann. Surg. 2021, 273, 1087–1093. [Google Scholar] [CrossRef]
- Alex, J.C.; Weaver, D.L.; Fairbank, J.T.; Rankin, B.S.; Krag, D.N. Gamma-Probe-Guided Lymph Node Localization in Malignant Melanoma. Surg. Oncol. 1993, 2, 303–308. [Google Scholar] [CrossRef]
- Krag, D.N.; Weaver, D.L.; Alex, J.C.; Fairbank, J.T. Surgical Resection and Radiolocalization of the Sentinel Lymph Node in Breast Cancer Using a Gamma Probe. Surg. Oncol. 1993, 2, 335–340. [Google Scholar] [CrossRef]
- Lützen, U.; Naumann, C.M.; Dischinger, J.; Marx, M.; Baumann, R.; Zhao, Y.; Jüptner, M.; Osmonov, D.; Bothe, K.; Jünemann, K.P.; et al. 10-Year Experience Regarding the Reliability and Morbidity of Radio Guided Lymph Node Biopsy in Penile Cancer Patients and the Associated Radiation Exposure of Medical Staff in This Procedure. BMC Urol. 2016, 16, 47. [Google Scholar] [CrossRef]
- Thongvitokomarn, S.; Polchai, N. Indocyanine Green Fluorescence versus Blue Dye or Radioisotope Regarding Detection Rate of Sentinel Lymph Node Biopsy and Nodes Removed in Breast Cancer: A Systematic Review and Meta-Analysis. Asian Pac. J. Cancer Prev. 2020, 21, 1187–1195. [Google Scholar] [CrossRef]
- He, P.S.; Li, F.; Li, G.H.; Guo, C.; Chen, T.J. The Combination of Blue Dye and Radioisotope versus Radioisotope Alone during Sentinel Lymph Node Biopsy for Breast Cancer: A Systematic Review. BMC Cancer 2016, 16, 107. [Google Scholar] [CrossRef]
- Van Leeuwen, F.W.B.; Hardwick, J.C.H.; Van Erkel, A.R. Luminescence-Based Imaging Approaches in the Field of Interventional Molecular Imaging. Radiology 2015, 276, 12–29. [Google Scholar] [CrossRef] [PubMed]
- Baeten, I.G.T.; Hoogendam, J.P.; Jeremiasse, B.; Braat, A.J.A.T.; Veldhuis, W.B.; Jonges, G.N.; Jürgenliemk-Schulz, I.M.; van Gils, C.H.; Zweemer, R.P.; Gerestein, C.G. Indocyanine Green versus Technetium-99m with Blue Dye for Sentinel Lymph Node Detection in Early-Stage Cervical Cancer: A Systematic Review and Meta-Analysis. Cancer Rep. 2022, 5, e1401. [Google Scholar] [CrossRef]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel Lymph Node Assessment in Endometrial Cancer: A Systematic Review and Meta-Analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476.e10. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lillo, J.; Villegas-Tovar, E.; Momblan-Garcia, D.; Turrado-Rodriguez, V.; Ibarzabal-Olano, A.; De Lacy, B.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Martinez-Portilla, R.J.; Lacy, A. Performance of Indocyanine-Green Imaging for Sentinel Lymph Node Mapping and Lymph Node Metastasis in Esophageal Cancer: Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2021, 28, 4869–4877. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.Y.L.; Dang, J.T.; Modasi, A.; Nasralla, A.; Switzer, N.J.; Birch, D.; Turner, S.R.; Karmali, S. Diagnostic Accuracy of Sentinel Lymph Node Biopsy Using Indocyanine Green in Lung Cancer: A Systematic Review and Meta-Analysis. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 905–913. [Google Scholar] [CrossRef]
- Villegas-Tovar, E.; Jimenez-Lillo, J.; Jimenez-Valerio, V.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Otero-Piñeiro, A.; De Lacy, F.B.; Martinez-Portilla, R.J.; Lacy, A.M. Performance of Indocyanine Green for Sentinel Lymph Node Mapping and Lymph Node Metastasis in Colorectal Cancer: A Diagnostic Test Accuracy Meta-Analysis. Surg. Endosc. 2020, 34, 1035–1047. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; de Vries, H.M.; Mazzone, E.; KleinJan, G.H.; Donswijk, M.L.; van der Poel, H.G.; Horenblas, S.; van Leeuwen, F.W.B.; Brouwer, O.R. Hybrid Indocyanine Green–99mTc-Nanocolloid for Single-Photon Emission Computed Tomography and Combined Radio- and Fluorescence-Guided Sentinel Node Biopsy in Penile Cancer: Results of 740 Inguinal Basins Assessed at a Single Institution. Eur. Urol. 2020, 78, 865–872. [Google Scholar] [CrossRef]
- KleinJan, G.H.; van Werkhoven, E.; van den Berg, N.S.; Karakullukcu, M.B.; Zijlmans, H.J.M.A.A.; van der Hage, J.A.; van de Wiel, B.A.; Buckle, T.; Klop, W.M.C.; Horenblas, S.; et al. The Best of Both Worlds: A Hybrid Approach for Optimal Pre- and Intraoperative Identification of Sentinel Lymph Nodes. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1915–1925. [Google Scholar] [CrossRef]
- Wit, E.M.K.; KleinJan, G.H.; Berrens, A.-C.; van Vliet, R.; van Leeuwen, P.J.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. A Hybrid Radioactive and Fluorescence Approach Is More than the Sum of Its Parts; Outcome of a Phase II Randomized Sentinel Node Trial in Prostate Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2023. [Google Scholar] [CrossRef]
- Naumann, C.M.; Colberg, C.; Jüptner, M.; Marx, M.; Zhao, Y.; Jiang, P.; Hamann, M.F.; Jünemann, K.P.; Zuhayra, M.; Lützen, U. Evaluation of the Diagnostic Value of Preoperative Sentinel Lymph Node (SLN) Imaging in Penile Carcinoma Patients without Palpable Inguinal Lymph Nodes via Single Photon Emission Computed Tomography/Computed Tomography (SPECT/CT) as Compared to Planar Sci. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 92.e17–92.e24. [Google Scholar] [CrossRef]
- Quartuccio, N.; Garau, L.M.; Arnone, A.; Pappalardo, M.; Rubello, D.; Arnone, G.; Manca, G. Comparison Of99mTc-Labeled Colloid SPECT/CT and Planar Lymphoscintigraphy in Sentinel Lymph Node Detection in Patients with Melanoma: A Meta-Analysis. J. Clin. Med. 2020, 9, 1680. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, R.; Wang, W.; Zhao, Y.; Liu, X. 68Ga-PSMA PET/CT for the Evaluation of Metastasis in Patients with Prostate Cancer: A Systematic Review and Meta-Analysis. Hell. J. Nucl. Med. 2022, 25, 297–311. [Google Scholar]
- Mehralivand, S.; Van Der Poel, H.; Winter, A.; Choyke, P.L.; Pinto, P.A.; Turkbey, B. Sentinel Lymph Node Imaging in Urologic Oncology. Transl. Androl. Urol. 2018, 7, 887–902. [Google Scholar] [CrossRef]
- De Loosdrecht, M.M.V.; Molenaar, L.; Krooshoop, E.J.G.; Ten Haken, B.; Meijerink, W.J.H.J.; Alic, L.; Broeders, I.A.M.J. Laparoscopic Probe for Sentinel Lymph Node Harvesting Using Magnetic Nanoparticles. IEEE Trans. Biomed. Eng. 2022, 69, 286–293. [Google Scholar] [CrossRef]
- Winter, A.; Engels, S.; Goos, P.; Süykers, M.C.; Gudenkauf, S.; Henke, R.P.; Wawroschek, F. Accuracy of Magnetometer-Guided Sentinel Lymphadenectomy after Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Prostate Cancer: The SentiMag Pro II Study. Cancers 2020, 12, 32. [Google Scholar] [CrossRef]
- Geißen, W.; Engels, S.; Aust, P.; Schiffmann, J.; Gerullis, H.; Wawroschek, F.; Winter, A. Diagnostic Accuracy of Magnetometer-Guided Sentinel Lymphadenectomy after Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Intermediate- And High-Risk Prostate Cancer Using the Magnetic Activity of Sentinel Nodes. Front. Pharmacol. 2019, 10, 1123. [Google Scholar] [CrossRef]
- Jazrawi, A.; Pantiora, E.; Abdsaleh, S.; Bacovia, D.V.; Eriksson, S.; Leonhardt, H.; Wärnberg, F.; Karakatsanis, A. Magnetic-Guided Axillary Ultrasound (Magus) Sentinel Lymph Node Biopsy and Mapping in Patients with Early Breast Cancer. a Phase 2, Single-Arm Prospective Clinical Trial. Cancers 2021, 13, 4285. [Google Scholar] [CrossRef]
- Kosaka, N.; Bernardo, M.; Mitsunaga, M.; Choyke, P.L.; Kobayashi, H. MR and Optical Imaging of Early Micrometastases in Lymph Nodes: Triple Labeling with Nano-Sized Agents Yielding Distinct Signals. Contrast Media Mol. Imaging 2012, 7, 247–253. [Google Scholar] [CrossRef]
- Cox, K.; Sever, A.; Jones, S.; Weeks, J.; Mills, P.; Devalia, H.; Fish, D.; Jones, P. Validation of a Technique Using Microbubbles and Contrast Enhanced Ultrasound (CEUS) to Biopsy Sentinel Lymph Nodes (SLN) in Pre-Operative Breast Cancer Patients with a Normal Grey-Scale Axillary Ultrasound. Eur. J. Surg. Oncol. 2013, 39, 760–765. [Google Scholar] [CrossRef]
- Ferrucci, M.; Franceschini, G.; Douek, M. New Techniques for Sentinel Node Biopsy in Breast Cancer. Transl. Cancer Res. 2018, 7, S405–S417. [Google Scholar] [CrossRef]
- Cui, Q.; Dai, L.; Li, J.; Xue, J. Accuracy of CEUS-Guided Sentinel Lymph Node Biopsy in Early-Stage Breast Cancer: A Study Review and Meta-Analysis. World J. Surg. Oncol. 2020, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Horenblas, S.; Van Tinteren, H. Squamous Cell Carcinoma of the Penis. IV. Prognostic Factors of Survival: Analysis of Tumor, Nodes and Metastasis Classification System. J. Urol. 1994, 151, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Gopman, J.M.; Djajadiningrat, R.S.; Baumgarten, A.S.; Espiritu, P.N.; Horenblas, S.; Zhu, Y.; Protzel, C.; Pow-Sang, J.M.; Kim, T.; Sexton, W.J.; et al. Predicting Postoperative Complications of Inguinal Lymph Node Dissection for Penile Cancer in an International Multicentre Cohort. BJU Int. 2015, 116, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Spiess, P.E.; Hernandez, M.S.; Pettaway, C.A. Contemporary Inguinal Lymph Node Dissection: Minimizing Complications. World J. Urol. 2009, 27, 205–212. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Tagawa, S.T.; Albersen, M.; Ayres, B.; Crook, J.; Van Der Heijden, M.S.; Johnstone, P.A.S.; Necchi, A.; Oliveira, P.; Pagliaro, L.C.; et al. EAU-ASCO Penile Cancer Guidelines. Edn. Presented at the EAU Annual Congress Milan 2023; EAU Guidelines Office: Arnhem, The Netherlands, 2023; ISBN 978-94-92671-19-6. [Google Scholar]
- Hughes, B.; Leijte, J.; Shabbir, M.; Watkin, N.; Horenblas, S. Non-Invasive and Minimally Invasive Staging of Regional Lymph Nodes in Penile Cancer. World J. Urol. 2009, 27, 197–203. [Google Scholar] [CrossRef]
- Leijte, J.A.P.; Kroon, B.K.; Valdés Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Reliability and Safety of Current Dynamic Sentinel Node Biopsy for Penile Carcinoma. Eur. Urol. 2007, 52, 170–177. [Google Scholar] [CrossRef]
- Kroon, B.K.; Lont, A.P.; Valdés Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Morbidity of Dynamic Sentinel Node Biopsy in Penile Carcinoma. J. Urol. 2005, 173, 813–815. [Google Scholar] [CrossRef]
- Tanis, P.J.; Lont, A.P.; Meinhardt, W.; Valdés Olmos, R.A.; Nieweg, O.E.; Horenblas, S. Dynamic Sentinel Node Biopsy for Penile Cancer: Reliability of a Staging Technique. J. Urol. 2002, 168, 76–80. [Google Scholar] [CrossRef]
- Dimopoulos, P.; Christopoulos, P.; Shilito, S.; Gall, Z.; Murby, B.; Ashworth, D.; Taylor, B.; Carrington, B.; Shanks, J.; Clarke, N.; et al. Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Comparison between 1- and 2-Day Protocols. BJU Int. 2016, 117, 890–896. [Google Scholar] [CrossRef]
- Wever, L.; de Vries, H.M.; van der Poel, H.; van Leeuwen, F.; Horenblas, S.; Brouwer, O. Minimally Invasive Evaluation of the Clinically Negative Inguinal Node in Penile Cancer: Dynamic Sentinel Node Biopsy. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 209–214. [Google Scholar] [CrossRef]
- Tabatabaei, S.; Harisinghani, M.; McDougal, W.S. Regional Lymph Node Staging Using Lymphotropic Nanoparticle Enhanced Magnetic Resonance Imaging with Ferumoxtran-10 in Patients with Penile Cancer. J. Urol. 2005, 174, 923–927. [Google Scholar] [CrossRef]
- Cleaveland, P.; Lau, M.; Parnham, A.; Murby, B.; Ashworth, D.; Manohoran, P.; Taylor, B.; Bell, J.; Najran, P.; Oliveira, P.; et al. Testing the Feasibility of SentiMag/Sienna+ for Detecting Inguinal Sentinel Nodes in Penile Cancer (SentiPen): An EUROGEN and National Cancer Research Institute Trial. Eur. Urol. 2019, 76, 874–875. [Google Scholar] [CrossRef]
- Winter, A.; Kowald, T.; Engels, S.; Wawroschek, F. Magnetic Resonance Sentinel Lymph Node Imaging and Magnetometer-Guided Intraoperative Detection in Penile Cancer, Using Superparamagnetic Iron Oxide Nanoparticles: First Results. Urol. Int. 2020, 104, 177–180. [Google Scholar] [CrossRef]
- Azargoshasb, S.; Molenaar, L.; Rosiello, G.; Buckle, T.; van Willigen, D.M.; van de Loosdrecht, M.M.; Welling, M.M.; Alic, L.; van Leeuwen, F.W.B.; Winter, A.; et al. Advancing Intraoperative Magnetic Tracing Using 3D Freehand Magnetic Particle Imaging. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 211–218. [Google Scholar] [CrossRef]
- Fallara, G.; Pozzi, E.; Onur Cakir, O.; Tandogdu, Z.; Castiglione, F.; Salonia, A.; Alnajjar, H.M.; Muneer, A. Diagnostic Accuracy of Dynamic Sentinel Lymph Node Biopsy for Penile Cancer: A Systematic Review and Meta-Analysis. Eur. Urol. Focus 2022, in press. [CrossRef]
- de Vries, H.M.; Lee, H.J.; Lam, W.; Djajadiningrat, R.S.; Ottenhof, S.R.; Roussel, E.; Kroon, B.K.; de Jong, I.J.; Oliveira, P.; Alnajjar, H.M.; et al. Clinicopathological Predictors of Finding Additional Inguinal Lymph Node Metastases in Penile Cancer Patients after Positive Dynamic Sentinel Node Biopsy: A European Multicentre Evaluation. BJU Int. 2022, 130, 126–132. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Eggener, S.E.; Scardino, P.T.; Walsh, P.C.; Han, M.; Partin, A.W.; Trock, B.J.; Feng, Z.; Wood, D.P.; Eastham, J.A.; Yossepowitch, O.; et al. Predicting 15-Year Prostate Cancer Specific Mortality after Radical Prostatectomy. J. Urol. 2011, 185, 869–875. [Google Scholar] [CrossRef]
- Joniau, S.; Briganti, A.; Gontero, P.; Gandaglia, G.; Tosco, L.; Fieuws, S.; Tombal, B.; Marchioro, G.; Walz, J.; Kneitz, B.; et al. Stratification of High-Risk Prostate Cancer into Prognostic Categories: A European Multi-Institutional Study. Eur. Urol. 2015, 67, 157–164. [Google Scholar] [CrossRef]
- Gandaglia, G.; Mazzone, E.; Stabile, A.; Pellegrino, A.; Cucchiara, V.; Barletta, F.; Scuderi, S.; Robesti, D.; Leni, R.; Samanes Gajate, A.M.; et al. Prostate-Specific Membrane Antigen Radioguided Surgery to Detect Nodal Metastases in Primary Prostate Cancer Patients Undergoing Robot-Assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection: Results of a Planned Interim Analysis of a Pro. Eur. Urol. 2022, 82, 411–418. [Google Scholar] [CrossRef]
- Lucciola, S.; Pisciotti, M.L.; Frisenda, M.; Magliocca, F.; Gentilucci, A.; Del Giudice, F.; Canale, V.; Scarrone, E.; Busetto, G.M.; Carrieri, G.; et al. Predictive Role of Node-Rads Score in Patients with Prostate Cancer Candidates for Radical Prostatectomy with Extended Lymph Node Dissection: Comparative Analysis with Validated Nomograms. Prostate Cancer Prostatic Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Dekalo, S.; Kuten, J.; Campbell, J.; Mintz, I.; Bar-Yosef, Y.; Keizman, D.; Sarid, D.; Even-Sapir, E.; Yossepowitch, O.; Mano, R. 68Ga-Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Patients with Favorable Intermediate-Risk Prostate Cancer. Can. Urol. Assoc. J. 2022, 16, E381. [Google Scholar] [CrossRef] [PubMed]
- Małkiewicz, B.; Bugla, B.; Czarnecki, M.; Karwacki, J.; Długosz, P.; Gurwin, A.; Kiełb, P.; Lemiński, A.; Krajewski, W.; Jędrzejuk, D.; et al. Diagnostic Value of Radio-Guided Sentinel Node Detection in Patients with Prostate Cancer Undergoing Radical Prostatectomy with Modified-Extended Lymphadenectomy. Cancers 2022, 14, 5012. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Chun, F.K.H.; Salonia, A.; Gallina, A.; Farina, E.; Da Pozzo, L.F.; Rigatti, P.; Montorsi, F.; Karakiewicz, P.I. Validation of a Nomogram Predicting the Probability of Lymph Node Invasion Based on the Extent of Pelvic Lymphadenectomy in Patients with Clinically Localized Prostate Cancer. BJU Int. 2006, 98, 788–793. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated Nomogram Predicting Lymph Node Invasion in Patients with Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection: The Essential Importance of Percentage of Positive Cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Maas, M.; Nassiri, N.; Ortega, D.; Gill, K.; Dell’Oglio, P.; Thalmann, G.N.; Heidenreich, A.; Eastham, J.A.; Evans, C.P.; et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-Analysis. Eur. Urol. Oncol. 2021, 4, 134–149. [Google Scholar] [CrossRef]
- Fossati, N.; Willemse, P.P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R.; Montorsi, F.; Studer, U.E. Pelvic Lymph Node Dissection in Prostate Cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Wang, J.; Bi, J. Different Lymph Node Dissection Ranges during Radical Prostatectomy for Patients with Prostate Cancer: A Systematic Review and Network Meta-Analysis. World J. Surg. Oncol. 2023, 21, 80. [Google Scholar] [CrossRef]
- Touijer, K.A.; Sjoberg, D.D.; Benfante, N.; Laudone, V.P.; Ehdaie, B.; Eastham, J.A.; Scardino, P.T.; Vickers, A. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur. Urol. Oncol. 2021, 4, 532–539. [Google Scholar] [CrossRef]
- Meinhardt, W.; van der Poel, H.G.; Valdés Olmos, R.A.; Bex, A.; Brouwer, O.R.; Horenblas, S. Laparoscopic Sentinel Lymph Node Biopsy for Prostate Cancer: The Relevance of Locations Outside the Extended Dissection Area. Prostate Cancer 2012, 2012, 751753. [Google Scholar] [CrossRef]
- Acar, C.; Kleinjan, G.H.; van den Berg, N.S.; Wit, E.M.; van Leeuwen, F.W.; van der Poel, H.G. Advances in Sentinel Node Dissection in Prostate Cancer from a Technical Perspective. Int. J. Urol. 2015, 22, 898–909. [Google Scholar] [CrossRef]
- Wit, E.M.K.; Acar, C.; Grivas, N.; Yuan, C.; Horenblas, S.; Liedberg, F.; Valdes Olmos, R.A.; van Leeuwen, F.W.B.; van den Berg, N.S.; Winter, A.; et al. Sentinel Node Procedure in Prostate Cancer: A Systematic Review to Assess Diagnostic Accuracy. Eur. Urol. 2017, 71, 596–605. [Google Scholar] [CrossRef]
- Ramírez-Backhaus, M.; Mira Moreno, A.; Gómez Ferrer, A.; Calatrava Fons, A.; Casanova, J.; Solsona Narbón, E.; Ortiz Rodríguez, I.M.; Rubio Briones, J. Indocyanine Green Guided Pelvic Lymph Node Dissection: An Efficient Technique to Classify the Lymph Node Status of Patients with Prostate Cancer Who Underwent Radical Prostatectomy. J. Urol. 2016, 196, 1429–1435. [Google Scholar] [CrossRef]
- Wawroschek, F.; Vogt, H.; Weckermann, D.; Wagner, T.; Harzmann, R. The Sentinel Lymph Node Concept in Prostate Cancer—First Results of Gamma Probe-Guided Sentinel Lymph Node Identification. Eur. Urol. 1999, 36, 595–600. [Google Scholar] [CrossRef]
- Wawroschek, F.; Vogt, H.; Weckermann, D.; Wagner, T.; Hamm, M.; Harzmann, R. Radioisotope Guided Pelvic Lymph Node Dissection for Prostate Cancer. J. Urol. 2001, 166, 1715–1719. [Google Scholar] [CrossRef]
- Rudoni, M.; Sacchetti, G.M.; Leva, L.; Inglese, E.; Monesi, G.; Minocci, D.; Frea, B. Recent Applications of the Sentinel Lymph Node Concept: Preliminary Experience in Prostate Cancer. Tumori 2002, 88, S16–S17. [Google Scholar] [CrossRef]
- Fumadó, L.; Abascal, J.M.; Mestre-Fusco, A.; Vidal-Sicart, S.; Aguilar, G.; Juanpere, N.; Cecchini, L. Sentinel Lymph Node Biopsy in Prostate Cancer Patients: Results From an Injection Technique Targeting the Index Lesion in the Prostate Gland. Front. Med. 2022, 9, 931867. [Google Scholar] [CrossRef]
- Motomura, K.; Inaji, H.; Komoike, Y.; Kasugai, T.; Noguchi, S.; Koyama, H. Sentinel Node Biopsy Guided by Indocyanin Green Dye in Breast Cancer Patients. Jpn. J. Clin. Oncol. 1999, 29, 604–607. [Google Scholar] [CrossRef]
- Inoue, S.; Shiina, H.; Arichi, N.; Mitsui, Y.; Hiraoka, T.; Wake, K.; Sumura, M.; Honda, S.; Yasumoto, H.; Urakami, S.; et al. Identification of Lymphatic Pathway Involved in the Spreading of Prostate Cancer by Fluorescence Navigation Approach with Intraoperatively Injected Indocyanine Green. J. Can. Urol. Assoc. 2011, 5, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Van Der Poel, H.G.; Buckle, T.; Brouwer, O.R.; Valdés Olmos, R.A.; Van Leeuwen, F.W.B. Intraoperative Laparoscopic Fluorescence Guidance to the Sentinel Lymph Node in Prostate Cancer Patients: Clinical Proof of Concept of an Integrated Functional Imaging Approach Using a Multimodal Tracer. Eur. Urol. 2011, 60, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, L.; Valdés Olmos, R.A.; Meinhardt, W.; Bex, A.; Van Der Poel, H.G.; Vogel, W.V.; Sivro, F.; Hoefnagel, C.A.; Horenblas, S. Intraoperative Radioguidance with a Portable Gamma Camera: A Novel Technique for Laparoscopic Sentinel Node Localisation in Urological Malignancies. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, S.; Lusuardi, L.; Myatt, A.; Hruby, S.; Pirich, C.; Janetschek, G. Visualisation of the Lymph Node Pathway in Real Time by Laparoscopic Radioisotope- and Fluorescence-Guided Sentinel Lymph Node Dissection in Prostate Cancer Staging. Urology 2012, 80, 1080–1087. [Google Scholar] [CrossRef]
- Harisinghani, M.G.; Barentsz, J.; Hahn, P.F.; Deserno, W.M.; Tabatabaei, S.; van de Kaa, C.H.; de la Rosette, J.; Weissleder, R. Noninvasive Detection of Clinically Occult Lymph-Node Metastases in Prostate Cancer. N. Engl. J. Med. 2003, 348, 2491–2499. [Google Scholar] [CrossRef]
- Turkbey, B.; Hoyt, R.F.; Agarwal, H.K.; Bernardo, M.; Sankineni, S.; Johnson, L.; Grant, K.B.; Rais-Bahrami, S.; Kobayashi, H.; Wood, B.J.; et al. Magnetic Resonance Sentinel Lymph Node Imaging of the Prostate with Gadofosveset Trisodium-Albumin: Preliminary Results in a Canine Model. Acad. Radiol. 2015, 22, 646–652. [Google Scholar] [CrossRef]
- Winter, A.; Woenkhaus, J.; Wawroschek, F. A Novel Method for Intraoperative Sentinel Lymph Node Detection in Prostate Cancer Patients Using Superparamagnetic Iron Oxide Nanoparticles and a Handheld Magnetometer: The Initial Clinical Experience. Ann. Surg. Oncol. 2014, 21, 4390–4396. [Google Scholar] [CrossRef]
- Kleinjan, G.H.; Van Den Berg, N.S.; Brouwer, O.R.; De Jong, J.; Acar, C.; Wit, E.M.; Vegt, E.; Van Der Noort, V.; Valdés Olmos, R.A.; Van Leeuwen, F.W.B.; et al. Optimisation of Fluorescence Guidance during Robot-Assisted Laparoscopic Sentinel Node Biopsy for Prostate Cancer. Eur. Urol. 2014, 66, 991–998. [Google Scholar] [CrossRef]
- Manny, T.B.; Patel, M.; Hemal, A.K. Fluorescence-Enhanced Robotic Radical Prostatectomy Using Real-Time Lymphangiography and Tissue Marking with Percutaneous Injection of Unconjugated Indocyanine Green: The Initial Clinical Experience in 50 Patients. Eur. Urol. 2014, 65, 1162–1168. [Google Scholar] [CrossRef]
- Povoski, S.R.; Neff, R.L.; Mojzisik, C.M.; O’Malley, D.M.; Hinkle, G.H.; Hall, N.C.; Murrey, D.A.; Knopp, M.V.; Martin, E.W. A Comprehensive Overview of Radioguided Surgery Using Gamma Detection Probe Technology. World J. Surg. Oncol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Buckle, T.; Bunschoten, A.; Kuil, J.; Vahrmeijer, A.L.; Wendler, T.; Valdés-Olmos, R.A.; Van Der Poel, H.G.; Van Leeuwen, F.W.B. Image Navigation as a Means to Expand the Boundaries of Fluorescence-Guided Surgery. Phys. Med. Biol. 2012, 57, 3123–3136. [Google Scholar] [CrossRef]
- Abascal Junquera, J.M.; Mestre-Fusco, A.; Grootendorst, M.R.; Vidal-Sicart, S.; Fumado, L. Sentinel Lymph Node Biopsy in Prostate Cancer Using the SENSEI® Drop-In Gamma Probe. Clin. Nucl. Med. 2022, 47, 86–87. [Google Scholar] [CrossRef]
- Abascal Junquera, J.M.; Harke, N.N.; Walz, J.C.; Hadaschik, B.; Adshead, J.; Everaerts, W.; Goffin, K.; Grootendorst, M.R.; Oldfield, F.; Vyas, K.; et al. A Drop-in Gamma Probe for Minimally Invasive Sentinel Lymph Node Dissection in Prostate Cancer: Preclinical Evaluation and Interim Results From a Multicenter Clinical Trial. Clin. Nucl. Med. 2023, 48, 213–220. [Google Scholar] [CrossRef]
- Dell’Oglio, P.; Meershoek, P.; Maurer, T.; Wit, E.M.K.; van Leeuwen, P.J.; van der Poel, H.G.; van Leeuwen, F.W.B.; van Oosterom, M.N. A DROP-IN Gamma Probe for Robot-Assisted Radioguided Surgery of Lymph Nodes During Radical Prostatectomy. Eur. Urol. 2021, 79, 124–132. [Google Scholar] [CrossRef]
- de Korne, C.M.; Wit, E.M.; de Jong, J.; Valdés Olmos, R.A.; Buckle, T.; van Leeuwen, F.W.B.; van der Poel, H.G. Anatomical Localization of Radiocolloid Tracer Deposition Affects Outcome of Sentinel Node Procedures in Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2558–2568. [Google Scholar] [CrossRef]
- Aoun, F.; Albisinni, S.; Zanaty, M.; Hassan, T.; Janetschek, G.; van Velthoven, R. Indocyanine Greenfluorescence-Guidedsentinel Lymph Node Identification in Urologic Cancers: A Systematic Review and Meta-Analysis. Minerva Urol. Nefrol. 2018, 70, 361–369. [Google Scholar] [CrossRef]
- Urabe, F.; Kimura, S.; Yasue, K.; Yanagisawa, T.; Tsuzuki, S.; Kimura, T.; Miki, J.; Egawa, S. Performance of Indocyanine Green Fluorescence for Detecting Lymph Node Metastasis in Prostate Cancer: A Systematic Review and Meta-Analysis. Clin. Genitourin. Cancer 2021, 19, 466.e1–466.e9. [Google Scholar] [CrossRef]
- Claps, F.; Ramírez-Backhaus, M.; Mir Maresma, M.C.; Gómez-Ferrer, Á.; Mascarós, J.M.; Marenco, J.; Collado Serra, A.; Casanova Ramón-Borja, J.; Calatrava Fons, A.; Trombetta, C.; et al. Indocyanine Green Guidance Improves the Efficiency of Extended Pelvic Lymph Node Dissection during Laparoscopic Radical Prostatectomy. Int. J. Urol. 2021, 28, 566–572. [Google Scholar] [CrossRef]
- Grivas, N.; Wit, E.M.K.; Kuusk, T.; KleinJan, G.H.; Donswijk, M.L.; Van Leeuwen, F.W.B.; Van Der Poel, H.G. The Impact of Adding Sentinel Node Biopsy to Extended Pelvic Lymph Node Dissection on Biochemical Recurrence in Prostate Cancer Patients Treated with Robot-Assisted Radical Prostatectomy. J. Nucl. Med. 2018, 59, 204–209. [Google Scholar] [CrossRef]
- Hinsenveld, F.J.; Wit, E.M.K.; van Leeuwen, P.J.; Brouwer, O.R.; Donswijk, M.L.; Tillier, C.N.; Vegt, E.; van Muilekom, E.; van Oosterom, M.N.; van Leeuwen, F.W.B.; et al. Prostate-Specific Membrane Antigen PET/CT Combined with Sentinel Node Biopsy for Primary Lymph Node Staging in Prostate Cancer. J. Nucl. Med. 2020, 61, 540–545. [Google Scholar] [CrossRef]
- Lannes, F.; Baboudjian, M.; Ruffion, A.; Rouy, M.; Giammarile, F.; Rousseau, T.; Kraeber-Bodéré, F.; Rousseau, C.; Rusu, D.; Colombié, M.; et al. Radioisotope-Guided Lymphadenectomy for Pelvic Lymph Node Staging in Patients With Intermediate- and High-Risk Prostate Cancer (The Prospective SENTINELLE Study). J. Urol. 2023, 209, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mattei, A.; Fuechsel, F.G.; Bhatta Dhar, N.; Warncke, S.H.; Thalmann, G.N.; Krause, T.; Studer, U.E. The Template of the Primary Lymphatic Landing Sites of the Prostate Should Be Revisited: Results of a Multimodality Mapping Study. Eur. Urol. 2008, 53, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Joniau, S.; Van Den Bergh, L.; Lerut, E.; Deroose, C.M.; Haustermans, K.; Oyen, R.; Budiharto, T.; Ameye, F.; Bogaerts, K.; Van Poppel, H. Mapping of Pelvic Lymph Node Metastases in Prostate Cancer. Eur. Urol. 2013, 63, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Chennamsetty, A.; Zhumkhawala, A.; Tobis, S.B.; Ruel, N.; Lau, C.S.; Yamzon, J.; Wilson, T.G.; Yuh, B.E. Lymph Node Fluorescence During Robot-Assisted Radical Prostatectomy With Indocyanine Green: Prospective Dosing Analysis. Clin. Genitourin. Cancer 2017, 15, e529–e534. [Google Scholar] [CrossRef]
- Nguyen, D.P.; Huber, P.M.; Metzger, T.A.; Genitsch, V.; Schudel, H.H.; Thalmann, G.N. A Specific Mapping Study Using Fluorescence Sentinel Lymph Node Detection in Patients with Intermediate- and High-Risk Prostate Cancer Undergoing Extended Pelvic Lymph Node Dissection. Eur. Urol. 2016, 70, 734–737. [Google Scholar] [CrossRef]
- Perez-Ardavin, J.; Martinez-Sarmiento, M.; Monserrat-Monfort, J.J.; Vera-Pinto, V.; Sopena-Novales, P.; Bello-Arqués, P.; Boronat-Tormo, F.; Vera-Donoso, C.D. The Sentinel Node with Technetium-99m for Prostate Cancer. A Safe and, Mature New Gold Standard? Q. J. Nucl. Med. Mol. Imaging 2022. ahead of print. [Google Scholar] [CrossRef]
- Wit, E.M.K.; van Beurden, F.; Kleinjan, G.H.; Grivas, N.; de Korne, C.M.; Buckle, T.; Donswijk, M.L.; Bekers, E.M.; van Leeuwen, F.W.B.; van der Poel, H.G. The Impact of Drainage Pathways on the Detection of Nodal Metastases in Prostate Cancer: A Phase II Randomized Comparison of Intratumoral vs Intraprostatic Tracer Injection for Sentinel Node Detection. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1743–1753. [Google Scholar] [CrossRef]
- Winter, A.; Chavan, A.; Wawroschek, F. Magnetic Resonance Imaging of Sentinel Lymph Nodes Using Intraprostatic Injection of Superparamagnetic Iron Oxide Nanoparticles in Prostate Cancer Patients: First-in-Human Results. Eur. Urol. 2018, 73, 813–814. [Google Scholar] [CrossRef]
- Winter, A.; Engels, S.; Reinhardt, L.; Wasylow, C.; Gerullis, H.; Wawroschek, F. Magnetic Marking and Intraoperative Detection of Primary Draining Lymph Nodes in High-Risk Prostate Cancer Using Superparamagnetic Iron Oxide Nanoparticles: Additional Diagnostic Value. Molecules 2017, 22, 2192. [Google Scholar] [CrossRef]
- Engels, S.; Michalik, B.; Meyer, L.M.; Nemitz, L.; Wawroschek, F.; Winter, A. Magnetometer-Guided Sentinel Lymph Node Dissection in Prostate Cancer: Rate of Lymph Node Involvement Compared with Radioisotope Marking. Cancers 2021, 13, 5821. [Google Scholar] [CrossRef]
- Sinha, A.; West, A.; Hayes, J.; Teoh, J.; Decaestecker, K.; Vasdev, N. Methods of Sentinel Lymph Node Detection and Management in Urinary Bladder Cancer—A Narrative Review. Curr. Oncol. 2022, 29, 1335–1348. [Google Scholar] [CrossRef]
- Sherif, A.; De La Torre, M.; Malmström, P.U.; Thörn, M. Lymphatic Mapping and Detection of Sentinel Nodes in Patients with Bladder Cancer. J. Urol. 2001, 166, 812–815. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M.; Sadeghi, R. Sentinel Node Biopsy in Urothelial Carcinoma of the Bladder: Systematic Review and Meta-Analysis. Urol. Int. 2019, 103, 373–382. [Google Scholar] [CrossRef]
- Liedberg, F.; Chebil, G.; Davidsson, T.; Gudjonsson, S.; Månsson, W. Intraoperative Sentinel Node Detection Improves Nodal Staging in Invasive Bladder Cancer. J. Urol. 2006, 175, 84–88. [Google Scholar] [CrossRef]
- Sadeghi, R.; Hasanzadeh, M. Sentinel Lymph Node Biopsy Algorithm: Can It Be a Universal Method for Midline Tumors? Gynecol. Oncol. 2014, 132, 273–274. [Google Scholar] [CrossRef]
- Inoue, S.; Shiina, H.; Mitsui, Y.; Yasumoto, H.; Matsubara, A.; Igawa, M. Identification of Lymphatic Pathway Involved in the Spread of Bladder Cancer: Evidence Obtained from Fluorescence Navigation with Intraoperatively Injected Indocyanine Green. J. Can. Urol. Assoc. 2013, 7, E322. [Google Scholar] [CrossRef]
- Assadi, M.; Yarani, M.; Zakavi, S.R.; Jangjoo, A.; Memar, B.; Treglia, G.; Aliakbarian, M.; Mehrabibahar, M.; Sadeghi, R. Sentinel Node Mapping in Papillary Thyroid Carcinoma Using Combined Radiotracer and Blue Dye Methods. Endokrynol. Pol. 2014, 65, 281–286. [Google Scholar] [CrossRef]
- Dabbagh Kakhki, V.R.; Bagheri, R.; Tehranian, S.; Shojaei, P.; Gholami, H.; Sadeghi, R.; Krag, D.N. Accuracy of Sentinel Node Biopsy in Esophageal Carcinoma: A Systematic Review and Meta-Analysis of the Pertinent Literature. Surg. Today 2014, 44, 607–619. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadeghi, R.; Sadri, K.; Tavakkoli, M.; Keshvari, M.; Salehi, M. Sentinel Lymph Node Biopsy in Muscle-Invasive Bladder Cancer: Single-Center Experience. Ann. Nucl. Med. 2020, 34, 718–724. [Google Scholar] [CrossRef]
- Alvaeus, J.; Rosenblatt, R.; Johansson, M.; Alamdari, F.; Jakubczyk, T.; Holmström, B.; Hemdan, T.; Huge, Y.; Aljabery, F.; Gabrielsson, S.; et al. Fewer Tumour Draining Sentinel Nodes in Patients with Progressing Muscle Invasive Bladder Cancer, after Neoadjuvant Chemotherapy and Radical Cystectomy. World J. Urol. 2020, 38, 2207–2213. [Google Scholar] [CrossRef]
- Polom, W.; Markuszewski, M.; Cytawa, W.; Czapiewski, P.; Lass, P.; Matuszewski, M. Fluorescent Versus Radioguided Lymph Node Mapping in Bladder Cancer. Clin. Genitourin. Cancer 2017, 15, e405–e409. [Google Scholar] [CrossRef] [PubMed]
- Rietbergen, D.D.D.; Van Gennep, E.J.; Kleinjan, G.H.; Donswijk, M.; Valdés Olmos, R.A.; Van Rhijn, B.W.; Van Der Poel, H.G.; Van Leeuwen, F.W.B. Evaluation of the Hybrid Tracer Indocyanine Green-99mTc-Nanocolloid for Sentinel Node Biopsy in Bladder Cancer—A Prospective Pilot Study. Clin. Nucl. Med. 2022, 47, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.H.M.; van Poppel, H.; Maréchal, J.M.; Jacqmin, D.; Schröder, F.H.; de Prijck, L.; Sylvester, R. Radical Nephrectomy with and without Lymph-Node Dissection: Final Results of European Organization for Research and Treatment of Cancer (EORTC) Randomized Phase 3 Trial 30881. Eur. Urol. 2009, 55, 28–34. [Google Scholar] [CrossRef]
- Freedland, S.J.; Dekernion, J.B. Role of Lymphadenectomy for Patients Undergoing Radical Nephrectomy for Renal Cell Carcinoma. Rev. Urol. 2003, 5, 191–195. [Google Scholar] [PubMed]
- Bex, A.; Vermeeren, L.; De Windt, G.; Prevoo, W.; Horenblas, S.; Olmos, R.A.V. Feasibility of Sentinel Node Detection in Renal Cell Carcinoma: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1117–1123. [Google Scholar] [CrossRef]
- Bex, A.; Vermeeren, L.; Meinhardt, W.; Prevoo, W.; Horenblas, S.; Olmos, R.A.V. Intraoperative Sentinel Node Identification and Sampling in Clinically Node-Negative Renal Cell Carcinoma: Initial Experience in 20 Patients. World J. Urol. 2011, 29, 793–799. [Google Scholar] [CrossRef]
- Kuusk, T.; Donswijk, M.L.; Valdés Olmos, R.A.; De Bruijn, R.E.; Brouwer, O.R.; Hendricksen, K.; Horenblas, S.; Jóźwiak, K.; Prevoo, W.; Van Der Poel, H.G.; et al. An Analysis of SPECT/CT Non-Visualization of Sentinel Lymph Nodes in Renal Tumors. EJNMMI Res. 2018, 8, 105. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Noe, A.; Olmos, R.A.V.; Bex, A. Lymphatic Drainage from Renal Cell Carcinoma along the Thoracic Duct Visualized with SPECT/CT. Lymphat. Res. Biol. 2013, 11, 233–238. [Google Scholar] [CrossRef]
- Kuusk, T.; De Bruijn, R.; Brouwer, O.R.; De Jong, J.; Donswijk, M.; Grivas, N.; Hendricksen, K.; Horenblas, S.; Prevoo, W.; Valdés Olmos, R.A.; et al. Lymphatic Drainage from Renal Tumors In Vivo: A Prospective Sentinel Node Study Using SPECT/CT Imaging. J. Urol. 2018, 199, 1426–1432. [Google Scholar] [CrossRef]
- Pathak, R.A.; Hemal, A.K. Intraoperative ICG-Fluorescence Imaging for Robotic-Assisted Urologic Surgery: Current Status and Review of Literature. Int. Urol. Nephrol. 2019, 51, 765–771. [Google Scholar] [CrossRef]
- Kuusk, T.; Brouwer, O.; Graafland, N.; Hendricksen, K.; Donswijk, M.; Bex, A. Sentinel Lymph Node Biopsy in Renal Tumors: Surgical Technique and Safety. Urology 2019, 130, 186–190. [Google Scholar] [CrossRef]
- Tanis, P.J.; Horenblas, S.; Olmos, R.A.; Hoefnagel, C.A.; Nieweg, O.E. Feasibility of Sentinel Node Lymphoscintigraphy in Stage I Testicular Cancer. Eur. J. Nucl. Med. 2002, 29, 670–673. [Google Scholar] [CrossRef]
- Ohyama, C.; Chiba, Y.; Yamazaki, T.; Endoh, M.; Hoshi, S.; Arai, Y. Lymphatic Mapping and Gamma Probe Guided Laparoscopic Biopsy of Sentinel Lymph Node in Patients with Clinical Stage I Testicular Tumor. J. Urol. 2002, 168, 1390–1395. [Google Scholar] [CrossRef]
- Satoh, M.; Ito, A.; Kaiho, Y.; Nakagawa, H.; Saito, S.; Endo, M.; Ohyama, C.; Arai, Y. Intraoperative, Radio-Guided Sentinel Lymph Node Mapping in Laparoscopic Lymph Node Dissection for Stage I Testicular Carcinoma. Cancer 2005, 103, 2067–2072. [Google Scholar] [CrossRef]
- Brouwer, O.R.; Olmos, R.A.V.; Vermeeren, L.; Hoefnagel, C.A.; Nieweg, O.E.; Horenblas, S. SPECT/CT and a Portable γ-Camera for Image-Guided Laparoscopic Sentinel Node Biopsy in Testicular Cancer. J. Nucl. Med. 2011, 52, 551–554. [Google Scholar] [CrossRef]
- Blok, J.M.; Kerst, J.M.; Vegt, E.; Brouwer, O.R.; Meijer, R.P.; Bosch, J.L.H.R.; Bex, A.; van der Poel, H.G.; Horenblas, S. Sentinel Node Biopsy in Clinical Stage I Testicular Cancer Enables Early Detection of Occult Metastatic Disease. BJU Int. 2019, 124, 424–430. [Google Scholar] [CrossRef]
- Tandstad, T.; Smaaland, R.; Solberg, A.; Bremnes, R.M.; Langberg, C.W.; Laurell, A.; Stierner, U.K.; Ståhl, O.; Cavallin-Ståhl, E.K.; Klepp, O.H.; et al. Management of Seminomatous Testicular Cancer: A Binational Prospective Population-Based Study from the Swedish Norwegian Testicular Cancer Study Group. J. Clin. Oncol. 2011, 29, 719–725. [Google Scholar] [CrossRef]
- Zarifmahmoudi, L.; Ghorbani, H.; Sadeghi, R.; Sadri, K.; Soltani, S.; Aghaee, A. Sentinel Lymph Node Mapping in Post Chemotherapy Nonseminoma Testicular Cancer Patients Undergoing Retroperitoneal Lymph Node Dissection: A Series of Nine Cases. Asia Ocean. J. Nucl. Med. Biol. 2022, 10, 36–42. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline. Part III: Principles of Radiation and Future Directions. J. Urol. 2022, 208, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.B.; Albertsen, P.C.; Barry, M.J.; Etzioni, R.; Freedland, S.J.; Greene, K.L.; Holmberg, L.; Kantoff, P.; Konety, B.R.; Murad, M.H.; et al. Early Detection of Prostate Cancer: AUA Guideline. J. Urol. 2013, 190, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non–Muscle-Invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef]
- Chang, S.S.; Bochner, B.H.; Chou, R.; Dreicer, R.; Kamat, A.M.; Lerner, S.P.; Lotan, Y.; Meeks, J.J.; Michalski, J.M.; Morgan, T.M.; et al. Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guideline. J. Urol. 2017, 198, 552–559. [Google Scholar] [CrossRef]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef]
- Campbell, S.C.; Clark, P.E.; Chang, S.S.; Karam, J.A.; Souter, L.; Uzzo, R.G. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-Up: AUA Guideline: Part I. J. Urol. 2021, 206, 199–208. [Google Scholar] [CrossRef]
- Campbell, S.C.; Uzzo, R.G.; Karam, J.A.; Chang, S.S.; Clark, P.E.; Souter, L. Renal Mass and Localized Renal Cancer: Evaluation, Management, and Follow-up: AUA Guideline: Part II. J. Urol. 2021, 206, 209–218. [Google Scholar] [CrossRef]
- Albers, P.; Albrecht, W.; Algaba, F.; Bokemeyer, C.; Cohn-Cedermark, G.; Fizazi, K.; Horwich, A.; Laguna, M.P.; Nicolai, N.; Oldenburg, J. Guidelines on Testicular Cancer: 2015 Update. Eur. Urol. 2015, 68, 1054–1068. [Google Scholar] [CrossRef]
- Stephenson, A.; Eggener, S.E.; Bass, E.B.; Chelnick, D.M.; Daneshmand, S.; Feldman, D.; Gilligan, T.; Karam, J.A.; Leibovich, B.; Liauw, S.L.; et al. Diagnosis and Treatment of Early Stage Testicular Cancer: AUA Guideline. J. Urol. 2019, 202, 272–281. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).