Cardiovascular Immunotoxicity Associated with Immune Checkpoint Inhibitors in Metastatic Melanoma
Abstract
Simple Summary
Abstract
1. Introduction
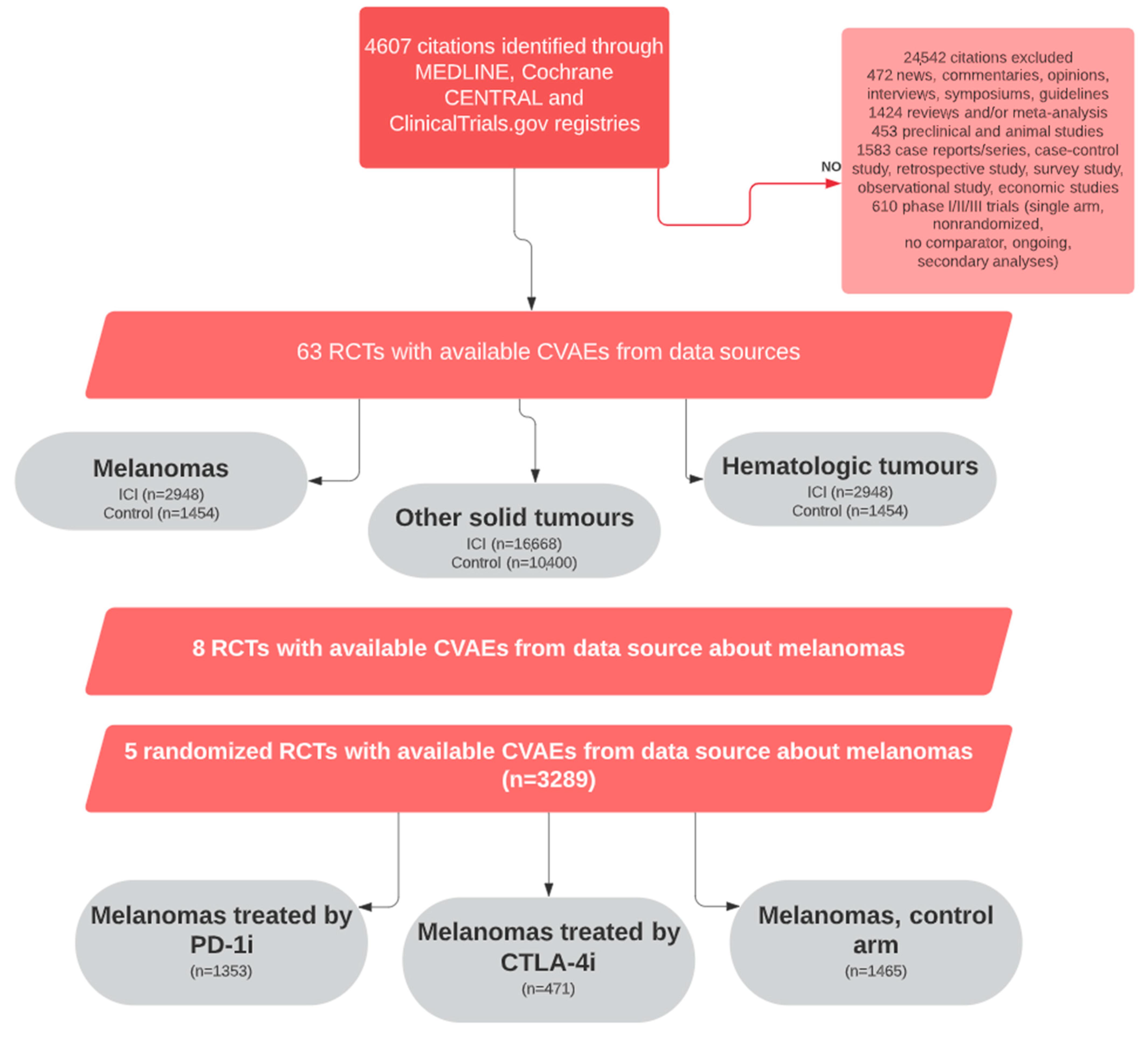
2. Materials and Methods
2.1. Registration
2.2. Data Sources, Search Strategy, and Data Extraction
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Descriptions of Included Studies
3.2. Risk of CVAEs Associated with ICI Exposure and Incidence of CV irAEs with an Increased Risk Associated with ICI Exposure in Melanoma
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lipson, E.J.; Sharfman, W.H.; Drake, C.G.; Wollner, I.; Taube, J.M.; Anders, R.A.; Xu, H.; Yao, S.; Pons, A.; Chen, L.; et al. Durable Cancer Regression Off-Treatment and Effective Reinduction Therapy with an Anti-PD-1 Antibody. Clin. Cancer Res. 2013, 19, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Diamantopoulos, P.T.; Tsatsou, K.; Benopoulou, O.; Bonou, M.; Anastasopoulou, A.; Mastrogianni, E.; Gogas, H. Concomitant development of neurologic and cardiac immune-related adverse effects in patients treated with immune checkpoint inhibitors for melanoma. Melanoma Res. 2020, 30, 484–491. [Google Scholar] [CrossRef]
- Reuben, A.; de Macedo, M.P.; McQuade, J.; Joon, A.; Ren, Z.; Calderone, T.; Conner, B.; Wani, K.; Cooper, Z.; Tawbi, H.; et al. Comparative immunologic charac-terization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 2017, 6, e1361097. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Jen, T.; Skulski, R. Late-onset pericardial tamponade, bilateral pleural effusions and recurrent immune monoarthritis induced by ipilimumab use for metastatic melanoma. J. Oncol. Pharm. Pr. 2016, 23, 231–234. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Morimoto, R.; Okumura, T.; Yamashita, Y.; Haga, T.; Kuwayama, T.; Yokoi, T.; Hiraiwa, H.; Kondo, T.; Sugiura, Y.; et al. Late-Onset Fulminant Myocar-ditis With Immune Checkpoint Inhibitor Nivolumab. Can. J. Cardiol. 2018, 34, 812.e1–812.e3. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Gottfridsson, C.; Asteggiano, R.; Atar, D.; Badimon, L.; Bax, J.J.; Cardinale, D.; Cardone, A.; Feijen, E.A.M.; Ferdinandy, P.; et al. The cancer patient and cardiology. Eur. J. Hear. Fail. 2020, 22, 2290–2309. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Munoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.; Lyon, A.R.; et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Dolladille, C.; Akroun, J.; Morice, P.-M.; Dompmartin, A.; Ezine, E.; Sassier, M.; Da-Silva, A.; Plane, A.-F.; Legallois, D.; L’Orphelin, J.-M.; et al. Cardiovascular immunotoxicities as-sociated with immune checkpoint inhibitors: A safety meta-analysis. Eur. Heart J. 2021, 42, 4964–4977. [Google Scholar] [CrossRef] [PubMed]
- Faillie, J.L.; Ferrer, P.; Gouverneur, A.; Driot, D.; Berkemeyer, S.; Vidal, X.; Martínez-Zapata, M.J.; Huerta, C.; Castells, X.; Rottenkolber, M.; et al. A new risk of bias checklist applicable to randomized trials, observational studies, and systematic reviews was developed and validated to be used for sys-tematic reviews focusing on drug adverse events. J. Clin. Epidemiol. 2017, 86, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Rockville, M.D. Methods Guide for Effectiveness and Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Ranganathan, P.; Aggarwal, R.; Pramesh, C. Common pitfalls in statistical analysis: Odds versus risk. Perspect. Clin. Res. 2015, 6, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, P. Relative risks versus odds ratios. BMJ 2014, 348, g1407. [Google Scholar] [CrossRef]
- Okazaki, T.; Tanaka, Y.; Nishio, R.; Mitsuiye, T.; Mizoguchi, A.; Wang, J.; Ishida, M.; Hiai, H.; Matsumori, A.; Minato, N.; et al. Autoantibodies against cardiac troponin I are responsible for dilated cardiomyopathy in PD-1-deficient mice. Nat. Med. 2003, 9, 1477–1483. [Google Scholar] [CrossRef]
- Escardio. Available online: www.escardio.org/static-file/Escardio/Subspecialty/EACPR/Documents/score-charts.pdf (accessed on 25 February 2023).
- Vincent, L.; Leedy, D.; Masri, S.C.; Cheng, R.K. Cardiovascular Disease and Cancer: Is There Increasing Overlap? Curr. Oncol. Rep. 2019, 21, 1–13. [Google Scholar] [CrossRef]
- Lutgens, E.; Seijkens, T.T. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J. Immunother. Cancer 2019, 8, e000300. [Google Scholar] [CrossRef]
- Tall, A.R.; Yvan-Charvet, L. Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 2015, 15, 104–116. [Google Scholar] [CrossRef]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef]
- Newman, J.L.; Stone, J.R. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol. 2019, 43, 107148. [Google Scholar] [CrossRef]
- Drobni, Z.D.; Alvi, R.M.; Taron, J.; Zafar, A.; Murphy, S.P.; Rambarat, P.K.; Mosarla, R.C.; Lee, C.; Zlotoff, D.A.; Raghu, V.K.; et al. Association Between Immune Checkpoint Inhibitors with Cardiovascular Events and Atherosclerotic Plaque. Circulation 2020, 142, 2299–2311. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.J.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.M.; Libby, P.; Raichlen, J.S.; Uno, K.; Borgman, M.; Wolski, K.; et al. Effect of two intensive statin regimens on progression of coronary disease. N. Engl. J. Med. 2011, 365, 2078–2087. [Google Scholar] [CrossRef]
- Strauss, L.; Mahmoud, M.A.A.; Weaver, J.D.; Tijaro-Ovalle, N.M.; Christofides, A.; Wang, Q.; Pal, R.; Yuan, M.; Asara, J.; Patsoukis, N.; et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci. Immunol. 2020, 5, eaay1863. [Google Scholar] [CrossRef]
- Perrone, F.; Minari, R.; Bersanelli, M.; Bordi, P.; Tiseo, M.; Favari, E.; Sabato, R.; Buti, S. The Prognostic Role of High Blood Cholesterol in Advanced Cancer Patients Treated with Immune Checkpoint Inhibitors. J. Immunother. 2020, 43, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Suo, A.; Chan, Y.; Beaulieu, C.; Kong, S.; Cheung, W.Y.; Monzon, J.G.; Smylie, M.; Walker, J.; Morris, D.; Cheng, T. Anti-PD1-Induced Immune-Related Adverse Events and Survival Outcomes in Advanced Melanoma. Oncology 2020, 25, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Orkaby, A.R.; Driver, J.A.; Ho, Y.L.; Lu, B.; Costa, L.; Honerlaw, J.; Galloway, A.; Vassy, J.L.; Forman, D.E.; Gaziano, J.M.; et al. Association of Statin Use with All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA 2020, 324, 68–78. [Google Scholar] [CrossRef]
- Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9, 895–905. [Google Scholar] [CrossRef]
- Sarrabayrouse, G.; Pich-Bavastro, C.; Teiti, I.; Tilkin-Mariame, A.F. Regulatory properties of statins and rho gtpases prenylation inhibitiors to stimulate melanoma immunogenicity and promote anti-melanoma immune response. Int. J. Cancer 2016, 140, 747–755. [Google Scholar] [CrossRef]
- Boudreau, D.M.; Yu, O.; Johnson, J. Statin use and cancer risk: A comprehensive review. Expert Opin. Drug Saf. 2010, 9, 603–621. [Google Scholar] [CrossRef]
- Li, X.; Wu, X.B.; Chen, Q. Statin use is not associated with reduced risk of skin cancer: A meta-analysis. Br. J. Cancer 2013, 110, 802–807. [Google Scholar] [CrossRef]
- Freeman, S.R.; Drake, A.L.; Heilig, L.F.; Graber, M.; McNealy, K.; Schilling, L.M.; Dellavalle, R.P. Statins, fibrates, and melanoma risk: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2006, 98, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fu, D.; Liu, H.; Peng, D. Independent association of PCSK9 with platelet reactivity in subjects without statin or antiplatelet agents. Front. Cardiovasc. Med. 2022, 9, 934914. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Bisceglia, I.; Berretta, M.; Iovine, M.; Canale, M.L.; Maurea, C.; Giordano, V.; Paccone, A.; Inno, A.; Maurea, N. PCSK9 Inhibitors in Cancer Patients Treated with Immune-Checkpoint Inhibitors to Reduce Cardiovascular Events: New Frontiers in Cardioncology. Cancers 2023, 15, 1397. [Google Scholar] [CrossRef] [PubMed]
- Gratton, J.; Finan, C.; Hingorani, A.D.; Humphries, S.E.; Futema, M. LDL-C Concentrations and the 12-SNP LDL-C Score for Polygenic Hypercholesterolaemia in Self-Reported South Asian, Black and Caribbean Participants of the UK Biobank. Front. Genet. 2022, 13, 845498. [Google Scholar] [CrossRef]
- Mahmood, S.S.; Fradley, M.G.; Cohen, J.V.; Nohria, A.; Reynolds, K.L.; Heinzerling, L.M.; Sullivan, R.J.; Damrongwatanasuk, R.; Chen, C.L.; Gupta, D.; et al. Myocarditis in Patients Treated with Immune Checkpoint Inhibitors. J. Am. Coll. Cardiol. 2018, 71, 1755–1764. [Google Scholar] [CrossRef]
- L’Orphelin, J.M.; Varey, E.; Khammari, A.; Dreno, B.; Dompmartin, A. Severe Late-Onset Grade III-IV Adverse Events un-der Immunotherapy: A Retrospective Study of 79 Cases. Cancers 2021, 13, 4928. [Google Scholar] [CrossRef]

| ClinicalTrials.gov Identifier | Study | Study Design | Comparison | Advanced or Metastatic Cancer | Prior Systemic Therapy (%) | Mean Patient Age (years) | Follow up Duration (months) | Number of Patients in the ICI Mono Therapy Group | Number of Patients in the Combination ICI Therapy Group | Number of Patients in the Control Group |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT00636168 | Eggermont Lancet Oncol. 2015 | Phase 3 RCT | Ipilimumab 10 mg/kg vs. placebo | No | 0 | 51.1 | 32.8 | 471 | - | 474 |
| NCT01704287 | Ribas Lancet Oncol. 2015 | Phase 2 RCT | Pembrolizumab 2 or 10 mg/kg vs. chemotherapy (paclitaxel + carboplatin, paclitaxel, carboplatin, dacarbazine or temozolomide) | Yes | 46–50 | 60.1 | 10 | 357 | - | 171 |
| NCT01721746 | Weber Lancet Oncol. 2015 | Phase 3 RCT | Nivolumab 3 mg/kg vs. chemotherapy (dacarbazine, or carboplatin + paclitaxel) | Yes | 100 | 59.2 | 8.4 | 268 | - | 102 |
| NCT01721772 | Robert N. Engl. J. Med. 2015 | Phase 3 RCT | Nivolumab 3 mg/kg + placebo vs. dacarbazine + placebo | Yes | 16.8 | 62.7 | 5.2 | 206 | - | 205 |
| NCT01844505 | Larkin N. Engl. J Med. 2015 | Phase 3 RCT | Nivolumab 3 mg/kg vs. Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg vs. Ipilimumab 3 mg/kg | Yes | 0 | 59.6 | 12.2 | 624 | 313 | - |
| NCT01927419 | Postow N. Engl. J. Med. 2015 | Phase 3 RCT | Ipilimumab 3 mg/kg + placebo vs. nivolumab 1 mg/kg + Ipilimumab 3 mg/kg | Yes | 0 | 63.7 | 11 | 46 | 94 | - |
| NCT02362594 | Eggermont N. Engl. J. Med. 2018 | Phase 3 RCT | Pembrolizumab 200 mg vs. placebo | No | 0 | 53.8 | 15 | 509 | - | 502 |
| NCT02374242 | Long Lancet Oncol. 2018 | Phase 2 RCT | Nivolumab 3 mg/kg vs. Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg | Yes | - | 61.1 | 14 | 25 | 35 |
| Immune Checkpoint Inhibitors Arm (n/N) | Control Arm (n/N) | n Study/.I2 | OR (IC95%) | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Melanomas | Other Tumors | Melanomas | Other Tumors | Melanomas | Other Tumors | Melanomas | Other Tumors | ||
| Supraventricular arrhythmias | 13/1811 | 85/11,217 | 5/1454 | 60/7406 | 5/0% | 29/27% | 1.74 (0.66–4.58) | 0.77 (0.50–1.20) | 0.13 |
| Ventricular arrhythmias | 2/777 | 4/2591 | 0/604 | 2/1572 | 2/0% | 6/31% | 5.57 (0.30–103.56) | 1.18 (0.16–8.80) | 0.39 |
| Cardiogenic shock | 7/1605 | 60/10,338 | 2/1249 | 27/6873 | 4/0% | 26/0% | 1.88 (0.47–7.58) | 1.45 (0.93–2.24) | 0.73 |
| Dyslipidemia | 30/625 | 9/1422 | 0/273 | 2/661 | 2/0% | 2/0% | 4.74 (2.16–10.41) | 1.91 (0.54–6.79) | 0.23 |
| Venous thromboembolic events | 15/1811 | 231/12,154 | 10/1454 | 162/8303 | 5/52% | 34/26% | 1.01 (0.30–3.39) | 0.97 (0.74–1.25) | 0.95 |
| High blood pressure | 122/1811 | 376/7668 | 87/1454 | 208/4758 | 5/62% | 18/76% | 1.71 (0.86–3.40) | 1.12 (0.74–1.70) | 0.30 |
| High pulmonary pressure | 2/2710 | 5/1696 | 6/3% | 0.26 (0.05–1.23) | 0.42 | ||||
| Myocardial infarction | 4/1605 | 66/11,093 | 1/1249 | 30/7631 | 3/0% | 28/0% | 2.50 (0.40–15.57) | 1.47 (0.97–2.22) | 0.58 |
| Cerebral ischemia | 5/1605 | 93/10,731 | 5/1249 | 36/7454 | 4/0% | 29/0% | 0.59 (0.16–2.16) | 1.68 (1.17–2.40) | 0.13 |
| Heart failure | 7/1811 | 82/9903 | 1/1454 | 28/6634 | 5/0% | 27/0% | 3.62 (0.87–15.07) | 1.90 (1.28–2.80) | 0.39 |
| Myocarditis | 1/509 | 13/4857 | 0/502 | 1/3587 | 1/NA% | 11/0% | 7.29 (0.14–367.33) | 4.25 (1.44–12.51) | 0.79 |
| Pericarditis | 1/357 | 70/8906 | 0/171 | 22/6702 | 1/NA% | 23/2% | 4.39 (0.07–289.28) | 2.17 (1.41–3.32) | 0.74 |
| Torsades de pointes/QT prolongation | 1/509 | 2/1211 | 0/502 | 1/589 | 1/NA% | 3/37% | 7.29 (0.14–367.33) | 0.96 (0.05–19.87) | 0.42 |
| Conduction disturbance | 1/828 | 2/3004 | 1/645 | 4/1782 | 2/29% | 6/0% | 0.71 (0.02–21.34) | 0.29 (0.05–1.52) | 0.64 |
| Valvulopathy | 1/1769 | 2/1363 | 3/41% | 0.37 (0.02–7.64) | 0.39 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
L’Orphelin, J.-M.; Dollalille, C.; Akroun, J.; Alexandre, J.; Dompmartin, A. Cardiovascular Immunotoxicity Associated with Immune Checkpoint Inhibitors in Metastatic Melanoma. Cancers 2023, 15, 2170. https://doi.org/10.3390/cancers15072170
L’Orphelin J-M, Dollalille C, Akroun J, Alexandre J, Dompmartin A. Cardiovascular Immunotoxicity Associated with Immune Checkpoint Inhibitors in Metastatic Melanoma. Cancers. 2023; 15(7):2170. https://doi.org/10.3390/cancers15072170
Chicago/Turabian StyleL’Orphelin, Jean-Matthieu, Charles Dollalille, Julia Akroun, Joachim Alexandre, and Anne Dompmartin. 2023. "Cardiovascular Immunotoxicity Associated with Immune Checkpoint Inhibitors in Metastatic Melanoma" Cancers 15, no. 7: 2170. https://doi.org/10.3390/cancers15072170
APA StyleL’Orphelin, J.-M., Dollalille, C., Akroun, J., Alexandre, J., & Dompmartin, A. (2023). Cardiovascular Immunotoxicity Associated with Immune Checkpoint Inhibitors in Metastatic Melanoma. Cancers, 15(7), 2170. https://doi.org/10.3390/cancers15072170





