18F-FDG PET/CT Maximum Tumor Dissemination (Dmax) in Lymphoma: A New Prognostic Factor?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol
2.2. Literature Search Strategy
2.3. Study Selection Process
2.4. Data Collection Process and Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
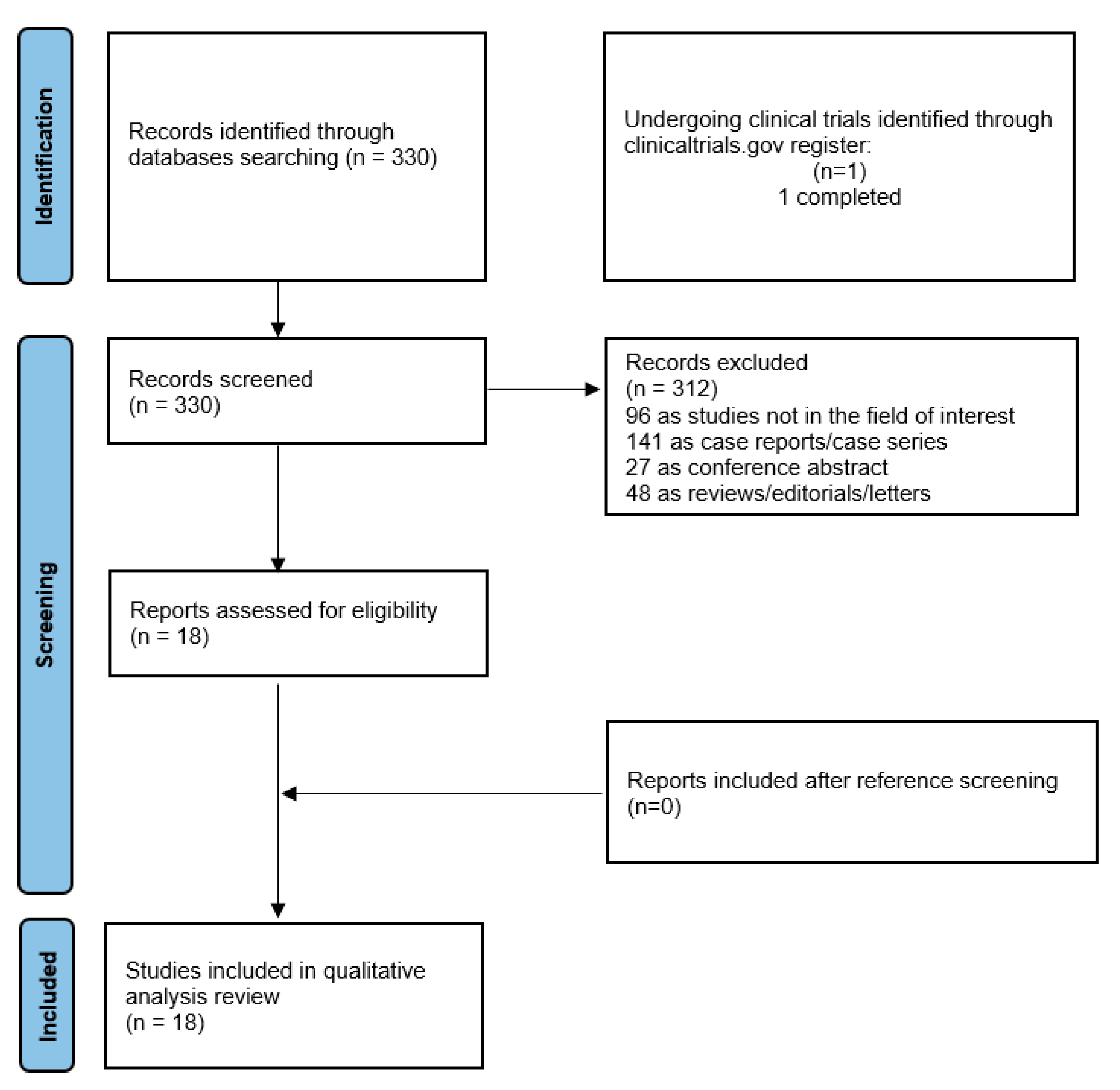
3.1. Literature Search and Study Selection
3.2. Study Characteristics
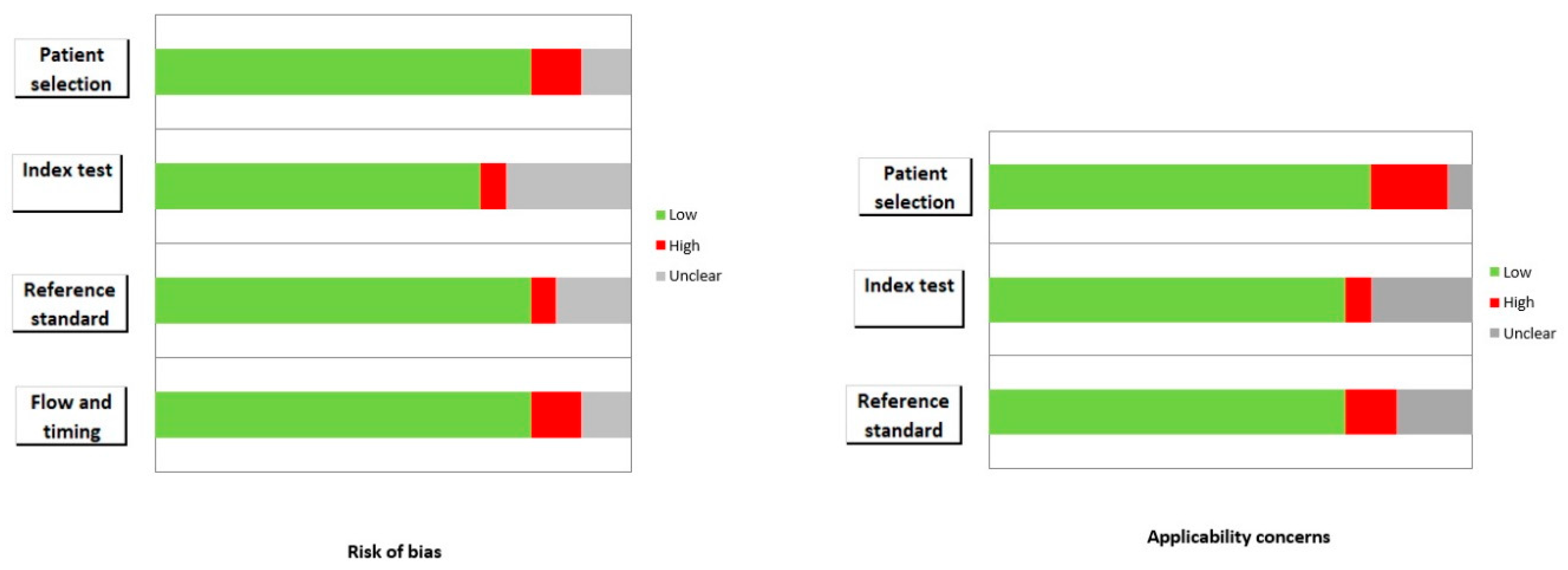
3.3. Risk of Bias and Applicability
3.4. Prognostic Role
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of imaging in the staging and response assessment of lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Albano, D.; Treglia, G.; Gazzilli, M.; Cerudelli, E.; Giubbini, R.; Bertagna, F. 18F-FDG PET or PET/CT in Mantle Cell Lymphoma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Durmo, R.; Treglia, G.; Giubbini, R.; Bertagna, F. 18F-FDG PET/CT or PET Role in MALT Lymphoma: An Open Issue not Yet Solved—A Critical Review. Clin. Lymphoma Myeloma Leuk. 2020, 20, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Bertagna, F.; Giubbini, R. 18F-FDG PET/CT role in Burkitt lymphoma. Clin. Transl. Imaging 2020, 8, 39–45. [Google Scholar] [CrossRef]
- Frood, R.; Burton, C.; Tsoumpas, C.; Frangi, A.F.; Gleeson, F.; Patel, C.; Scarsbrook, A. Baseline PET/CT imaging parameters for prediction of treatment outcome in Hodgkin and diffuse large B cell lymphoma: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3198–3220. [Google Scholar] [CrossRef] [PubMed]
- Aldin, A.; Umlauff, L.; Estcourt, L.J.; Collins, G.; Moons, K.G.; Engert, A.; Kobe, C.; von Tresckow, B.; Haque, M.; Foroutan, F.; et al. Interim PET-results for prognosis in adults with Hodgkin lymphoma: A systematic review and meta-analysis of prognostic factor studies. Cochrane Database Syst. Rev. 2020, 1, Cd012643. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, A.; Ji, Z.; Tian, M.; Zhang, H. Role of Radiomics-Based Baseline PET/CT Imaging in Lymphoma: Diagnosis, Prognosis, and Response Assessment. Mol. Imaging Biol. 2022, 24, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Albano, D.; Dondi, F.; Ravanelli, M.; Tucci, A.; Farina, D.; Giubbini, R.; Treglia, G.; Bertagna, F. Prognostic Role of “Radiological” Sarcopenia in Lymphoma: A Systematic Review. Clin. Lymphoma Myeloma Leuk. 2022, 22, e340–e349. [Google Scholar] [CrossRef]
- Cottereau, A.S.; Nioche, C.; Dirand, A.S.; Clerc, J.; Morschhauser, F.; Casasnovas, O.; Meignan, M.; Buvat, I. 18F-FDG PET Dissemination Features in Diffuse Large B-Cell Lymphoma Are Predictive of Outcome. J. Nucl. Med. 2020, 61, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, L.; Zucca, E. Dmax: A simple and reliable PET/CT-derived new biomarker of lymphoma outcome? Hematol. Oncol. 2022, 40, 843–845. [Google Scholar] [CrossRef] [PubMed]
- McInnes, M.D.F.; Moher, D.; Thombs, B.D.; McGrath, T.A.; Bossuyt, P.M.; Clifford, T.; Cohen, J.F.; Deeks, J.J.; Gatsonis, C.; Hooft, L.; et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies. The PRISMA-DTA Statement. JAMA 2018, 319, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Weisman, A.J.; Kim, J.; Lee, I.; McCarten, K.M.; Kessel, S.; Schwartz, C.L.; Kelly, K.M.; Jeraj, R.; Cho, S.Y.; Bradshaw, T.J. Automated quantification of baseline imaging PET metrics on FDG PET/CT images of pediatric Hodgkin lymphoma patients. EJNMMI Phys. 2020, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Cottereau, A.S.; Meignan, M.; Nioche, C.; Capobianco, N.; Clerc, J.; Chartier, L.; Vercellino, L.; Casasnovas, O.; Thieblemont, C.; Buvat, I. Risk stratification in diffuse large B-cell lymphoma using lesion dissemination and metabolic tumor burden calculated from baseline PET/CT. Ann. Oncol. 2021, 32, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, Y.; Chen, Z.; Li, J.; Sang, S.; Deng, S. Radiomic Features of 18F-FDG PET in Hodgkin Lymphoma Are Predictive of Outcomes. Contrast Media Mol. Imaging 2021, 2021, 6347404. [Google Scholar] [CrossRef]
- Cottereau, A.S.; Meignan, M.; Nioche, C.; Clerc, J.; Chartier, L.; Vercellino, L.; Casasnovas, O.; Thieblemont, C.; Buvat, I. New Approaches in Characterization of Lesions Dissemination in DLBCL Patients on Baseline PET/CT. Cancers 2021, 13, 3998. [Google Scholar] [CrossRef] [PubMed]
- Vergote, V.K.J.; Verhoef, G.; Janssens, A.; Woei-A-Jin, F.S.H.; Laenen, A.; Tousseyn, T.; Dierickx, D.; Deroose, C.M. [18F]FDG-PET/CT volumetric parameters can predict outcome in untreated mantle cell lymphoma. Leuk. Lymphoma 2023, 64, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Durmo, R.; Donati, B.; Rebaud, L.; Cottereau, A.S.; Ruffini, A.; Nizzoli, M.E.; Ciavarella, S.; Vegliante, M.C.; Nioche, C.; Meignan, M.; et al. Prognostic value of lesion dissemination in doxorubicin, bleomycin, vinblastine, and dacarbazine-treated, interimPET-negative classical Hodgkin Lymphoma patients: A radio-genomic study. Hematol. Oncol. 2022, 40, 645–657. [Google Scholar] [CrossRef]
- Li, H.; Wang, M.; Zhang, Y.; Hu, F.; Wang, K.; Wang, C.; Gao, Z. Prediction of prognosis and pathologic grade in follicular lymphoma using 18F-FDG PET/CT. Front. Oncol. 2022, 12, 943151. [Google Scholar] [CrossRef]
- Ceriani, L.; Milan, L.; Cascione, L.; Gritti, G.; Dalmasso, F.; Esposito, F.; Pirosa, M.C.; Schär, S.; Bruno, A.; Dirnhofer, S.; et al. Generation and validation of a PET radiomics model that predicts survival in diffuse large B cell lymphoma treated with R-CHOP14: A SAKK 38/07 trial post-hoc analysis. Hematol. Oncol. 2022, 40, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Drees, E.E.; Driessen, J.; Zwezerijnen, G.J.; Verkuijlen, S.A.; Eertink, J.J.; van Eijndhoven, M.A.; Groenewegen, N.J.; Vallés-Martí, A.; de Jong, D.; Boellaard, R.; et al. Blood-circulating EV-miRNAs, serum TARC, and quantitative FDG-PET features in classical Hodgkin lymphoma. EJHaem 2022, 3, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Driessen, J.; Zwezerijnen, G.J.; Schöder, H.; Drees, E.E.; Kersten, M.J.; Moskowitz, A.J.; Moskowitz, C.H.; Eertink, J.J.; De Vet, H.C.; Hoekstra, O.S.; et al. The Impact of Semiautomatic Segmentation Methods on Metabolic Tumor Volume, Intensity, and Dissemination Radiomics in 18F-FDG PET Scans of Patients with Classical Hodgkin Lymphoma. J. Nucl. Med. 2022, 63, 1424–1430. [Google Scholar] [CrossRef]
- Eertink, J.J.; van de Brug, T.; Wiegers, S.E.; Zwezerijnen, G.J.; Pfaehler, E.A.; Lugtenburg, P.J.; van der Holt, B.; de Vet, H.C.; Hoekstra, O.S.; Boellaard, R.; et al. 18F-FDG PET baseline radiomics features improve the prediction of treatment outcome in diffuse large B-cell lymphoma. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 932–942. [Google Scholar] [CrossRef]
- Eertink, J.J.; Zwezerijnen, G.J.; Cysouw, M.C.; Wiegers, S.E.; Pfaehler, E.A.; Lugtenburg, P.J.; van der Holt, B.; Hoekstra, O.S.; de Vet, H.C.; Zijlstra, J.M.; et al. Comparing lesion and feature selections to predict progression in newly diagnosed DLBCL patients with FDG PET/CT radiomics features. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 4642–4651. [Google Scholar] [CrossRef] [PubMed]
- Girum, K.B.; Rebaud, L.; Cottereau, A.S.; Meignan, M.; Clerc, J.; Vercellino, L.; Casasnovas, O.; Morschhauser, F.; Thieblemont, C.; Buvat, I. 18F-FDG PET Maximum-Intensity Projections and Artificial Intelligence: A Win-Win Combination to Easily Measure Prognostic Biomarkers in DLBCL Patients. J. Nucl. Med. 2022, 63, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Tang, B.; Li, T.; Li, J.; Tang, L.; Ding, C. The added prognostic values of baseline PET dissemination parameter in patients with angioimmunoblastic T-cell lymphoma. EJHaem 2022, 4, 67–77. [Google Scholar] [CrossRef]
- Jo, J.H.; Chung, H.W.; Kim, S.Y.; Lee, M.H.; So, Y. FDG PET/CT Maximum Tumor Dissemination to Predict Recurrence in Patients with Diffuse Large B-Cell Lymphoma. Nucl. Med. Mol. Imaging 2023, 57, 26–33. [Google Scholar] [CrossRef]
- Xie, Y.; Teng, Y.; Jiang, C.; Ding, C.; Zhou, Z. Prognostic value of 18F-FDG lesion dissemination features in patients with peripheral T-cell lymphoma (PTCL). Jpn. J. Radiol. 2023; published online ahead of print. [Google Scholar] [CrossRef]
- Eertink, J.J.; Zwezerijnen, G.J.; Wiegers, S.E.; Pieplenbosch, S.; Chamuleau, M.E.; Lugtenburg, P.J.; de Jong, D.; Ylstra, B.; Mendeville, M.; Dührsen, U.; et al. Baseline radiomics features and MYC rearrangement status predict progression in aggressive B-cell lymphoma. Blood Adv. 2023, 7, 214–223. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Barrington, S.F.; Meignan, M. Time to prepare for risk adaptation in lymphoma by standardizing measurement of metabolic tumor burden. J. Nucl. Med. 2019, 60, 1096–1102. [Google Scholar] [CrossRef]
- Fornacon-Wood, I.; Mistry, H.; Ackermann, C.J.; Blackhall, F.; McPartlin, A.; Faivre.Finn, C.; Prie, G.J.; O’Connor, J.P.B. Reliability and prognostic value of radiomic features are highly dependent on choice of feature extraction platform. Eur. Radiol. 2020, 30, 6241–6250. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Zwarthoed, C. Interim FDG-PET Imaging in Lymphoma. Semin. Nucl. Med. 2018, 48, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Meignan, M.; Gallamini, A.; Haioun, C.; Polliack, A. Report on the Second International Workshop on interim positron emission tomography in lymphoma held in Menton, France, 8–9 April 2010. Leuk. Lymph. 2010, 51, 2171–2180. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
| First Author | Year | Country | Study Design | Funding Sources |
|---|---|---|---|---|
| Cottereau, A.S. [10] | 2020 | France | Retrospective | None declared |
| Weisman, A.J. [14] | 2020 | USA | Retrospective | GE Healthcare; National Institutes of Health to the Children’s Oncology Group (U10CA098543), Statistics & Data Center Grant (U10CA098413), NCTN Operations Center Grant (U10CA180886), NCTN Statistics & Data Center Grant (U10CA180899), QARC (CA29511) IROC RI (U24CA180803); and St. Baldricks Foundation |
| Cottereau, A.S. [15] | 2021 | France | Retrospective | None declared |
| Zhou, Y. [16] | 2021 | China | Retrospective | None declared |
| Cottereau, A.S. [17] | 2021 | France | Retrospective | None declared |
| Vergote, V.K.J. [18] | 2022 | Belgium | Retrospective | None declared |
| Durmo, R. [19] | 2022 | Italy | Retrospective | GRADE Onlus; Associazione Italiana per la Ricerca sul Cancro; Italian Ministry of Health Ricerca Corrente Annual Program 2023 |
| Li, H. [20] | 2022 | China | Retrospective | National Natural Science Foundation of China (No. 81771866). |
| Ceriani, L. [21] | 2022 | Switzerland | Prospective | Ente Ospedaliero Cantonale, Grant/Award Number: ABREOC 22008-262; Amgen; Oncosuisse, Grant/Award Number: OCS-02270-08-2008 |
| Drees, E.E.E. [22] | 2022 | The Netherland | Retrospective | The Dutch Cancer Society, Grant/Award Number: KWF-5510; Cancer Center Amsterdam Foundation, Grant/Award Number: CCA-2013; Technology Foundation STW, Grant/Award Number: CANCER-ID |
| Driessen, J. [23] | 2022 | The Netherland/USA | Retrospective | None declared |
| Eertink, J.J. [24] | 2022 | The Netherland | Prospective | Dutch Cancer Society (# VU 2018–11648) |
| Eertink, J.J. [25] | 2022 | The Netherland | Prospective | Dutch Cancer Society (# VU 2018–11648) |
| Girum, K.B. [26] | 2022 | France | Retrospective | None declared |
| Gong, H. [27] | 2022 | China | Retrospective | None declared |
| Jo, J.H. [28] | 2023 | Korea | Retrospective | None declared |
| Xie, Y. [29] | 2023 | China | Retrospective | None declared |
| Eertink, J.J. [30] | 2023 | Netherland | Retrospective | None declared |
| First Author | N Pts | Lymphoma Variant | Early (I–II)/Advanced (III–IV) Stage Acc Ann Arbor | M:F | Median Age (Range) | Main Results |
|---|---|---|---|---|---|---|
| Cottereau, A.S. [10] | 95 | DLBCL | 0:95 | 53:42 | 46 (18–59) | Dmax was significantly associated with PFS and OS. The combination of MTV and Dmax helped to stratify patients |
| Weisman, A.J. [14] | 100 | HL | 0:100 | 60:40 | 15.8 (5.2–21.4) | Moderate reproducibility in the Dmax measurement between fully automated software and physicians |
| Cottereau, A.S. [15] | 290 | DLBCL | 26:264 | 170:120 | Nr (60–80) | SDmax was significantly associated with PFS and OS. The combination of MTV and SDmax helped to stratify patients |
| Zhou, Y. [16] | 65 | HL | 36:29 | 45:20 | 29 (8–72) | Dmax was significantly associated with PFS and OS |
| Cottereau, A.S. [17] | 290 | DLBCL | 26:264 | 170:120 | Nr (60–80) | Comparison of different ways to calculate dissemination features |
| Vergote, V.K.J. [18] | 83 | MCL | 12:71 | 62:21 | 66 (58–72) | Dmax was not associated with prognosis |
| Durmo, R. [19] | 155 | HL | 77:78 | 79:76 | Nr | Dmax was significantly associated with PFS. Dmax and interim metabolic treatment response helped to stratify patients |
| Li, H. [20] | 126 | FL | 22:104 | 63:63 | 53 (21–76) | Dmax and TLG were significantly associated with PFS |
| Ceriani, L. [21] | 240 | DLBCL | 104:136 | 119:121 | Nr | SDmax was included in a radiomics model with a prognostic value |
| Drees, E.E.E. [22] | 30 | HL | Nr | Nr | 36 * (18–66) | Blood-based markers, EV-miRNA, and sTARC were moderately related to dissemination features |
| Driessen, J. [23] | 105 | HL | Nr | 47:58 | 30 (13–66) | Good reproducibility of Dmax between 6 different segmentation methods |
| Eertink, J.J. [24] | 317 | DLBCL | 51:266 | 161:156 | 65 (23–80) | Dmaxbulk was one of the best predictors of treatment outcome |
| Eertink, J.J. [25] | 296 | DLBCL | 48:248 | 152:144 | 65 (55–72) | Dissemination features were the best predictors of progression |
| Girum, K.B. [26] | 382 | DLBCL | Nr | 207:175 | 62.1 * (34–73) | Dmax was significantly associated with PFS and OS. The combination of MTV and Dmax helped to stratify patients |
| Gong, H. [27] | 81 | AITL | 5:76 | 53:28 | 63 | Dmax was significantly associated with PFS and OS. The combination of MTV and Dmax helped to stratify patients |
| Jo, J.H. [28] | 63 | DLBCL | 26:39 | 28:35 | 57.3 * (21–87) | Dmax and end-of-treatment metabolic treatment response were significantly associated with TTP |
| Xie, Y. [29] | 95 | PTCL | 10:85 | 59:46 | 64 (16–84) | Dmax and bone marrow biopsy were significantly associated with PFS and OS |
| Eertink, J.J. [30] | 323 | DLBCL | 77:246 | 185:138 | 63 (53–71) | Baseline radiomics features were significantly associated with PFS |
| First Author | PET Features | Software | Dmax Cut-Off | Dmax Median |
|---|---|---|---|---|
| Cottereau, A.S. [10] | SUVmax, MTV, TLG, Dmaxpatient. Dmaxbulk, SPREADbulk, and SPREADpatient | LIFEx | 45 cm | 45 cm |
| Weisman, A.J. [14] | SUVmax, MTV, TLG, SA/MTV, and Dmax | Deepmedic | Nr | Nr |
| Cottereau, A.S. [15] | MTV, Dmax, and SDmax | LIFEx | 47 cm for Dmax 0.32 m−1 for SDmax | 42 cm for Dmax 0.23 m−1 for SDmax |
| Zhou, Y. [16] | SUVmin, SUVmax, SUVmean, SUVpeak, SUVst, MTV, TLG, Dmax, histogram-derived features, shape-derived features, and texture features | LIFEx | 57.4 cm | Nr |
| Cottereau, A.S. [17] | SDmax | LIFEx | Nr | Nr |
| Vergote, V.K.J. [18] | SUVmax, SUVmean, SUVpeak, MTV, TLG, Dmax, and SDmax | MIM | Nr | 0.6 m for Dmax 0.3 m−1 for SDmax |
| Durmo, R. [19] | MTV, TLG, and Dmax | FIJI and LIFEx | 20 cm | 20 cm |
| Li, H. [20] | SUVmax, MTV, TLG, and Dmax | R | 56.73 cm | 64 cm |
| Ceriani, L. [21] | SUVmax, SUVmean, MTV, TLG, SDmax, and texture features | PyRadiomics Python | Nr | Nr |
| Drees, E.E.E. [22] | SUVmax, SUVpeak, MTV, TLG, DmaxPatient, DmaxBulk, SpreadPatient, and SpreadBulk | RaCat | Nr | Nr |
| Driessen, J. [23] | SUVmax, SUVmean, SUVpeak, MTV, TLG, and Dmax | RaCat | Nr | Nr |
| Eertink, J.J. [24] | SUVmax, SUVmean, SUVpeak, MTV, TLG, Dmaxpatient, Dmaxbulk, SPREADbulk, SPREADpatient, and texture features | RaCat | Nr | Nr |
| Eertink, J.J. [25] | SUVmax, SUVmean, SUVpeak, MTV, TLG, Dmaxpatient, Dmaxbulk, SPREADbulk, SPREADpatient, and texture features | RaCat | Nr | Nr |
| Girum, K.B. [26] | MTV and Dmax | LIFEx | 59 cm | 98 cm for REMARC 116.4 cm for LNH073B |
| Gong, H. [27] | MTV and Dmax | LIFEx | 65.7 cm | 66.4 cm |
| Jo, J.H. [28] | SUVmax, SUVmean, MTV, TLG, and Dmax | LIFEx | 27.5 cm | Nr |
| Xie, Y. [29] | SUV, MTV, TLG, and Dmax | LIFEx | 65.95 cm | 69.3 cm |
| Eertink, J.J. [30] | SUVmax, SUVmean, SUVpeak, MTV, TLG, and 12 dissemination features | RaCat | Nr | Nr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albano, D.; Treglia, G.; Dondi, F.; Calabrò, A.; Rizzo, A.; Annunziata, S.; Guerra, L.; Morbelli, S.; Tucci, A.; Bertagna, F. 18F-FDG PET/CT Maximum Tumor Dissemination (Dmax) in Lymphoma: A New Prognostic Factor? Cancers 2023, 15, 2494. https://doi.org/10.3390/cancers15092494
Albano D, Treglia G, Dondi F, Calabrò A, Rizzo A, Annunziata S, Guerra L, Morbelli S, Tucci A, Bertagna F. 18F-FDG PET/CT Maximum Tumor Dissemination (Dmax) in Lymphoma: A New Prognostic Factor? Cancers. 2023; 15(9):2494. https://doi.org/10.3390/cancers15092494
Chicago/Turabian StyleAlbano, Domenico, Giorgio Treglia, Francesco Dondi, Anna Calabrò, Alessio Rizzo, Salvatore Annunziata, Luca Guerra, Silvia Morbelli, Alessandra Tucci, and Francesco Bertagna. 2023. "18F-FDG PET/CT Maximum Tumor Dissemination (Dmax) in Lymphoma: A New Prognostic Factor?" Cancers 15, no. 9: 2494. https://doi.org/10.3390/cancers15092494
APA StyleAlbano, D., Treglia, G., Dondi, F., Calabrò, A., Rizzo, A., Annunziata, S., Guerra, L., Morbelli, S., Tucci, A., & Bertagna, F. (2023). 18F-FDG PET/CT Maximum Tumor Dissemination (Dmax) in Lymphoma: A New Prognostic Factor? Cancers, 15(9), 2494. https://doi.org/10.3390/cancers15092494










