TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach
Abstract
Simple Summary
Abstract
1. Introduction
2. Lung Cancer: Molecular Mechanisms
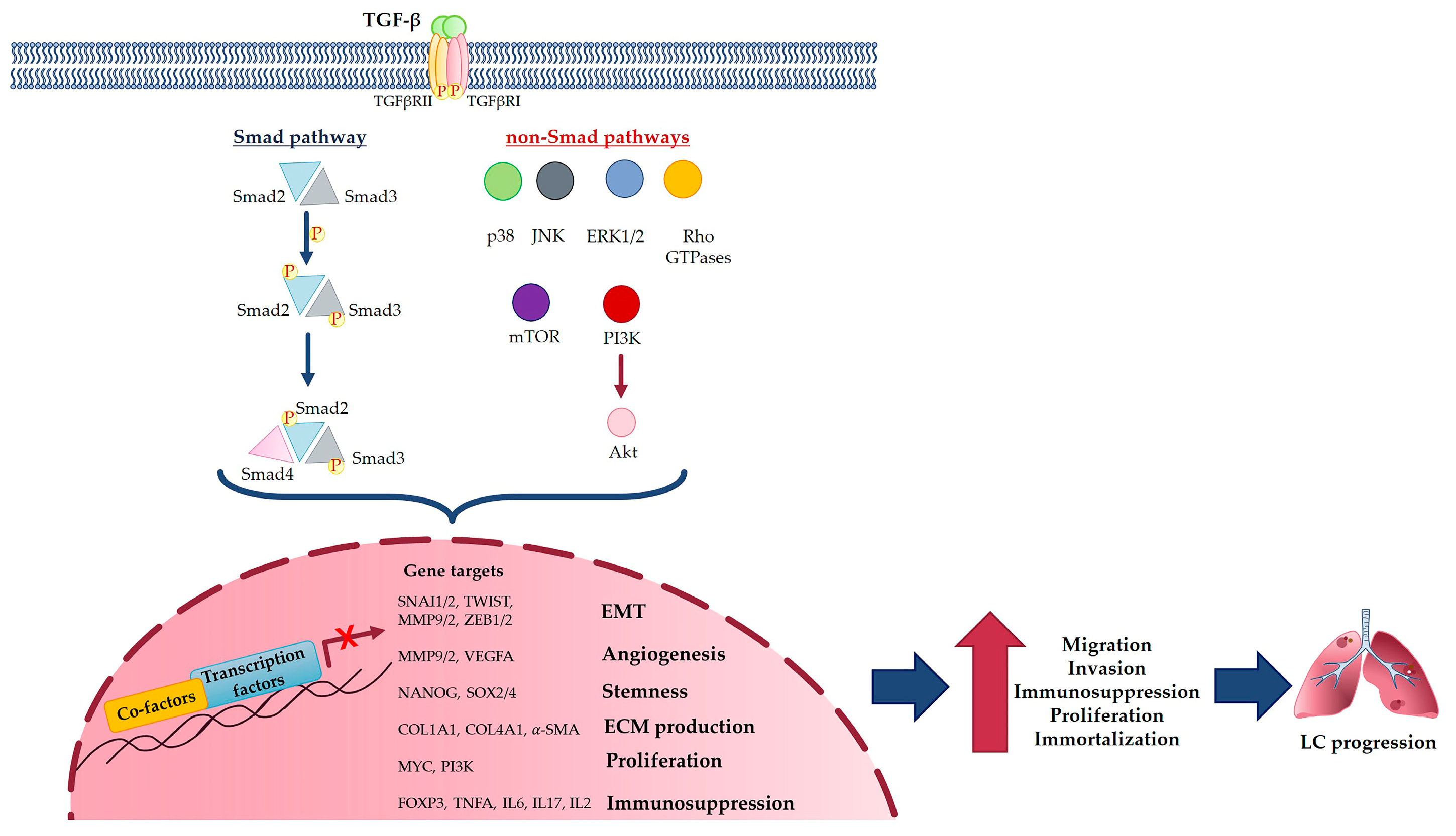
3. TGF-β Signaling Pathway in Cancer
4. TGF-β Molecular Mechanism in Lung Cancer
TGF-β and EMT in Lung Cancer
5. TGF-β in Lung Cancer Development and Metastasis
5.1. TGF-β in Lung Cancer Development
5.2. TGF-β in Lung Cancer Metastasis
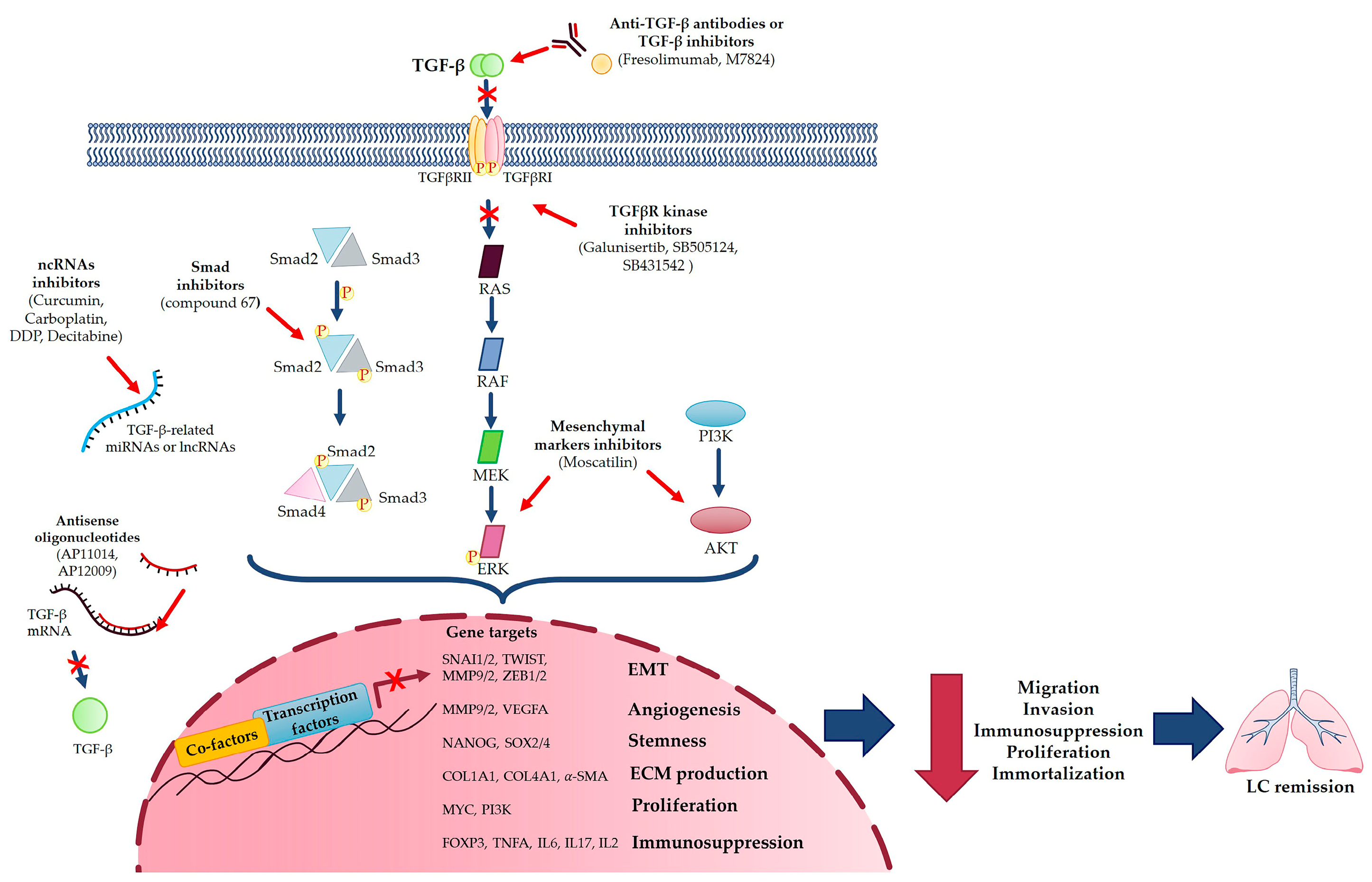
6. Targeting TGF-β in Lung Cancer Approach
6.1. TGF-β as Predictive Marker in Lung Cancer
6.2. TGF-β as Pharmacological Target in Lung Cancer
- -
- TGF-β inhibitors, such as Fresolimumab, a TGF-β-specific human monoclonal antibody [14];
- -
- -
- TGF-β-induced EMT inhibitor (compound 67) that specifically inhibits migration and invasion, blocking Smad2 phosphorylation or some EMT markers, such as MMP-2 and MMP-9 [30];
- -
- Targeting Smad6, which could reduce tumor growth via a protein which promotes LC survival [27];
- -
- Using a bifunctional TGF-β ligand trap such as M7824, a fusion protein that simultaneously targets both PD-L1 and TGF-β [72];
- -
- -
- Targeting miRNAs and lncRNAs involved in TGF-β-mediated LC progression with different compounds, such as curcumin, carboplatin, DDP or Decitabine [3], and, moreover, exploiting the inhibitory effects of miRNA-454-3p against TGFB2 and miR-454-3p on NSCLC, which reversed TGF-β overexpression, thus providing a promising strategy for treating NSCLC [73];
- -
- Blocking TGF-β-induced EMT with different molecules, such as Bufalin or CX-4945, direct inhibitors of TGF-β-induced EMT, or Moscatilin, an inhibitor of mesenchymal markers, via suppressing ERK and Akt signaling pathways [2].
6.3. Targeting TGF-β in Lung Cancer Approach
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of Lung Cancer. Contemp. Oncol. Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef]
- Otsuki, Y.; Saya, H.; Arima, Y. Prospects for New Lung Cancer Treatments That Target EMT Signaling. Dev. Dyn. 2018, 247, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.-N.; Li, J.; Tang, L.-B.; Chen, W.-T.; Zhang, L.; Xiong, L.-X. MiRNAs and LncRNAs: Dual Roles in TGF-β Signaling-Regulated Metastasis in Lung Cancer. Int. J. Mol. Sci. 2020, 21, 1193. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; He, J. Epithelial Mesenchymal Transition and Lung Cancer. J. Thorac. Dis. 2010, 2, 154. [Google Scholar] [PubMed]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.-T. The Role of Tumor Stroma in Cancer Progression and Prognosis: Emphasis on Carcinoma-Associated Fibroblasts and Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Santibañez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β/TGF-β Receptor System and Its Role in Physiological and Pathological Conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef]
- Vázquez, P.F.; Carlini, M.J.; Daroqui, M.C.; Colombo, L.; Dalurzo, M.L.; Smith, D.E.; Grasselli, J.; Pallotta, M.G.; Ehrlich, M.; Bal de Kier Joffé, E.D.; et al. TGF-Beta Specifically Enhances the Metastatic Attributes of Murine Lung Adenocarcinoma: Implications for Human Non-Small Cell Lung Cancer. Clin. Exp. Metastasis 2013, 30, 993–1007. [Google Scholar] [CrossRef]
- Ramundo, V.; Zanirato, G.; Aldieri, E. The Epithelial-to-Mesenchymal Transition (EMT) in the Development and Metastasis of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 12216. [Google Scholar] [CrossRef]
- Lee, H.-W.; Jose, C.C.; Cuddapah, S. Epithelial-Mesenchymal Transition: Insights into Nickel-Induced Lung Diseases. Semin. Cancer Biol. 2021, 76, 99–109. [Google Scholar] [CrossRef]
- Ma, M.; Shi, F.; Zhai, R.; Wang, H.; Li, K.; Xu, C.; Yao, W.; Zhou, F. TGF-β Promote Epithelial-Mesenchymal Transition via NF-ΚB/NOX4/ROS Signal Pathway in Lung Cancer Cells. Mol. Biol. Rep. 2021, 48, 2365–2375. [Google Scholar] [CrossRef]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Horie, M.; Micke, P.; Nagase, T. The Role of TGF-β Signaling in Lung Cancer Associated with Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2018, 19, 3611. [Google Scholar] [CrossRef]
- Eser, P.Ö.; Jänne, P.A. TGFβ Pathway Inhibition in the Treatment of Non-Small Cell Lung Cancer. Pharmacol. Ther. 2018, 184, 112–130. [Google Scholar] [CrossRef] [PubMed]
- Saji, H.; Nakamura, H.; Awut, I.; Kawasaki, N.; Hagiwara, M.; Ogata, A.; Hosaka, M.; Saijo, T.; Kato, Y.; Kato, H. Significance of Expression of TGF--β in Pulmonary Metastasis in Non-Small Cell Lung Cancer Tissues. Ann. Thorac. Cardiovasc. Surg. 2003, 9, 295–300. [Google Scholar] [PubMed]
- Zhou, Y.; Hill, C.; Yao, L.; Li, J.; Hancock, D.; Downward, J.; Jones, M.G.; Davies, D.E.; Ewing, R.M.; Skipp, P.; et al. Quantitative Proteomic Analysis in Alveolar Type II Cells Reveals the Different Capacities of RAS and TGF-β to Induce Epithelial-Mesenchymal Transition. Front. Mol. Biosci. 2021, 8, 595712. [Google Scholar] [CrossRef]
- Zhang, J.; Qi, J.; Wei, H.; Lei, Y.; Yu, H.; Liu, N.; Zhao, L.; Wang, P. TGFβ1 in Cancer-Associated Fibroblasts Is Associated with Progression and Radiosensitivity in Small-Cell Lung Cancer. Front. Cell Dev. Biol. 2021, 9, 667645. [Google Scholar] [CrossRef]
- Miyazono, K.; Katsuno, Y.; Koinuma, D.; Ehata, S.; Morikawa, M. Intracellular and Extracellular TGF-β Signaling in Cancer: Some Recent Topics. Front. Med. 2018, 12, 387–411. [Google Scholar] [CrossRef]
- Kang, Y.; Prentice, M.A.; Mariano, J.M.; Davarya, S.; Linnoila, R.I.; Moody, T.W.; Wakefield, L.M.; Jakowlew, S.B. Transforming Growth Factor-Beta 1 and Its Receptors in Human Lung Cancer and Mouse Lung Carcinogenesis. Exp. Lung Res. 2000, 26, 685–707. [Google Scholar] [CrossRef]
- Neel, J.-C.; Humbert, L.; Lebrun, J.-J. The Dual Role of TGFβ in Human Cancer: From Tumor Suppression to Cancer Metastasis. ISRN Mol. Biol. 2012, 2012, 1–28. [Google Scholar] [CrossRef]
- Zhang, Y.E. Non-Smad Pathways in TGF-β Signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Sundar, R.; Thakur, N.; Ekman, M.; Gudey, S.K.; Yakymovych, M.; Hermansson, A.; Dimitriou, H.; Bengoechea-Alonso, M.T.; Ericsson, J.; et al. TRAF6 Ubiquitinates TGFβ Type I Receptor to Promote Its Cleavage and Nuclear Translocation in Cancer. Nat. Commun. 2011, 2, 330. [Google Scholar] [CrossRef] [PubMed]
- Gudey, S.K.; Sundar, R.; Mu, Y.; Wallenius, A.; Zang, G.; Bergh, A.; Heldin, C.-H.; Landström, M. TRAF6 Stimulates the Tumor-Promoting Effects of TGFβ Type I Receptor through Polyubiquitination and Activation of Presenilin 1. Sci. Signal. 2014, 7, ra2. [Google Scholar] [CrossRef]
- Mu, Y.; Gudey, S.K.; Landström, M. Non-Smad Signaling Pathways. Cell Tissue Res. 2012, 347, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Wang, Z.-G.; Akhtari, M.; Zhao, W.; Seth, P. Targeting TGF Beta Signaling for Cancer Therapy. Cancer Biol. Ther. 2005, 4, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-G.; Chae, M.H.; Park, J.M.; Kim, E.J.; Park, J.H.; Kam, S.; Cha, S.I.; Kim, C.H.; Park, R.-W.; Park, S.H.; et al. Polymorphisms in TGF-Beta1 Gene and the Risk of Lung Cancer. Lung Cancer Amst. Neth. 2006, 52, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-S.; Jen, J. TGF-Beta Signaling and the Role of Inhibitory Smads in Non-Small Cell Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2010, 5, 417–419. [Google Scholar] [CrossRef]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20, 150. [Google Scholar] [CrossRef]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.-J.; Lee, S.H.; Park, M.S.; Yim, H.W.; et al. TGF-β Induced EMT and Stemness Characteristics Are Associated with Epigenetic Regulation in Lung Cancer. Sci. Rep. 2020, 10, 10597. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Jang, H.J.; Kwak, S.; Sung, G.-J.; Park, S.-H.; Song, J.-H.; Kim, H.; Na, Y.; Choi, K.-C. Novel TGF-Β1 Inhibitor Antagonizes TGF-Β1-Induced Epithelial-Mesenchymal Transition in Human A549 Lung Cancer Cells. J. Cell. Biochem. 2019, 120, 977–987. [Google Scholar] [CrossRef]
- Serrano-Gomez, S.J.; Maziveyi, M.; Alahari, S.K. Regulation of Epithelial-Mesenchymal Transition through Epigenetic and Post-Translational Modifications. Mol. Cancer 2016, 15, 18. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. The SUMO Guards for SNAIL. Oncotarget 2017, 8, 97701–97702. [Google Scholar] [CrossRef]
- Yao, L.; Conforti, F.; Hill, C.; Bell, J.; Drawater, L.; Li, J.; Liu, D.; Xiong, H.; Alzetani, A.; Chee, S.J.; et al. Paracrine Signalling during ZEB1-Mediated Epithelial–Mesenchymal Transition Augments Local Myofibroblast Differentiation in Lung Fibrosis. Cell Death Differ. 2019, 26, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhong, C.; Duan, S. The Tumorigenic Function of LINC00858 in Cancer. Biomed. Pharmacother. 2021, 143, 112235. [Google Scholar] [CrossRef]
- Chen, G.; Ye, B. The Key MicroRNAs Regulated the Development of Non-Small Cell Lung Cancer by Targeting TGF-β-Induced Epithelial-Mesenchymal Transition. Comb. Chem. High Throughput Screen. 2019, 22, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Feng, Y.; Zhao, J.; Lei, J.; Qiao, T.; Zhou, Y.; Lu, Q.; Jiang, T.; Jia, L.; Han, Y. TFAP2A Potentiates Lung Adenocarcinoma Metastasis by a Novel MiR-16 Family/TFAP2A/PSG9/TGF-β Signaling Pathway. Cell Death Dis. 2021, 12, 352. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, J.; Wang, S.; Sun, Z.; Lei, Z.; Zhang, H.-T.; Huang, J. RGS6 Suppresses TGF-β-Induced Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancers via a Novel Mechanism Dependent on Its Interaction with SMAD4. Cell Death Dis. 2022, 13, 656. [Google Scholar] [CrossRef]
- Wang, S.; Tong, X.; Li, C.; Jin, E.; Su, Z.; Sun, Z.; Zhang, W.; Lei, Z.; Zhang, H.-T. Quaking 5 Suppresses TGF-β-Induced EMT and Cell Invasion in Lung Adenocarcinoma. EMBO Rep. 2021, 22, e52079. [Google Scholar] [CrossRef]
- Xue, Q.; Jiang, H.; Wang, J.; Wei, D. LASP1 Induces Epithelial-Mesenchymal Transition in Lung Cancer through the TGF-Β1/Smad/Snail Pathway. Can. Respir. J. 2021, 2021, 5277409. [Google Scholar] [CrossRef]
- Sundar, R.; Gudey, S.K.; Heldin, C.-H.; Landström, M. TRAF6 Promotes TGFβ-Induced Invasion and Cell-Cycle Regulation via Lys63-Linked Polyubiquitination of Lys178 in TGFβ Type I Receptor. Cell Cycle 2015, 14, 554–565. [Google Scholar] [CrossRef]
- Chae, Y.K.; Chang, S.; Ko, T.; Anker, J.; Agte, S.; Iams, W.; Choi, W.M.; Lee, K.; Cruz, M. Epithelial-Mesenchymal Transition (EMT) Signature Is Inversely Associated with T-Cell Infiltration in Non-Small Cell Lung Cancer (NSCLC). Sci. Rep. 2018, 8, 2918. [Google Scholar] [CrossRef]
- Kim, H.; Choi, P.; Kim, T.; Kim, Y.; Song, B.G.; Park, Y.-T.; Choi, S.-J.; Yoon, C.H.; Lim, W.-C.; Ko, H.; et al. Ginsenosides Rk1 and Rg5 Inhibit Transforming Growth Factor-Β1-Induced Epithelial-Mesenchymal Transition and Suppress Migration, Invasion, Anoikis Resistance, and Development of Stem-like Features in Lung Cancer. J. Ginseng Res. 2021, 45, 134–148. [Google Scholar] [CrossRef]
- Sun, M.; Zhuang, X.; Lv, G.; Lin, Z.; Huang, X.; Zhao, J.; Lin, H.; Wang, Y. Ginsenoside CK Inhibits TGF-β-Induced Epithelial-Mesenchymal Transition in A549 Cell via SIRT1. BioMed Res. Int. 2021, 2021, 9140191. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, M.-S.; Kim, G.J.; Kwon, H.; Lee, M.J.; Han, A.-R.; Nam, J.-W.; Jung, C.-H.; Kang, K.S.; Choi, H. Inhibition of A549 Lung Cancer Cell Migration and Invasion by Ent-Caprolactin C via the Suppression of Transforming Growth Factor-β-Induced Epithelial-Mesenchymal Transition. Mar. Drugs 2021, 19, 465. [Google Scholar] [CrossRef] [PubMed]
- Nakasuka, F.; Tabata, S.; Sakamoto, T.; Hirayama, A.; Ebi, H.; Yamada, T.; Umetsu, K.; Ohishi, M.; Ueno, A.; Goto, H.; et al. TGF-β-Dependent Reprogramming of Amino Acid Metabolism Induces Epithelial-Mesenchymal Transition in Non-Small Cell Lung Cancers. Commun. Biol. 2021, 4, 782. [Google Scholar] [CrossRef]
- Yang, H.; Wang, L.; Zhao, J.; Chen, Y.; Lei, Z.; Liu, X.; Xia, W.; Guo, L.; Zhang, H.-T. TGF-β-Activated SMAD3/4 Complex Transcriptionally Upregulates N-Cadherin Expression in Non-Small Cell Lung Cancer. Lung Cancer Amst. Neth. 2015, 87, 249–257. [Google Scholar] [CrossRef]
- Wang, L.-N.; Zhang, Z.-T.; Wang, L.; Wei, H.-X.; Zhang, T.; Zhang, L.-M.; Lin, H.; Zhang, H.; Wang, S.-Q. TGF-Β1/SH2B3 Axis Regulates Anoikis Resistance and EMT of Lung Cancer Cells by Modulating JAK2/STAT3 and SHP2/Grb2 Signaling Pathways. Cell Death Dis. 2022, 13, 472. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-Y.; Liao, J.-J.; Xue, W.-R. FMNL1 Down-Regulation Suppresses Bone Metastasis through Reducing TGF-Β1 Expression in Non-Small Cell Lung Cancer (NSCLC). Biomed. Pharmacother. 2019, 117, 109126. [Google Scholar] [CrossRef]
- Hao, Y.; Baker, D.; ten Dijke, P. TGF-β-Mediated Epithelial-Mesenchymal Transition and Cancer Metastasis. Int. J. Mol. Sci. 2019, 20, 2767. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.J.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- El-Baz, L.M.F.; Shoukry, N.M.; Hafez, H.S.; Guzy, R.D.; Salem, M.L. Fibroblast Growth Factor 2 Augments Transforming Growth Factor Beta 1 Induced Epithelial-Mesenchymal Transition in Lung Cell Culture Model. Iran. J. Allergy Asthma Immunol. 2020, 19, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Sohal, S.S.; Mahmood, M.Q.; Walters, E.H. Clinical Significance of Epithelial Mesenchymal Transition (EMT) in Chronic Obstructive Pulmonary Disease (COPD): Potential Target for Prevention of Airway Fibrosis and Lung Cancer. Clin. Transl. Med. 2014, 3, 33. [Google Scholar] [CrossRef]
- Willis, B.C.; Borok, Z. TGF-β-Induced EMT: Mechanisms and Implications for Fibrotic Lung Disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Shen, W.; Liu, Y.; Gao, W.; Zhou, W.; Li, J.; Zhao, S.; Chen, C.; Chen, Y.; Liu, Y.; et al. TGIF2 Promotes the Progression of Lung Adenocarcinoma by Bridging EGFR/RAS/ERK Signaling to Cancer Cell Stemness. Signal Transduct. Target. Ther. 2019, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Shen, S.; Huang, Q.; Fu, J.; Wang, T.; Pan, L.; Zhang, P.; Chen, G.; Huang, T.; Li, K.; et al. Proteasome-Dependent Degradation of Smad7 Is Critical for Lung Cancer Metastasis. Cell Death Differ. 2020, 27, 1795–1806. [Google Scholar] [CrossRef]
- Sun, Z.; Su, Z.; Zhou, Z.; Wang, S.; Wang, Z.; Tong, X.; Li, C.; Wang, Y.; Chen, X.; Lei, Z.; et al. RNA Demethylase ALKBH5 Inhibits TGF-β-Induced EMT by Regulating TGF-β/SMAD Signaling in Non-Small Cell Lung Cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22283. [Google Scholar] [CrossRef]
- Khan, G.J.; Sun, L.; Abbas, M.; Naveed, M.; Jamshaid, T.; Baig, M.M.F.A.; Yuan, S. In-Vitro Pre-Treatment of Cancer Cells with TGF-Β1: A Novel Approach of Tail Vein Lung Cancer Metastasis Mouse Model for Anti-Metastatic Studies. Curr. Mol. Pharmacol. 2019, 12, 249–260. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Chen, C.-H.; Yen, C.-H.; Tung, C.-W.; Chen, C.-J.; Chen, Y.-M.A.; Huang, M.-S. Human Immunodeficiency Virus Tat-TIP30 Interaction Promotes Metastasis by Enhancing the Nuclear Translocation of Snail in Lung Cancer Cell Lines. Cancer Sci. 2018, 109, 3105–3114. [Google Scholar] [CrossRef]
- Plou, J.; Juste-Lanas, Y.; Olivares, V.; Del Amo, C.; Borau, C.; García-Aznar, J.M. From Individual to Collective 3D Cancer Dissemination: Roles of Collagen Concentration and TGF-β. Sci. Rep. 2018, 8, 12723. [Google Scholar] [CrossRef]
- Stappenbeck, F.; Wang, F.; Tang, L.-Y.; Zhang, Y.E.; Parhami, F. Inhibition of Non-Small Cell Lung Cancer Cells by Oxy210, an Oxysterol-Derivative That Antagonizes TGFβ and Hedgehog Signaling. Cells 2019, 8, 1297. [Google Scholar] [CrossRef]
- Singhal, M.; Gengenbacher, N.; Abdul Pari, A.A.; Kamiyama, M.; Hai, L.; Kuhn, B.J.; Kallenberg, D.M.; Kulkarni, S.R.; Camilli, C.; Preuß, S.F.; et al. Temporal Multi-Omics Identifies LRG1 as a Vascular Niche Instructor of Metastasis. Sci. Transl. Med. 2021, 13, eabe6805. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, X.; Zhu, J. LSD1-Mediated Stabilization of SEPT6 Protein Activates the TGF-Β1 Pathway and Regulates Non-Small-Cell Lung Cancer Metastasis. Cancer Gene Ther. 2022, 29, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.; Sin, M.-J.; Kim, M.-J.; Kim, H.-J.; Kim, Y.-S.; Choi, E.-K.; Kim, M.-Y. Involvement of Cellular Prion Protein in Invasion and Metastasis of Lung Cancer by Inducing Treg Cell Development. Biomolecules 2021, 11, 285. [Google Scholar] [CrossRef]
- Miao, Z.-F.; Li, W.-Y.; Wang, Z.-N.; Zhao, T.-T.; Xu, Y.-Y.; Song, Y.-X.; Huang, J.-Y.; Xu, H.-M. Lung Cancer Cells Induce Senescence and Apoptosis of Pleural Mesothelial Cells via Transforming Growth Factor-Beta1. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 2657–2665. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cho, E.A.; Chun, J.N.; Lee, D.Y.; Lee, S.; Kim, M.Y.; Bae, S.M.; Jo, S.I.; Lee, S.H.; Park, H.H.; et al. Crizotinib Attenuates Cancer Metastasis by Inhibiting TGFβ Signaling in Non-Small Cell Lung Cancer Cells. Exp. Mol. Med. 2022, 54, 1225–1235. [Google Scholar] [CrossRef]
- Ko, J.-H.; Nam, D.; Um, J.-Y.; Jung, S.H.; Sethi, G.; Ahn, K.S. Bergamottin Suppresses Metastasis of Lung Cancer Cells through Abrogation of Diverse Oncogenic Signaling Cascades and Epithelial-to-Mesenchymal Transition. Mol. Basel Switz. 2018, 23, 1601. [Google Scholar] [CrossRef]
- de Miguel-Perez, D.; Russo, A.; Gunasekaran, M.; Buemi, F.; Hester, L.; Fan, X.; Carter-Cooper, B.A.; Lapidus, R.G.; Peleg, A.; Arroyo-Hernández, M.; et al. Baseline Extracellular Vesicle TGF-β Is a Predictive Biomarker for Response to Immune Checkpoint Inhibitors and Survival in Non-Small Cell Lung Cancer. Cancer 2023, 129, 521–530. [Google Scholar] [CrossRef]
- Li, J.; Shen, C.; Wang, X.; Lai, Y.; Zhou, K.; Li, P.; Liu, L.; Che, G. Prognostic Value of TGF-β in Lung Cancer: Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 691. [Google Scholar] [CrossRef]
- Seok, Y.; Lee, W.K.; Park, J.Y.; Kim, D.S. TGFBI Promoter Methylation Is Associated with Poor Prognosis in Lung Adenocarcinoma Patients. Mol. Cells 2019, 42, 161–165. [Google Scholar] [CrossRef]
- Gordian, E.; Welsh, E.A.; Gimbrone, N.; Siegel, E.M.; Shibata, D.; Creelan, B.C.; Cress, W.D.; Eschrich, S.A.; Haura, E.B.; Muñoz-Antonia, T. Transforming Growth Factor β-Induced Epithelial-to-Mesenchymal Signature Predicts Metastasis-Free Survival in Non-Small Cell Lung Cancer. Oncotarget 2019, 10, 810–824. [Google Scholar] [CrossRef]
- Mamdani, H.; Matosevic, S.; Khalid, A.B.; Durm, G.; Jalal, S.I. Immunotherapy in Lung Cancer: Current Landscape and Future Directions. Front. Immunol. 2022, 13, 823618. [Google Scholar] [CrossRef] [PubMed]
- David, J.M.; Dominguez, C.; McCampbell, K.K.; Gulley, J.L.; Schlom, J.; Palena, C. A Novel Bifunctional Anti-PD-L1/TGF-β Trap Fusion Protein (M7824) Efficiently Reverts Mesenchymalization of Human Lung Cancer Cells. OncoImmunology 2017, 6, e1349589. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Liang, Y.; Kang, L.; Xiao, Y.; Yu, T.; Wan, R. MiR-454-3p Inhibits Non-small Cell Lung Cancer Cell Proliferation and Metastasis by Targeting TGFB2. Oncol. Rep. 2021, 45, 67. [Google Scholar] [CrossRef] [PubMed]
- Marques da Fonseca, L.; Jacques da Silva, L.R.; Santos dos Reis, J.; Rodrigues da Costa Santos, M.A.; de Sousa Chaves, V.; Monteiro da Costa, K.; Sa-Diniz, J. de N.; Freire de Lima, C.G.; Morrot, A.; Nunes Franklim, T.; et al. Piperine Inhibits TGF-β Signaling Pathways and Disrupts EMT-Related Events in Human Lung Adenocarcinoma Cells. Medicines 2020, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-N.; Yang, S.-F.; Yu, C.-C.; Lin, C.-Y.; Huang, S.-H.; Chu, S.-C.; Hsieh, Y.-S. Duchesnea Indica Extract Suppresses the Migration of Human Lung Adenocarcinoma Cells by Inhibiting Epithelial–Mesenchymal Transition. Environ. Toxicol. 2017, 32, 2053–2063. [Google Scholar] [CrossRef]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.-K.; Kim, B.-C. Kaempferol Suppresses Transforming Growth Factor-Β1–Induced Epithelial-to-Mesenchymal Transition and Migration of A549 Lung Cancer Cells by Inhibiting Akt1-Mediated Phosphorylation of Smad3 at Threonine-179. Neoplasia 2015, 17, 525–537. [Google Scholar] [CrossRef]
- Pattarayan, D.; Sivanantham, A.; Krishnaswami, V.; Loganathan, L.; Palanichamy, R.; Natesan, S.; Muthusamy, K.; Rajasekaran, S. Tannic Acid Attenuates TGF-Β1-Induced Epithelial-to-Mesenchymal Transition by Effectively Intervening TGF-β Signaling in Lung Epithelial Cells. J. Cell. Physiol. 2018, 233, 2513–2525. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Tang, L.; Chen, H.; Wu, C.; Zhao, M.; Yang, Y.; Chen, X.; Liu, G. Resveratrol Inhibits TGF-Β1-Induced Epithelial-to-Mesenchymal Transition and Suppresses Lung Cancer Invasion and Metastasis. Toxicology 2013, 303, 139–146. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, Z.; Lee, S.-H. Arctigenin Represses TGF-β-Induced Epithelial Mesenchymal Transition in Human Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2017, 493, 934–939. [Google Scholar] [CrossRef]
- Richter, A.M.; Küster, M.M.; Woods, M.L.; Walesch, S.K.; Gökyildirim, M.Y.; Krueger, M.; Dammann, R.H. RASSF10 Is a TGFβ-Target That Regulates ASPP2 and E-Cadherin Expression and Acts as Tumor Suppressor That Is Epigenetically Downregulated in Advanced Cancer. Cancers 2019, 11, 1976. [Google Scholar] [CrossRef]
- Jacquet, M.; Hervouet, E.; Baudu, T.; Herfs, M.; Parratte, C.; Feugeas, J.-P.; Perez, V.; Reynders, C.; Ancion, M.; Vigneron, M.; et al. GABARAPL1 Inhibits EMT Signaling through SMAD-Tageted Negative Feedback. Biology 2021, 10, 956. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramundo, V.; Palazzo, M.L.; Aldieri, E. TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach. Cancers 2023, 15, 2295. https://doi.org/10.3390/cancers15082295
Ramundo V, Palazzo ML, Aldieri E. TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach. Cancers. 2023; 15(8):2295. https://doi.org/10.3390/cancers15082295
Chicago/Turabian StyleRamundo, Valeria, Maria Luisa Palazzo, and Elisabetta Aldieri. 2023. "TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach" Cancers 15, no. 8: 2295. https://doi.org/10.3390/cancers15082295
APA StyleRamundo, V., Palazzo, M. L., & Aldieri, E. (2023). TGF-β as Predictive Marker and Pharmacological Target in Lung Cancer Approach. Cancers, 15(8), 2295. https://doi.org/10.3390/cancers15082295







