Protective Effects of Influenza Vaccine against Colorectal Cancer in Populations with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
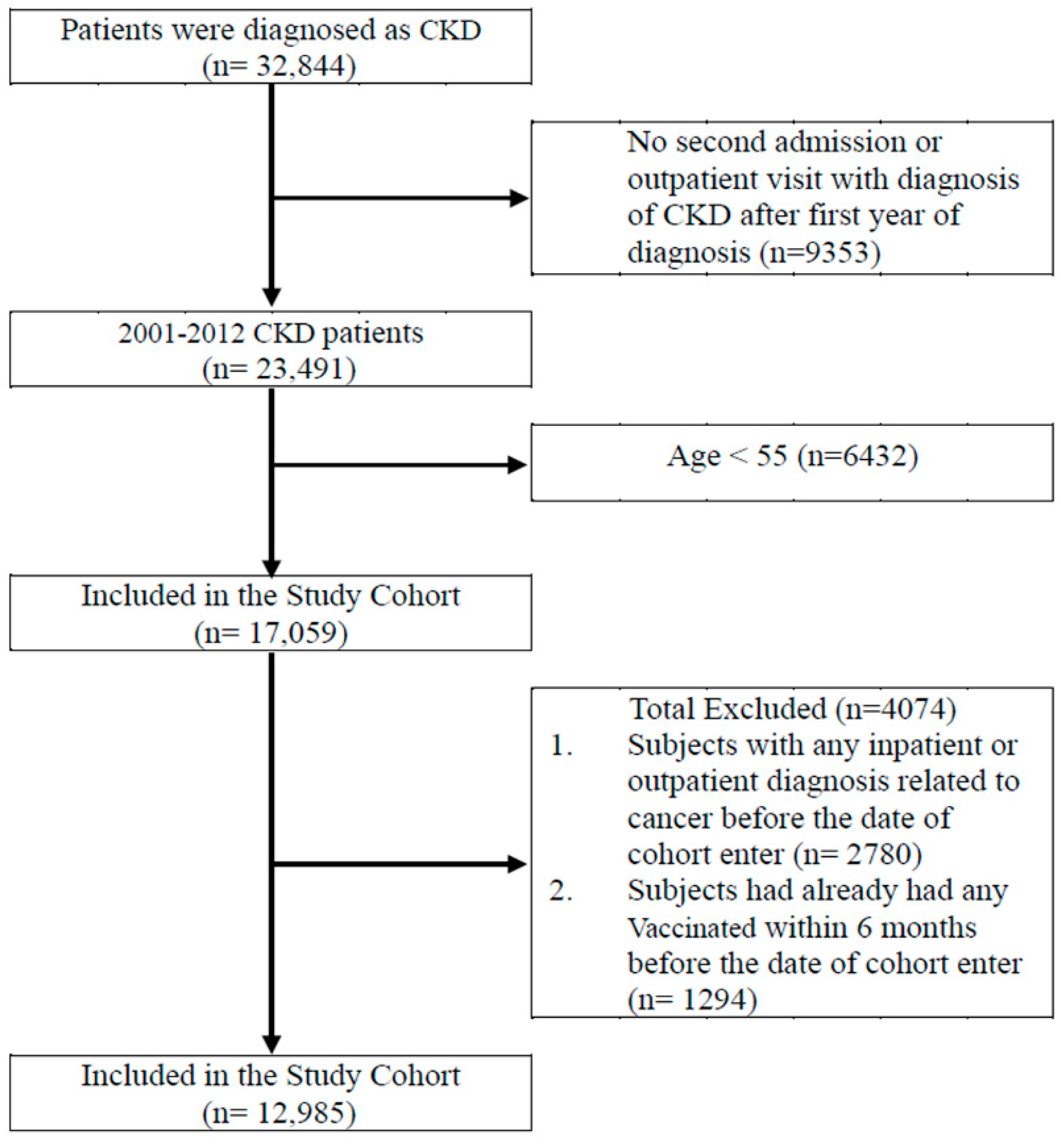
2.2. Participants
2.3. Potential Confounder
2.4. Statical Analysis
3. Result
3.1. Baseline Characteristics among Vaccinated and Unvaccinated Groups
3.2. Age and Sex among Vaccinated and Unvaccinated Groups
3.3. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- James, L.J.; Wong, G.; Craig, J.C.; Ju, A.; Williams, N.; Lim, W.H.; Cross, N.; Tong, A. Beliefs and Attitudes to Bowel Cancer Screening in Patients with CKD: A Semistructured Interview Study. Clin. J. Am. Soc. Nephrol. 2017, 12, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Au, E.H.; Wong, G.; Howard, K.; Chapman, J.R.; Castells, A.; Roger, S.D.; Bourke, M.J.; Macaskill, P.; Turner, R.; Lim, W.H.; et al. Factors Associated With Advanced Colorectal Neoplasia in Patients with CKD. Am. J. Kidney Dis. 2022, 79, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Lee, H.A.; Moon, C.M.; Ryu, D.-R. Incidence risk of various types of digestive cancers in patients with pre-dialytic chronic kidney disease: A nationwide population-based cohort study. PLoS ONE 2018, 13, e0207756. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Chang, T.-C.; Chao, T.-Y.; Huang, M.-T.; Lin, H.-W. Risk of Colorectal Cancer in Chronic Kidney Disease: A Matched Cohort Study Based on Administrative Data. Ann. Surg. Oncol. 2013, 20, 3885–3891. [Google Scholar] [CrossRef]
- Cobo, G.; Lindholm, B.; Stenvinkel, P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol. Dial. Transplant. 2018, 33 (Suppl. S3), iii35–iii40. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Evenepoel, P.; Poesen, R.; Meijers, B. The gut-kidney axis. Pediatr. Nephrol. 2017, 32, 2005–2014. [Google Scholar] [CrossRef]
- Arthur, J.C.; Perez-Chanona, M.E.; Mühlbauer, S.; Tomkovich, J.M.; Uronis, T.J.; Fan, B.J.; Campbell, T.; Abujamel, B.; Dogan, A.B.; Rogers, A.B.; et al. Intestinal Inflammation Targets Cancer-Inducing Activity of the Microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 3100. [Google Scholar] [CrossRef]
- Read, S.A.; Douglas, M.W. Virus induced inflammation and cancer development. Cancer Lett. 2014, 345, 174–181. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Wu, Z.; Chen, Y.; Gao, H.; Lv, L.; Guo, F.; Zhang, X.; Luo, R.; Huang, C.; et al. Alterations of the Gut Microbiota in Patients with Coronavirus Disease 2019 or H1N1 Influenza. Clin. Infect. Dis. 2020, 71, 2669–2678. [Google Scholar] [CrossRef]
- Guo, C.; Guo, D.; Fang, L.; Sang, T.; Wu, J.; Guo, C.; Wang, Y.; Wang, Y.; Chen, C.; Chen, J.; et al. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydr. Polym. 2021, 267, 118231. [Google Scholar] [CrossRef]
- Gögenur, M.; Fransgård, T.; Krause, T.G.; Thygesen, L.C.; Gögenur, I. Association of influenza vaccine and risk of recurrence in patients undergoing curative surgery for colorectal cancer. Acta. Oncol. 2021, 60, 1507–1512. [Google Scholar] [CrossRef]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef]
- Tai, L.-H.; Zhang, J.; Auer, R.C. Preventing surgery-induced NK cell dysfunction and cancer metastases with influenza vaccination. Oncoimmunology 2013, 2, e26618. [Google Scholar] [CrossRef]
- Tai, L.-H.; Zhang, J.; Scott, K.J.; de Souza, C.T.; Alkayyal, A.A.; Ananth, A.A.; Sahi, S.; Adair, R.A.; Mahmoud, A.B.; Sad, S.; et al. Perioperative Influenza Vaccination Reduces Postoperative Metastatic Disease by Reversing Surgery-Induced Dysfunction in Natural Killer Cells. Clin. Cancer Res. 2013, 19, 5104–5115. [Google Scholar] [CrossRef]
- Gögenur, M.; Fransgård, T.; Krause, T.G.; Thygesen, L.C.; Gögenur, I. Association of postoperative influenza vaccine on overall mortality in patients undergoing curative surgery for solid tumors. Int. J. Cancer 2021, 148, 1821–1827. [Google Scholar] [CrossRef]
- Chen, C.-C.; Lin, C.-H.; Yang, T.Y.; Wang, T.-J.; Li, S.-J.; Fang, Y.-A.; Chen, T.-J.; Tzeng, H.-E.; Chiu, C.-C.; Hao, W.-R.; et al. Association between sleep disorder and atrial fibrillation: A nationwide population-based cohort study. Sleep Med. 2022, 96, 50–56. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wu, C.-H.; Lin, C.-H.; Chiu, C.-C.; Yang, T.-Y.; Lei, M.-H.; Yeh, H.-T.; Jian, W.; Fang, Y.-A.; Hao, W.-R.; et al. Influenza Vaccination and Risk of Lung Cancer in Patients with Chronic Kidney Disease: A Nationwide, Population-Based Cohort Study. Cancers 2022, 14, 2926. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.M.; Wilson, M.; Elwin, C.-E.; Norrving, B.; Algra, A.; Warlow, C.P.; Meade, T.W. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010, 376, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Seretis, A.; Cividini, S.; Markozannes, G.; Tseretopoulou, X.; Lopez, D.S.; Ntzani, E.E.; Tsilidis, K.K. Association between blood pressure and risk of cancer development: A systematic review and meta-analysis of observational studies. Sci. Rep. 2019, 9, 8565. [Google Scholar] [CrossRef]
- Yao, X.; Tian, Z. Dyslipidemia and colorectal cancer risk: A meta-analysis of prospective studies. Cancer Causes Control. 2015, 26, 257–268. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, R.B., Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 1998, 17, 2265–2281. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef]
- Anders, H.-J.; Andersen, K.; Stecher, B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013, 83, 1010–1016. [Google Scholar] [CrossRef]
- Cremonesi, E.; Governa, V.; Garzon, J.F.G.; Mele, V.; Amicarella, F.; Muraro, M.G.; Trella, E.; Galati-Fournier, V.; Oertli, D.; Däster, S.R.; et al. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut 2018, 67, 1984–1994. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Wang, D.K.; Mucida, C.A.; Stewart, B.; Schnabl, D.; Jauch, K.; Taniguchi, G.Y.; Yu, C.H.; Osterreicher, K.E.; Hung, K.E.; et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012, 491, 254–258. [Google Scholar] [CrossRef]
- Song, X.; Gao, H.; Lin, Y.; Yao, Y.; Zhu, S.; Wang, J.; Liu, Y.; Yao, X.; Meng, G.; Shen, N.; et al. Alterations in the Microbiota Drive Interleukin-17C Production from Intestinal Epithelial Cells to Promote Tumorigenesis. Immunity 2014, 40, 140–152. [Google Scholar] [CrossRef]
- Hosainzadegan, M.; Eftekhari, A.; Khalilov, R.; Nasibova, A.; Hasanzadeh, A.; Vahedi, P. Are Microbial Infections and Some Antibiotics Causes Cancer? Adv. Biol. Earth Sci. 2020, 5, 58–61. [Google Scholar]
- Deriu, E.; Boxx, G.M.; He, X.; Pan, C.; Benavidez, S.D.; Cen, L.; Rozengurt, N.; Shi, W.; Cheng, G. Influenza Virus Affects Intestinal Microbiota and Secondary Salmonella Infection in the Gut through Type I Interferons. PLoS Pathog. 2016, 12, e1005572. [Google Scholar] [CrossRef]
- Wang, J.; Li, F.; Wei, H.; Lian, Z.X.; Sun, R.; Tian, Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota-mediated Th17 cell-dependent inflammation. J. Exp. Med. 2014, 211, 2397–2410. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; De Paula, R.; Da Silva, E.F.; Pral, L.P.; DOS Santos, A.; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 3273. [Google Scholar] [CrossRef]
- Haase, S.; Haghikia, A.; Wilck, N.; Müller, D.N.; Linker, R.A. Impacts of microbiome metabolites on immune regulation and autoimmunity. Immunology 2018, 154, 230–238. [Google Scholar] [CrossRef]
- Tsiakos, K.; Kyriakoulis, K.G.; Kollias, A.; Kyriakoulis, I.G.; Poulakou, G.; Syrigos, K. Influenza Vaccination in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. J. Immunother. 2022, 45, 291–298. [Google Scholar] [CrossRef]
- Bersanelli, M.; Buti, S.; Banna, G.L.; De Giorgi, U.; Cortellini, A.; Rebuzzi, S.E.; Tiseo, M.; Fornarini, G.; Mazzoni, F.; Panni, S.; et al. Impact of influenza syndrome and flu vaccine on survival of cancer patients during immunotherapy in the INVIDIa study. Immunotherapy 2020, 12, 151–159. [Google Scholar] [CrossRef]
- Puthillath, A.; Trump, D.L.; Andrews, C.; Bir, A.; Romano, K.; Wisniewski, M.; Fakih, M.G. Serological immune responses to influenza vaccine in patients with colorectal cancer. Cancer Chemother. Pharmacol. 2011, 67, 111–115. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]

| Whole Cohort (n = 12,985) | Unvaccinated (n = 7490) | Vaccinated (n = 5495) | p a | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Age, years (mean ± SD) | 70.98 (9.40) | 70.09 (10.26) | 72.18 (7.90) | <0.001 | |||
| 55–64 | 3989 | 30.72 | 2877 | 38.41 | 1112 | 20.24 | <0.001 |
| 65–74 | 4541 | 34.97 | 2139 | 28.56 | 2402 | 43.71 | |
| ≥75 | 4455 | 34.31 | 2474 | 33.03 | 1981 | 36.05 | |
| Gender | |||||||
| Female | 5712 | 43.99 | 3333 | 44.50 | 2379 | 43.29 | 0.172 |
| Male | 7273 | 56.01 | 4157 | 55.50 | 3116 | 56.71 | |
| CCI Index + | |||||||
| 0 | 1491 | 11.48 | 876 | 11.70 | 615 | 11.19 | 0.013 |
| 1 | 2043 | 15.73 | 1166 | 15.57 | 877 | 15.96 | |
| 2 | 2876 | 22.15 | 1589 | 21.21 | 1287 | 23.42 | |
| ≥3 | 6575 | 50.64 | 3859 | 51.52 | 2716 | 49.43 | |
| Diabetes | |||||||
| No | 6310 | 48.59 | 3355 | 44.79 | 2955 | 53.78 | <0.001 |
| Yes | 6675 | 51.41 | 4135 | 55.21 | 2540 | 46.22 | |
| Hypertension | |||||||
| No | 2555 | 19.68 | 1387 | 18.52 | 1168 | 21.26 | <0.001 |
| Yes | 10,430 | 80.32 | 6103 | 81.48 | 4327 | 78.74 | |
| Dyslipidemia | |||||||
| No | 6337 | 48.80 | 3386 | 45.21 | 2951 | 53.70 | <0.001 |
| Yes | 6648 | 51.20 | 4104 | 54.79 | 2544 | 46.30 | |
| Statin | |||||||
| <28 days | 7972 | 61.39 | 4786 | 63.90 | 3186 | 57.98 | <0.001 |
| 28–365 days | 2683 | 20.66 | 1576 | 21.04 | 1107 | 20.15 | |
| >365 days | 2330 | 17.94 | 1128 | 15.06 | 1202 | 21.87 | |
| Metformin | |||||||
| <28 days | 10,266 | 79.06 | 6045 | 80.71 | 4221 | 76.82 | <0.001 |
| 28–365 days | 1331 | 10.25 | 804 | 10.73 | 527 | 9.59 | |
| >365 days | 1388 | 10.69 | 641 | 8.56 | 747 | 13.59 | |
| RAA | |||||||
| <28 days | 4114 | 31.68 | 2792 | 37.28 | 1322 | 24.06 | <0.001 |
| 28–365 days | 3751 | 28.89 | 2344 | 31.30 | 1407 | 25.61 | |
| >365 days | 5120 | 39.43 | 2354 | 31.43 | 2766 | 50.34 | |
| Aspirin | |||||||
| <28 days | 6715 | 51.71 | 4478 | 59.79 | 2237 | 40.71 | <0.001 |
| 28–365 days | 3149 | 24.25 | 1702 | 22.72 | 1447 | 26.33 | |
| >365 days | 3121 | 24.04 | 1310 | 17.49 | 1811 | 32.96 | |
| Level of urbanization | |||||||
| Urban | 8785 | 67.65 | 5350 | 71.43 | 3435 | 62.51 | <0.001 |
| Suburban | 2806 | 21.61 | 1488 | 19.87 | 1318 | 23.99 | |
| Rural | 1394 | 10.74 | 652 | 8.70 | 742 | 13.50 | |
| Monthly income (NTD) | |||||||
| 0 | 1596 | 12.29 | 901 | 12.03 | 695 | 12.65 | <0.001 |
| 1–21,000 | 4486 | 34.55 | 2397 | 32.00 | 2089 | 38.02 | |
| 21,000–33,300 | 3788 | 29.17 | 1996 | 26.65 | 1792 | 32.61 | |
| ≥33,301 | 3115 | 23.99 | 2196 | 29.32 | 919 | 16.72 | |
| All Group (n = 12,985) | Unvaccinated (Total Follow-up 21,919.2 Person-Years) | Vaccinated (Total Follow-up 33,990.2 Person-Years) | χ2 | Adjusted HR † (95% C.I.) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients with Cancer | Incidence Rate (Per 105 Person-Years) (95% C.I.) | No. of Patients With Cancer | Incidence Rate (Per 105 Person-Years) (95% C.I.) | |||||||
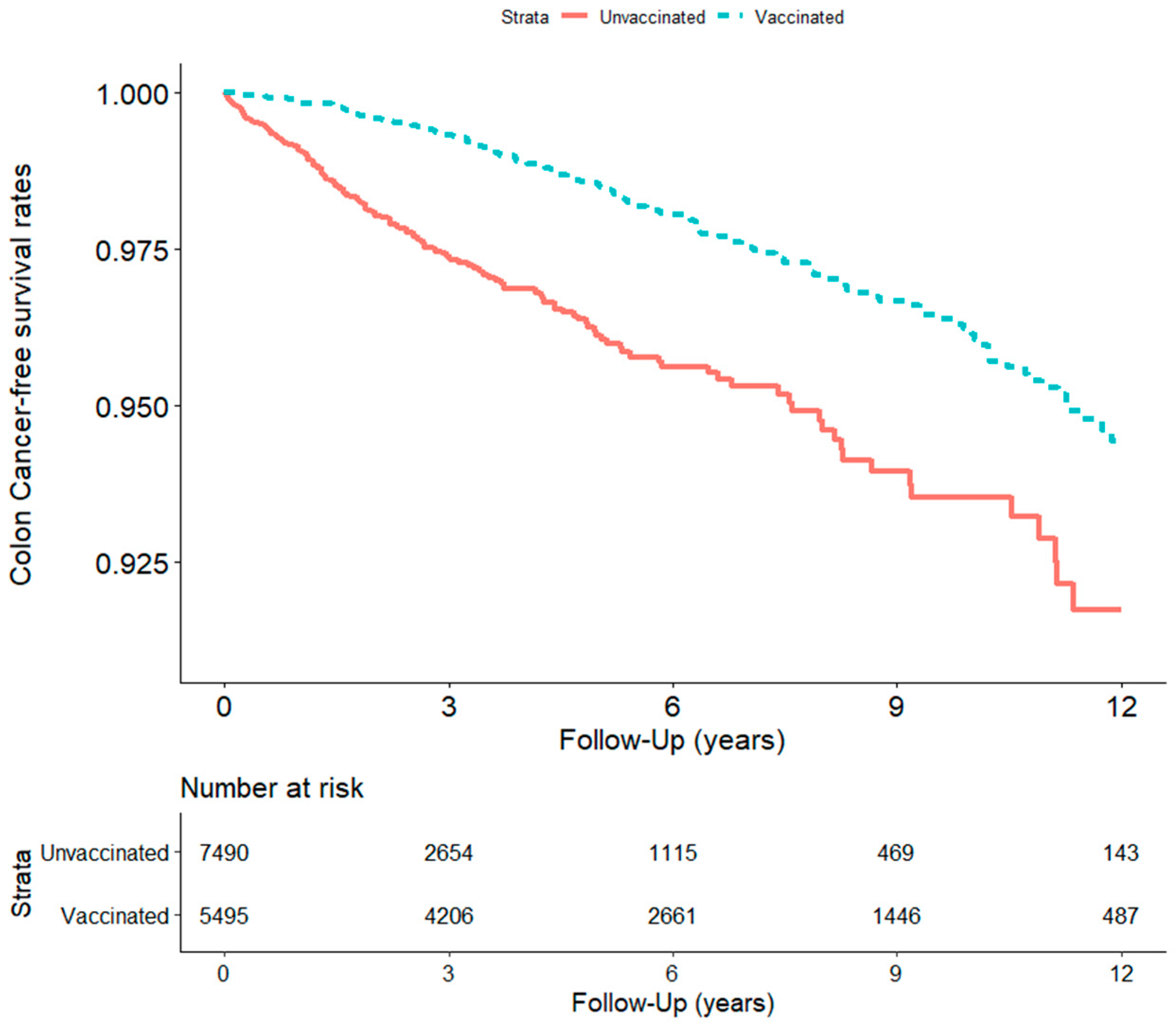
| 175 | 798.4 | (680.1, | 916.7) | 128 | 376.6 | (311.3, | 441.8) | 0.001 | 0.38 (0.30, 0.48) *** | |
| Age, 55–64 a | 39 | 361.5 | (248.1, | 475.0) | 25 | 277.8 | (168.9, | 386.8) | 3.502 | 0.65 (0.39, 1.09) |
| Age, 65–74 b | 75 | 1222.7 | (945.9, | 1499.4) | 62 | 391.9 | (294.3, | 489.4) | 3.001 | 0.31 (0.22, 0.44) *** |
| Age, ≥75 c | 61 | 1220.6 | (914.3, | 1526.9) | 41 | 447.1 | (310.2, | 584.0) | 0.604 | 0.35 (0.23, 0.52) *** |
| Female d | 62 | 643.4 | (483.2, | 803.5) | 37 | 247.0 | (167.4, | 326.6) | 0.589 | 0.34 (0.22, 0.52) *** |
| Male e | 113 | 920.0 | (750.4, | 1089.6) | 91 | 478.7 | (380.3, | 577.0) | 0.198 | 0.40 (0.30, 0.53) *** |
| Unvaccinated | Vaccinated | p for Trend | |||
|---|---|---|---|---|---|
| 1 | 2–3 | ≥4 | |||
| Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | Adjusted HR (95% C.I.) | ||
| Main model † | 1.00 | 0.74 (0.54, 1.00) * | 0.41 (0.30, 0.57) *** | 0.16 (0.11, 0.25) *** | <0.001 |
| Additional covariates ‡ | |||||
| Main model + statin | 1.00 | 0.75 (0.55, 1.01) | 0.42 (0.30, 0.59) *** | 0.17 (0.11, 0.26) *** | <0.001 |
| Main model + metformin | 1.00 | 0.74 (0.55, 1.01) | 0.42 (0.30, 0.58) *** | 0.17 (0.11, 0.25) *** | <0.001 |
| Main model + RAA | 1.00 | 0.76 (0.56, 1.04) | 0.43 (0.31, 0.60) *** | 0.18 (0.12, 0.27) *** | <0.001 |
| Main model + aspirin | 1.00 | 0.77 (0.57, 1.05) | 0.44 (0.32, 0.62) *** | 0.18 (0.12, 0.28) *** | <0.001 |
| Subgroup effects | |||||
| Age, years | |||||
| 55–64 | 1.00 | 0.86 (0.43, 1.74) | 0.76 (0.36, 1.57) | 0.39 (0.16, 0.94) * | 0.037 |
| 65–74 | 1.00 | 0.71 (0.45, 1.11) | 0.36 (0.22, 0.58) *** | 0.12 (0.07, 0.22) *** | <0.001 |
| ≥75 | 1.00 | 0.63 (0.38, 1.06) | 0.31 (0.17, 0.55) *** | 0.17 (0.08, 0.37) *** | <0.001 |
| Sex | |||||
| Female | 1.00 | 0.57 (0.32, 1.01) | 0.44 (0.25, 0.77) ** | 0.11 (0.05, 0.27) *** | <0.001 |
| Male | 1.00 | 0.84 (0.58, 1.21) | 0.40 (0.26, 0.60) *** | 0.19 (0.11, 0.30) *** | <0.001 |
| CCI Index + | |||||
| 0 | 1.00 | 0.66 (0.23, 1.94) | 0.80 (0.34, 1.84) | 0.07 (0.01, 0.49) ** | 0.004 |
| 1 | 1.00 | 0.75 (0.31, 1.80) | 0.26 (0.08, 0.90) * | 0.31 (0.12, 0.79) * | 0.005 |
| 2 | 1.00 | 1.23 (0.70, 2.17) | 0.57 (0.30, 1.09) | 0.30 (0.16, 0.59) *** | <0.001 |
| ≥3 | 1.00 | 0.55 (0.35, 0.84)** | 0.30 (0.18, 0.48) *** | 0.08 (0.03, 0.17) *** | <0.001 |
| Diabetes | |||||
| No | 1.00 | 0.67 (0.44, 1.02) | 0.38 (0.24, 0.59) *** | 0.19 (0.12, 0.31) *** | <0.001 |
| Yes | 1.00 | 0.80 (0.51, 1.25) | 0.44 (0.27, 0.73) ** | 0.10 (0.04, 0.25) *** | <0.001 |
| Dyslipidemia | |||||
| No | 1.00 | 0.72 (0.48, 1.09) | 0.47 (0.31, 0.72) *** | 0.22 (0.14, 0.36) *** | <0.001 |
| Yes | 1.00 | 0.74 (0.47, 1.16) | 0.31 (0.18, 0.54) *** | 0.07 (0.03, 0.18) *** | <0.001 |
| Hypertension | |||||
| No | 1.00 | 0.61 (0.32, 1.14) | 0.49 (0.27, 0.89) * | 0.22 (0.11, 0.43) *** | <0.001 |
| Yes | 1.00 | 0.78 (0.55, 1.10) | 0.38 (0.25, 0.56) *** | 0.14 (0.08, 0.23) *** | <0.001 |
| Statin | |||||
| <28 days | 1.00 | 0.67 (0.46, 0.98) * | 0.41 (0.27, 0.62) *** | 0.17 (0.10, 0.29) *** | <0.001 |
| 28–365 days | 1.00 | 0.95 (0.51, 1.76) | 0.28 (0.12, 0.64) ** | 0.20 (0.08, 0.46) *** | <0.001 |
| >365 days | 1.00 | 0.89 (0.34, 2.32) | 0.84 (0.36, 1.98) | 0.15 (0.04, 0.54) ** | 0.004 |
| Metformin | |||||
| <28 days | 1.00 | 0.73 (0.52, 1.02) | 0.40 (0.28, 0.58) *** | 0.15 (0.09, 0.24) *** | <0.001 |
| 28–365 days | 1.00 | 1.30 (0.44, 3.84) | 0.71 (0.21, 2.47) | 0.34 (0.07, 1.72) | 0.168 |
| >365 days | 1.00 | 0.61 (0.22, 1.64) | 0.37 (0.15, 0.96) * | 0.21 (0.08, 0.56) ** | 0.001 |
| RAA | |||||
| <28 days | 1.00 | 0.39 (0.20, 0.76) ** | 0.37 (0.20, 0.68) ** | 0.22 (0.11, 0.44) *** | <0.001 |
| 28–365 days | 1.00 | 0.91 (0.53, 1.58) | 0.34 (0.17, 0.68) ** | 0.14 (0.06, 0.36) *** | <0.001 |
| >365 days | 1.00 | 1.09 (0.67, 1.75) | 0.59 (0.35, 0.98) * | 0.19 (0.10, 0.35) *** | <0.001 |
| Aspirin | |||||
| <28 days | 1.00 | 0.61 (0.39, 0.94) * | 0.31 (0.18, 0.53) *** | 0.15 (0.08, 0.29) *** | <0.001 |
| 28–365 days | 1.00 | 0.95 (0.55, 1.67) | 0.60 (0.34, 1.06) | 0.12 (0.05, 0.31) *** | <0.001 |
| >365 days | 1.00 | 1.29 (0.61, 2.70) | 0.69 (0.32, 1.48) | 0.39 (0.19, 0.83) * | <0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-C.; Hao, W.-R.; Hong, H.-J.; Lin, K.-J.; Chiu, C.-C.; Yang, T.-Y.; Fang, Y.-A.; Jian, W.; Chen, M.-Y.; Hsu, M.-H.; et al. Protective Effects of Influenza Vaccine against Colorectal Cancer in Populations with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Cancers 2023, 15, 2398. https://doi.org/10.3390/cancers15082398
Chen C-C, Hao W-R, Hong H-J, Lin K-J, Chiu C-C, Yang T-Y, Fang Y-A, Jian W, Chen M-Y, Hsu M-H, et al. Protective Effects of Influenza Vaccine against Colorectal Cancer in Populations with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Cancers. 2023; 15(8):2398. https://doi.org/10.3390/cancers15082398
Chicago/Turabian StyleChen, Chun-Chao, Wen-Rui Hao, Hong-Jye Hong, Kuan-Jie Lin, Chun-Chih Chiu, Tsung-Yeh Yang, Yu-Ann Fang, William Jian, Ming-Yao Chen, Min-Huei Hsu, and et al. 2023. "Protective Effects of Influenza Vaccine against Colorectal Cancer in Populations with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study" Cancers 15, no. 8: 2398. https://doi.org/10.3390/cancers15082398
APA StyleChen, C.-C., Hao, W.-R., Hong, H.-J., Lin, K.-J., Chiu, C.-C., Yang, T.-Y., Fang, Y.-A., Jian, W., Chen, M.-Y., Hsu, M.-H., Lu, S.-C., Lai, Y.-H., Yang, T.-L., & Liu, J.-C. (2023). Protective Effects of Influenza Vaccine against Colorectal Cancer in Populations with Chronic Kidney Disease: A Nationwide Population-Based Cohort Study. Cancers, 15(8), 2398. https://doi.org/10.3390/cancers15082398









