Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Study Populations, and Data
2.2. Study Period Definition
2.3. Tumor Samples and Laboratorial Analysis
2.4. Outcome Variables and Baseline Characteristic
2.5. Staging
2.6. Treatment
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Lymphoma Treatment and Causes of Death
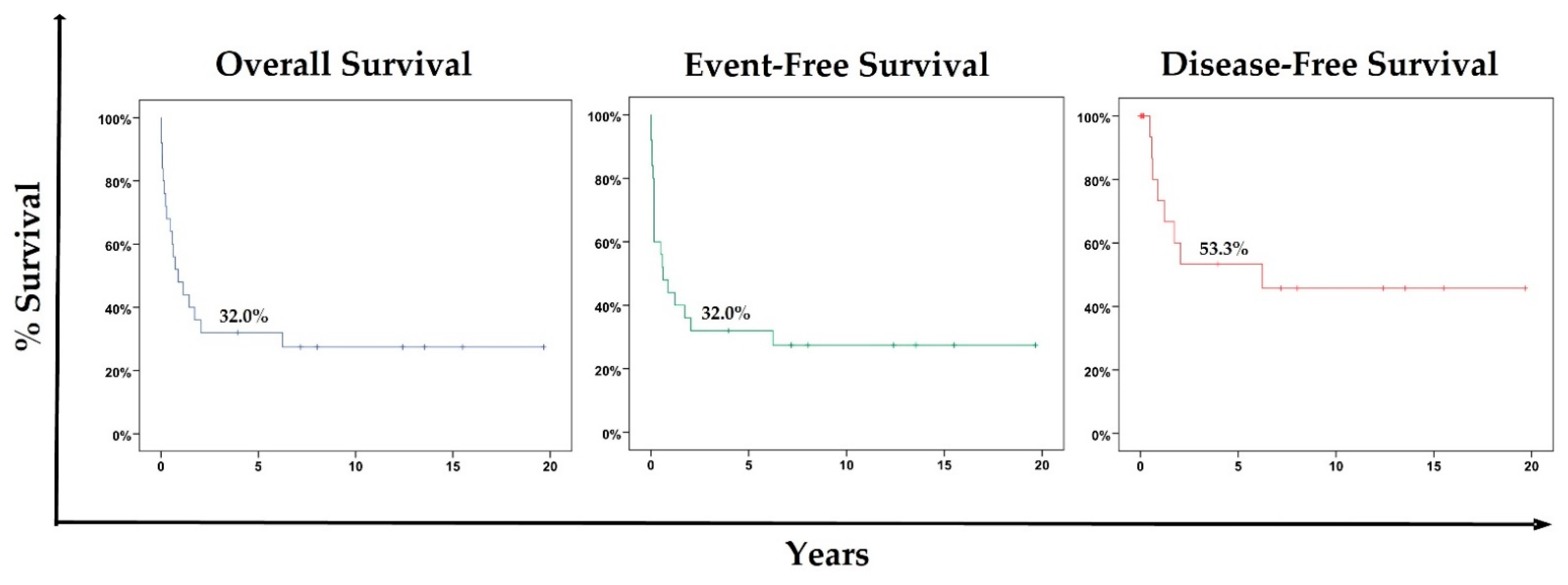
3.3. Overall Survival, Event-Free Survival, and Disease-Free Survival for the Cohort
3.4. Prognostic Features
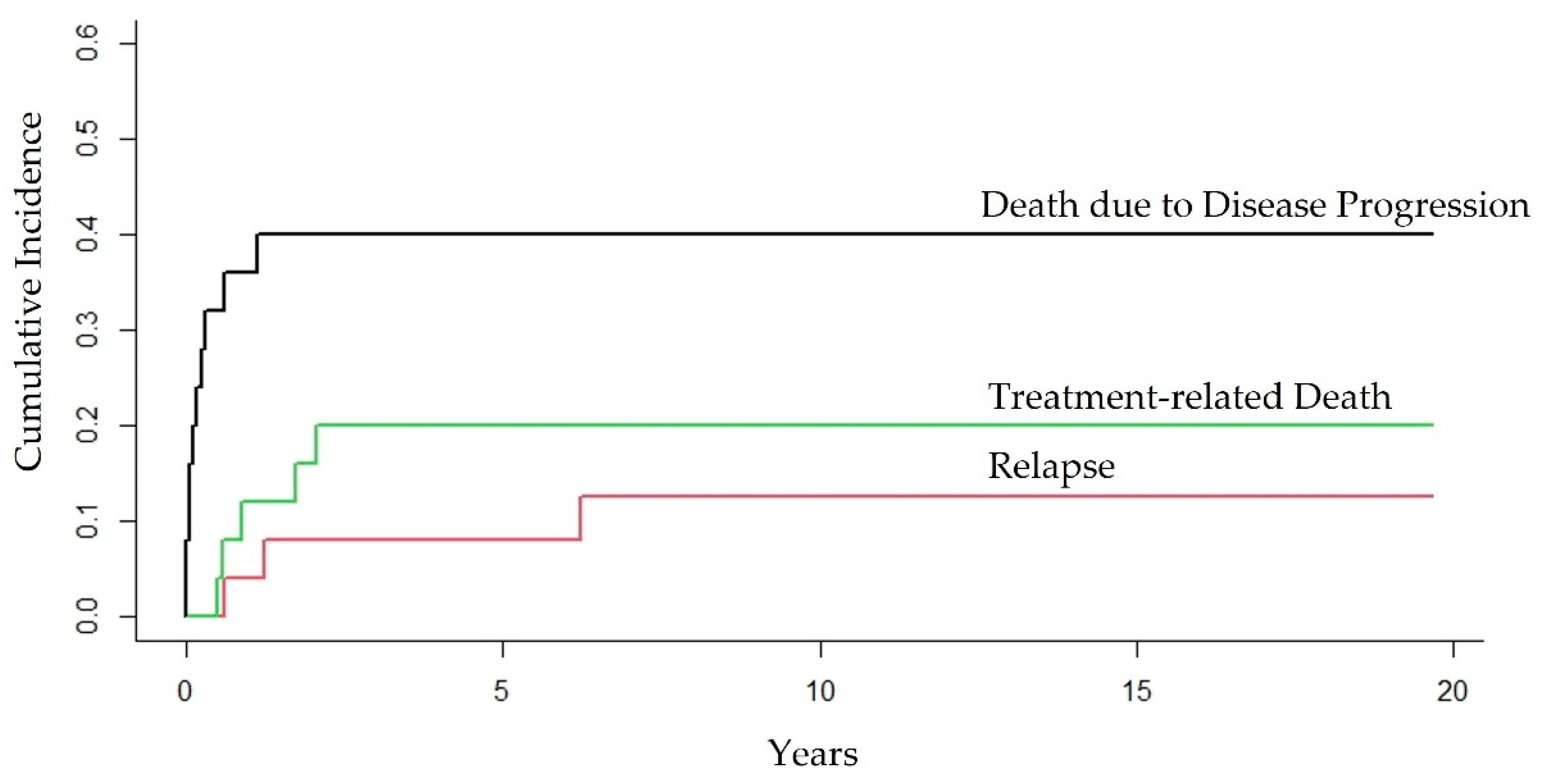
3.5. Competing Risk of Death due to Different Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pollock, B.H.; Jenson, H.; Leach, C.T.; McClain, K.; Hutchison, R.E.; Garzarella, L.; Joshi, V.V.; Parmley, R.T.; Murphy, S.B. Risk factors for pediatric human immunodeficiency virus–related malignancy. JAMA 2003, 289, 2393–2399. [Google Scholar] [CrossRef] [PubMed]
- Chiappini, E.; Berti, E.; Gianesin, K.; Petrara, M.R.; Galli, L.; Giaquinto, C.; de Martino, M.; De Rossi, A. Pediatric human immunodeficiency virus infection and cancer in the highly active antiretroviral treatment (HAART) era. Cancer Lett. 2014, 347, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune activation, inflammation, and non-AIDS co-morbidities in HIV-infected patients under long-term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Shindiapina, P.; Ahmed, E.H.; Mozhenkova, A.; Abebe, T.; Baiocchi, R.A. Immunology of EBV-related lymphoproliferative disease in HIV-positive individuals. Front. Oncol. 2020, 10, 1723. [Google Scholar] [CrossRef]
- Verdu-Bou, M.; Tapia, G.; Hernandez-Rodriguez, A.; Navarro, J.-T. Clinical and Therapeutic Implications of Epstein–Barr Virus in HIV-Related Lymphomas. Cancers 2021, 13, 5534. [Google Scholar] [CrossRef]
- Hatano, Y.; Ideta, T.; Hirata, A.; Hatano, K.; Tomita, H.; Okada, H.; Shimizu, M.; Tanaka, T.; Hara, A. Virus-driven carcinogenesis. Cancers 2021, 13, 2625. [Google Scholar] [CrossRef]
- Gougeon, M.; Montagnier, L. Programmed Cell Death as a Mechanism of CD4 and CD8 T Cell Deletion in AIDS: Molecular Control and Effect of Highly Active Antiretroviral Therapy. Ann N. Y. Acad. Sci. 1999, 887, 199–212. [Google Scholar] [CrossRef]
- Chanock, S.J.; Pizzo, P.A. Infection prevention strategies for children with cancer and AIDS: Contrasting dilemmas. J. Hosp. Infect. 1995, 30, 197–208. [Google Scholar] [CrossRef]
- Geel, J.A.; Eyal, K.C.; Hendricks, M.G.; Myezo, K.H.; Stones, D.K.; Omar, F.; Goga, Y.; van Zyl, A.; van Emmenes, B.; Vaithilingum, M.; et al. Prognostic factors affecting survival in children and adolescents with HIV and Hodgkin lymphoma in South Africa. Leuk. Lymphoma 2021, 62, 2854–2863. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. Morb. Mortal. Wkly. Rep. 1994, 43, 1–10. [Google Scholar]
- Simard, E.P.; Shiels, M.S.; Bhatia, K.; Engels, E.A. Long-term Cancer Risk among People Diagnosed with AIDS during Childhood Cancers among People Diagnosed with AIDS during Childhood. Cancer Epidemiol. Biomark. Prev. 2012, 21, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Carbone, A.; Vaccher, E.; Gloghini, A. Hematologic cancers in individuals infected by HIV. Blood 2022, 139, 995–1012. [Google Scholar] [CrossRef]
- Chiappini, E.; Galli, L.; Tovo, P.-A.; Gabiano, C.; Lisi, C.; Giaquinto, C.; Rampon, O.; Gattinara, G.C.; De Marco, G.; Osimani, P.; et al. Cancer rates after year 2000 significantly decrease in children with perinatal HIV infection: A study by the Italian Register for HIV Infection in Children. J. Clin. Oncol. 2007, 25, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Orem, J.; Mulumba, Y.; Algeri, S.; Bellocco, R.; Mangen, F.W.; Mbidde, E.K.; Weiderpass, E. Clinical characteristics, treatment and outcome of childhood Burkitt’s lymphoma at the Uganda Cancer Institute. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Stefan, D.C.; Stones, D.; Newton, R. Burkitt lymphoma in South African children: One or two entities? Transfus. Apher. Sci. 2011, 44, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Abrahão, R.; Li, Q.W.; Malogolowkin, M.H.; Alvarez, E.M.; Ribeiro, R.C.; Wun, T.; Keegan, T.H. Chronic medical conditions and late effects following non-Hodgkin lymphoma in HIV-uninfected and HIV-infected adolescents and young adults: A population-based study. Br. J. Haematol. 2020, 190, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Naidu, G.; Davies, M.A.; Bohlius, J. HIV-associated malignancies in children. Curr. Opin. HIV AIDS 2017, 12, 77. [Google Scholar] [CrossRef]
- Katumba, R.G.; Sensoy Bahar, O.; Johnson, K.J.; Ssewamala, F.M. Cancer in Youth Living With HIV (YLWHIV): A Narrative Review of the Access to Oncological Services Among YLWHIV and the Role of Economic Strengthening in Child Health. Front. Public Health 2020, 8, 409. [Google Scholar] [CrossRef]
- Westmoreland, K.D.; Stanley, C.C.; Montgomery, N.D.; Kaimila, B.; Kasonkanji, E.; El-Mallawany, N.K.; Wasswa, P.; Mtete, I.; Butia, M.; Itimu, S.; et al. Hodgkin lymphoma, HIV, and Epstein–Barr virus in Malawi: Longitudinal results from the Kamuzu Central Ho spital lymphoma study. Pediatr. Blood Cancer 2017, 64, e26302. [Google Scholar] [CrossRef]
- Chiappini, E.; Larotonda, F.; Lisi, C.; Giacomet, V.; Erba, P.; Bernardi, S.; Zangari, P.; Di Biagio, A.; Taramasso, L.; Giaquinto, C.; et al. Real-world analysis of survival and clinical events in a cohort of Italian perinatally HIV-1 infected children from 2001 to 2018. Front. Pediatr. 2021, 9, 665764. [Google Scholar] [CrossRef]
- Irira, M.; Ngocho, J.S.; Youze, J.; Shayo, I.; Komba, V.; Minja, L.; Karia, F.P.; Bartlett, J.; Mmbaga, B.T. Prevalence and outcome of HIV-associated malignancies among HIV-infected children enrolled into care at Kilimanjaro Christian Medical Center 2006-2014: A hospital-based retrospective analytical study. J. Pediatr. Hematol. Oncol. 2020, 42, 69. [Google Scholar] [CrossRef] [PubMed]
- Rabkin, C. AIDS and cancer in the era of highly active antiretroviral therapy (HAART). Eur. J. Cancer 2001, 37, 1316–1319. [Google Scholar] [CrossRef]
- Duarte, N.L.; Bueno, A.P.S.; Sanches, B.S.; Ramos, G.A.; dos Santos, J.M.B.; Silva, H.F.H.E.; Pondé, J.D.O.; de Sá, J.G.; Rossi, P.M.; Horn, P.R.C.B.; et al. Incidence and Clinical Description of Lymphomas in Children and Adolescents with Vertical Transmission of HIV in Rio de Janeiro, Brazil, in Pre-and Post-Combined Antiretroviral Therapy Eras: A Multicentric Hospital-Based Survival Analysis Study. Cancers 2022, 14, 6129. [Google Scholar] [CrossRef] [PubMed]
- de Martino, M.; Tovo, P.A.; Balducci, M.; Galli, L.; Gabiano, C.; Rezza, G.; Pezzotti, P.; Italian Register for HIV Infection in Children. Reduction in mortality with availability of antiretroviral therapy for children with perinatal HIV-1 infection. JAMA 2000, 284, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Álvaro-Meca, A.; Micheloud, D.; Jensen, J.; Díaz, A.; García-Alvarez, M.; Resino, S. Epidemiologic trends of cancer diagnoses among HIV-infected children in Spain from 1997 to 2008. Pediatr. Infect. Dis. J. 2011, 30, 764–768. [Google Scholar] [CrossRef]
- Carrasco, I.; Tarancon-Diez, L.; Vázquez-Alejo, E.; de Ory, S.J.; Sainz, T.; Apilanez, M.; Epalza, C.; Guillén, S.; Ramos, J.T.; Díez, C.; et al. Innate and adaptive abnormalities in youth with vertically acquired HIV through a multicentre cohort in Spain. J. Int. AIDS Soc. 2021, 24, e25804. [Google Scholar] [CrossRef]
- Chhabra, S.; Fidler, S.; Ayers, S.; Bower, M.; Lyall, H.; Foster, C. Malignancy and all-cause mortality; incidence in adolescents and young adults living with perinatally acquired HIV. J. Virus Erad. 2020, 6, 30–33. [Google Scholar] [CrossRef]
- Szwarcwald, C.L.; De Castilho, E.A. The HIV/AIDS epidemic in Brazil: Three decades. Cad. De Saúde Pública 2011, 27, s4–s5. [Google Scholar] [CrossRef]
- Grinsztejn, B.; Luz, P.M.; Pacheco, A.G.; Santos, D.V.G.; Velasque, L.; Moreira, R.I.; Guimarães, M.R.C.; Nunes, E.P.; Lemos, A.S.; Ribeiro, S.R.; et al. Changing mortality profile among HIV-infected patients in Rio de Janeiro, Brazil: Shifting from AIDS to Non-AIDS related conditions in the HAART era. PLoS ONE 2013, 8, e59768. [Google Scholar] [CrossRef]
- Pacheco, A.G.; Tuboi, S.H.; Faulhaber, J.C.; Harrison, L.H.; Schechter, M. Increase in non-AIDS related conditions as causes of death among HIV-infected individuals in the HAART era in Brazil. PLoS ONE 2008, 3, e1531. [Google Scholar] [CrossRef]
- Geel, J.A.; Chirwa, T.C.; Rowe, B.; Eyal, K.C.; Omar, F.; Stones, D.K.; Goga, Y.; Stefan, D.C.; van Zyl, A.; Van Emmenes, B.; et al. Treatment outcomes of children with Hodgkin lymphoma between 2000 and 2010: First report by the South African Children’s Cancer Study Group. Pediatr. Blood Cancer 2017, 64, e26536. [Google Scholar] [CrossRef] [PubMed]
- BRASIL. Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Manejo da Infecção Pelo HIV em Crianças e Adolescentes—Brasília: Ministério da Saúde. 2018. Available online: https://www.gov.br/aids/pt-br/centrais-de-conteudo/pcdts/2017/hiv-aids/pcdt_crianca_adolescentel_04_2019_web.pdf/view (accessed on 2 February 2023).
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: Myeloid and histiocytic/dendritic neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- WHO ICD 11. 2022. Available online: https://www.who.int/standards/classifications/classification-of-diseases (accessed on 2 February 2023).
- Murphy, S.B. Classification, staging and end results of treatment of childhood non-Hodgkin’s lymphomas: Dissimilarities from lymphomas in adults. Semin. Oncol. 1980, 7, 332–339. [Google Scholar]
- Lister, T.A.; Crowther, D.; Sutcliffe, S.B.; Glatstein, E.; Canellos, G.P.; Young, R.C.; Rosenberg, S.A.; Coltman, C.A.; Tubiana, M. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin’s disease: Cotswolds meeting. J. Clin. Oncol. 1989, 7, 1630–1636, Erratum in J. Clin. Oncol. 1990, 9, 1602. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059. [Google Scholar] [CrossRef] [PubMed]
- Younes, A.; Hilden, P.; Coiffier, B.; Hagenbeek, A.; Salles, G.; Wilson, W.; Seymour, J.F.; Kelly, K.; Gribben, J.; Pfreunschuh, M.; et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. 2017, 28, 1436–1447. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of monotone likelihood in Cox regression. Biometrics 2001, 57, 114–119. [Google Scholar] [CrossRef]
- da Silva, W.F.; Garibaldi, P.M.M.; Da Rosa, L.I.; Bellesso, M.; Clé, D.V.; Delamain, M.T.; Rego, E.M.; Pereira, J.; Rocha, V. Outcomes of HIV-associated Burkitt Lymphoma in Brazil: High treatment toxicity and refractoriness rates—A multicenter cohort study. Leuk. Res. 2020, 89, 106287. [Google Scholar] [CrossRef]
- Rabie, H.; Maskew, M.; Bohlius, J.; Poole, J.; Davidson, A.; Prozesky, H.; Stefan, D.C.; Sawry, S.; Spoerri, A.; Maxwell, N.; et al. Incidence of AIDS-defining and other cancers in HIV-positive children in South Africa: Record linkage study. Pediatr. Infect. Dis. J. 2016, 35, e164. [Google Scholar]
- Stefan, D.C.; Stones, D.K. Children with cancer and HIV infection: What is different about them? J. Pediatr. Hematol. Oncol. 2013, 35, 590–596. [Google Scholar] [CrossRef]
- Arzoo, K.K.; Bu, X.; Espina, B.M.; Seneviratne, L.; Nathwani, B.; Levine, A.M. T-Cell lymphoma in HIV-infected patients. JAIDS J. Acquir. Immune Defic. Syndr. 2004, 36, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Kuri, A.; Jacobs, B.M.; Vickaryous, N.; Pakpoor, J.; Middeldorp, J.; Giovannoni, G.; Dobson, R. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health 2020, 20, 912. [Google Scholar] [CrossRef] [PubMed]
- Besson, C.; Goubar, A.; Gabarre, J.; Rozenbaum, W.; Pialoux, G.; Châtelet, F.-P.; Katlama, C.; Charlotte, F.; Dupont, B.; Brousse, N.; et al. Changes in AIDS-related lymphoma since the era of highly active antiretroviral therapy. Blood J. Am. Soc. Hematol. 2001, 98, 2339–2344. [Google Scholar] [CrossRef] [PubMed]
- Haq, H.; Elyanu, P.; Bulsara, S.; Bacha, J.; Campbell, L.; El-Mallawany, N.; Keating, E.; Kisitu, G.; Mehta, P.; Rees, C.; et al. Association between antiretroviral therapy and cancers among children living with HIV in sub-Saharan Africa. Cancers 2021, 13, 1379. [Google Scholar] [CrossRef]
- Borges, H.; Neuhaus, J.; Babiker, A.G.; Henry, K.; Jain, M.K.; Palfreeman, A.; Mugyenyi, P.; Domingo, P.; Hoffmann, C.; Read, T.R.H.; et al. Immediate antiretroviral therapy reduces risk of infection-related cancer during early HIV infection. Clin. Infect. Dis. 2016, 63, 1668–1676. [Google Scholar] [CrossRef]
- Patel, K.; Hernán, M.A.; Williams, P.L.; Seeger, J.D.; McIntosh, K.; Van Dyke, R.B.; Seage, G.R.; Pediatric AIDS Clinical Trials Group. 219/219C Study Team. Long-term effects of highly active antiretroviral therapy on CD4+ cell evolution among children and adolescents infected with HIV: 5 years and counting. Clin. Infect. Dis. 2008, 46, 1751–1760. [Google Scholar] [CrossRef]
- Walker, A.S.; Doerholt, K.; Sharland, M.; Gibb, D.M. Response to highly active antiretroviral therapy varies with age: The UK and Ireland Collaborative HIV Paediatric Study. Aids 2004, 18, 1915–1924. [Google Scholar] [CrossRef]
- Prendergast, A.; Klenerman, P.; Goulder, P.J.R. The impact of differential antiviral immunity in children and adults. Nat. Rev. Immunol. 2012, 12, 636–648. [Google Scholar] [CrossRef]
- Elizabeth, C. Malignancies among children and young people with HIV in Western and Eastern Europe and Thailand. AIDS 2021, 35, 1973. [Google Scholar]
- Resino, S.; Resino, R.; Bellon, J.; Micheloud, D.; Gutierrez, M.D.G.; De Jose, M.I.; Ramos, J.T.; Fontelos, P.M.; Ciria, L.; MunozFernandez, M.A.; et al. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV Type-1–infected children. Clin. Infect. Dis. 2006, 43, 243–252. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.; Hullsiek, K.H.; Marconi, V.; Weintrob, A.; Ganesan, A.; Barthel, R.V.; Fraser, S.; Agan, B.; Wegner, S. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: A 20-year cohort study. Aids 2009, 23, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Montagnani, C.; Chiappini, E.; Bonsignori, F.; Galli, L.; de Martino, M. Long-Term effect of highly active antiretroviral therapy on immunologic features in children. Pediatr. Infect. Dis. J. 2015, 34, S3–S6. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.J.; Nachega, J.B.; Bangsberg, D.R.; Singh, S.; Rachlis, B.; Wu, P.; Wilson, K.; Buchan, I.; Gill, C.J.; Cooper, C. Adherence to HAART: A Systematic Review of Developed and Developing Nation Patient-Reported Barriers and Facilitators. PLoS Med. 2006, 3, e438. [Google Scholar] [CrossRef]
- WHO. Global Health Sector Strategies on, Respectively, HIV, Viral Hepatitis and Sexually Transmitted Infections for the Period 2022–2030. Available online: https://www.who.int/publications/i/item/9789240053779 (accessed on 2 February 2023).
- Orem, J.; Maganda, A.; Mbidde, E.K.; Weiderpass, E. Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infection. Pediatr. Blood Cancer 2009, 52, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Suri, D.; Bhattad, S.; Gupta, A.; Trehan, A.; Bansal, D.; Rajwanshi, A.; Das, A.; Singh, S.; Rawat, A. Malignancies in children with human immunodeficiency virus infection–our experience at Chandigarh, North India. J. Trop. Pediatr. 2017, 63, 210–216. [Google Scholar] [CrossRef]
- Howard, S.C.; Zaidi, A.; Cao, X.; Weil, O.; Bey, P.; Patte, C.; Samudio, A.; Haddad, L.; Lam, C.G.; Moreira, C.; et al. The My Child Matters programme: Effect of public–private partnerships on paediatric cancer care in low-income and middle-income countries. Lancet Oncol. 2018, 9, 252–266. [Google Scholar] [CrossRef]
| Features | n = 25 (100%) |
|---|---|
| Biological Sex | |
| Female | 11 (44%) |
| Male | 14 (56%) |
| Lymphoma Subtype | |
| BL (ADM) | 13 (52%) |
| DLBCL (ADM) | 6 (24%) |
| NSCHL (NADM) | 4 (16%) |
| ALCL (CD30+/ALK+) (NADM) | 1 (4%) |
| PTCL (NADM) | 1 (4%) |
| Site of Lymphoma at Disease Presentation | |
| Lymph node | 4 (16%) |
| Respiratory | 0 |
| Gastrointestinal | 1 (4%) |
| Bones | 3 (12%) |
| CNS | 0 |
| BM | 1 (4%) |
| Diffuse (two or more different sites) | 16 (64%) |
| Year of Lymphoma Diagnosis | |
| 1996 | 1 (4%) |
| 1998 | 2 (8%) |
| 1999 | 2 (8%) |
| 2000 | 3 (12%) |
| 2001 | 2 (8%) |
| 2002 | 4 (16%) |
| 2004 | 4 (16%) |
| 2005 | 3 (12%) |
| 2007 | 1 (4%) |
| 2010 | 2 (8%) |
| 2013 | 1 (4%) |
| Age at Lymphoma Diagnosis (years) | |
| Median (IQR) | 7.43 (4.55) |
| 0–4 | 5 (20%) |
| 5–9 | 13 (52%) |
| ≥10 | 7 (28%) |
| ART Prophylaxis | |
| Yes | 02 (8%) |
| No | 23 (92%) |
| ART Use? | |
| Yes | 20 (80%) |
| No | 5 (20%) |
| cART Use? | |
| Yes | 17 (68%) |
| No | 8 (32%) |
| cART Era | |
| Early-cART | 13 (52%) |
| Mid-cART | 11 (44%) |
| Late-cART | 1 (4%) |
| cART at Lymphoma Diagnosis? | |
| Yes | 13 (52%) |
| No | 12 (48%) |
| CD4+ T-cell Count at HIV Diagnosis (%) | |
| Median (IQR) | 16.00% (17.55%) |
| >25% | 4 (20%) |
| 15–25% | 7 (35%) |
| <15% | 9 (45%) |
| NP | 5 |
| HIV Load at HIV Diagnosis (Copies/mL) | |
| Median (IQR) | 330,000 (793,000) |
| CDC Category/Stage at HIV Diagnosis | |
| N2 | 1 (4%) |
| A1 | 2 (8%) |
| A2 | 4 (16%) |
| C | 5 (20%) |
| C1 | 2 (8%) |
| C2 | 2 (8%) |
| C3 | 9 (36%) |
| CD4+ T-cell Count at Lymphoma Diagnosis (%) | |
| Median (IQR) | 15.50 % (15.00%) |
| >25% | 2 (11.10%) |
| 15–25% | 8 (44.40%) |
| <15% | 8 (44.40%) |
| NP | 07 |
| HIV Load at Lymphoma Diagnosis (Copies/mL) | |
| Median (IQR) | 78,000 (274,000) |
| CDC Category/Stage at Lymphoma Diagnosis | |
| C | 3 (12%) |
| C1 | 1 (04%) |
| C2 | 5 (20%) |
| C3 | 16 (64%) |
| Performance Status (PS) | |
| 1 | 5 (20%) |
| 2 | 9 (36%) |
| 3 | 0 |
| 4 | 11 (44%) |
| Stage of Lymphoma | |
| I | 1 (4%) |
| IA | 1 (4%) |
| III | 2 (8%) |
| IIIB | 6 (24%) |
| IV | 6 (24%) |
| IVA | 1 (4%) |
| IVB | 8 (32%) |
| B Symptoms | |
| Yes | 14 (56%) |
| No | 11 (44%) |
| CNS Involvement | |
| Yes | 1 (4.54%) |
| No | 21 (95.45%) |
| NP | 3 |
| BM Involvement | |
| Yes | 6 (26.08%) |
| No | 17 (73.91%) |
| NP | 2 (8%) |
| Chemotherapy Protocols | |
| NHL-BFM 90 | 1 (4%) |
| GPOH-HD-95 | 1 (4%) |
| NHL-BFM 95 | 16 (64%) |
| B-NHL-BFM 04 | 1 (4%) |
| m-BACOD | 1 (4%) |
| DA-EPOCH-R | 1 (4%) |
| ABVD | 2 (8%) |
| NHL-BFM 95 + m-BACOD | 2 (8%) |
| Days of Chemotherapy | |
| Median (IQR) | 129.00 (167.00) |
| Chemotherapy Completion | |
| Yes | 11 (44%) |
| No (death) | 14 (56%) |
| cART at Chemotherapy? | |
| Yes | 13 (52%) |
| No | 12 (48%) |
| Surgery | |
| Yes | 2 (8%) |
| No | 23 (92%) |
| Outcomes | n = 25 (100%) |
| Complete Response | |
| Yes | 15 (60%) |
| No | 10 (40%) |
| Relapse | |
| Yes | 3 (20%) |
| No | 12 (80%) |
| Death | |
| Yes | 18 (72%) |
| No | 7 (28%) |
| Status at Moment of Death | |
| Progression of disease/non-complete response | 10 (55.56%) |
| After relapse | 3 (16.67%) |
| Complete response | 5 (27.78%) |
| Univariate | Multivariate | Multivariate Analysis of Imputed Dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables (n) | HR | Low | High | p | HR | Low | High | p | HR | Low | High | p |
| Biological Sex | ||||||||||||
| Female (11) | 0.88 | 0.55 | 1.41 | 0.547 | ||||||||
| Male (14) | 1 | |||||||||||
| Year of Lymphoma Diagnosis (25) | 0.98 | 0.88 | 1.09 | 0.760 | ||||||||
| Age at Lymphoma Diagnosis (25) | 0.99 | 0.87 | 1.15 | 0.976 | ||||||||
| Lymphoma Group | ||||||||||||
| ADM (19) | 0.62 | 0.22 | 1.79 | 0.380 | ||||||||
| NADM (6) | 1 | |||||||||||
| cART Era | ||||||||||||
| Early-cART (13) | 0.24 | 0.03 | 2.15 | 0.204 | ||||||||
| Mid-cART (11) | 0.34 | 0.04 | 2.95 | 0.326 | ||||||||
| Late-cART (1) | 1 | |||||||||||
| CD4+ T-Cell Count at Lymphoma Diagnosis (%) (18) | 0.91 | 0.84 | 0.98 | 0.014 | ||||||||
| NP (7) | ||||||||||||
| HIV Load at Lymphoma Diagnosis (Copies/mL) (17) | 1.00 | 1.00 | 1.00 | 0.797 | ||||||||
| PS at Lymphoma Diagnosis | ||||||||||||
| 4 (11) | 4.85 | 1.82 | 12.97 | 0.002 | 4.85 | 1.82 | 12.97 | 0.002 | 4.85 | 1.82 | 12.97 | 0.002 |
| <4 (14) | 1 | |||||||||||
| Lymphoma Stage | ||||||||||||
| I–III (10) | 1 | |||||||||||
| IV (15) | 1.53 | 0.57 | 4.09 | 0.398 | ||||||||
| cART at Chemotherapy | ||||||||||||
| Yes (13) | 0.50 | 0.19 | 1.26 | 0.142 | ||||||||
| No (12) | 1 | |||||||||||
| Univariate | Multivariate | Multivariate Analysis of Imputed Dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables (n) | HR | Low | High | p | HR | Low | High | p | HR | Low | High | p |
| Biological Sex | ||||||||||||
| Female (11) | 0.75 | 0.29 | 1.93 | 0.549 | ||||||||
| Male (14) | 1 | |||||||||||
| Year of Lymphoma Diagnosis (25) | 0.99 | 0.89 | 1.10 | 0.798 | ||||||||
| Age at Lymphoma Diagnosis (25) | 1.00 | 0.87 | 1.16 | 0.978 | ||||||||
| Lymphoma Group | ||||||||||||
| ADM (19) | 0.58 | 0.20 | 1.65 | 0.306 | ||||||||
| NADM (6) | 1 | |||||||||||
| cART Era | ||||||||||||
| Early-cART (13) | 0.37 | 0.04 | 3.16 | 0.367 | ||||||||
| Mid-cART (11) | 0.54 | 0.07 | 4.49 | 0.571 | ||||||||
| Late-cART (1) | 1 | |||||||||||
| CD4+ T-Cell Count at Lymphoma Diagnosis (%) (18) | 0.90 | 0.84 | 0.98 | 0.012 | ||||||||
| NP (7) | ||||||||||||
| HIV Load at Lymphoma Diagnosis (Copies/mL) (17) | 1.00 | 1.00 | 1.00 | 0.777 | ||||||||
| PS at Lymphoma Diagnosis | ||||||||||||
| 4 (11) | 4.95 | 1.84 | 13.34 | 0.002 | 4.95 | 1.84 | 13.34 | 0.002 | 4.95 | 1.84 | 13.34 | 0.002 |
| <4 (14) | 1 | |||||||||||
| Lymphoma Stage | ||||||||||||
| I–III (10) | 1 | |||||||||||
| IV (15) | 1.53 | 0.57 | 4.09 | 0.398 | ||||||||
| cART at Chemotherapy | ||||||||||||
| Yes (13) | 0.49 | 0.19 | 1.26 | 0.142 | ||||||||
| No (12) | 1 | |||||||||||
| Univariate | Multivariate | Multivariate Analysis of Imputed Dataset | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables (n) | HR | Low | High | p | HR | Low | High | p | HR | Low | High | p |
| Biological Sex | ||||||||||||
| Female (11) | 0.68 | 0.16 | 2.89 | 0.607 | ||||||||
| Male (14) | 1 | |||||||||||
| Year of Lymphoma Diagnosis (25) | 0.92 | 0.78 | 1.09 | 0.355 | ||||||||
| Age at Lymphoma Diagnosis (25) | 1.03 | 0.85 | 1.25 | 0.745 | ||||||||
| Lymphoma Group | ||||||||||||
| ADM (19) | 0.46 | 0.08 | 2.45 | 0.366 | ||||||||
| NADM (6) | 1 | |||||||||||
| cART Era * | ||||||||||||
| Early-cART (13) | 0.73 | 0.19 | 2.87 | 0.645 | ||||||||
| Mid-cART (11) | 1.36 | 0.35 | 5.35 | 0.645 | ||||||||
| Late-cART (1) ** | NA | NA | NA | NA | ||||||||
| CD4+ T-Cell Count at Lymphoma Diagnosis (%) (18) | 0.86 | 0.76 | 0.97 | 0.017 | 0.86 | 0.76 | 0.97 | 0.017 | 0.85 | 0.75 | 0.95 | 0.007 |
| NP (7) | ||||||||||||
| HIV Load at Lymphoma Diagnosis (Copies/mL) (17) | 1.00 | 1.00 | 1.00 | 0.942 | ||||||||
| PS at Lymphoma Diagnosis | ||||||||||||
| 4 (11) | 3.45 | 0.82 | 14.60 | 0.092 | ||||||||
| <4 (14) | 1 | |||||||||||
| Lymphoma Stage | ||||||||||||
| I–III (10) | 1 | |||||||||||
| IV (15) | 2.25 | 0.45 | 11.28 | 0.323 | ||||||||
| cART at Chemotherapy | ||||||||||||
| Yes (13) | 0.88 | 0.21 | 3.72 | 0.867 | ||||||||
| No (12) | 1 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duarte, N.L.; Bueno, A.P.S.; Sanches, B.S.; Ramos, G.A.; Santos, J.M.B.d.; Silva, H.F.H.e.; Pondé, J.d.O.; Sá, J.G.d.; Rossi, P.M.; Horn, P.R.C.B.; et al. Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study. Cancers 2023, 15, 2292. https://doi.org/10.3390/cancers15082292
Duarte NL, Bueno APS, Sanches BS, Ramos GA, Santos JMBd, Silva HFHe, Pondé JdO, Sá JGd, Rossi PM, Horn PRCB, et al. Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study. Cancers. 2023; 15(8):2292. https://doi.org/10.3390/cancers15082292
Chicago/Turabian StyleDuarte, Nathalia Lopez, Ana Paula Silva Bueno, Bárbara Sarni Sanches, Gabriella Alves Ramos, Julia Maria Bispo dos Santos, Henrique Floriano Hess e Silva, Janaina de Oliveira Pondé, José Gilberto de Sá, Priscila Mazucanti Rossi, Patricia Regina Cavalcanti Barbosa Horn, and et al. 2023. "Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study" Cancers 15, no. 8: 2292. https://doi.org/10.3390/cancers15082292
APA StyleDuarte, N. L., Bueno, A. P. S., Sanches, B. S., Ramos, G. A., Santos, J. M. B. d., Silva, H. F. H. e., Pondé, J. d. O., Sá, J. G. d., Rossi, P. M., Horn, P. R. C. B., Sztajnbok, D. C. d. N., Rubini, N. d. P. M., da Costa, E. S., Milito, C. B., de Abreu, T. F., & Land, M. G. P. (2023). Prognostic Factors in Children and Adolescents with Lymphomas and Vertical Transmission of HIV in Rio de Janeiro, Brazil: A Multicentric Hospital-Based Survival Analysis Study. Cancers, 15(8), 2292. https://doi.org/10.3390/cancers15082292









