New Automated Method for Lung Functional Volumes Delineation with Lung Perfusion PET/CT Imaging
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. 68Ga-PET/CT Perfusion Protocol
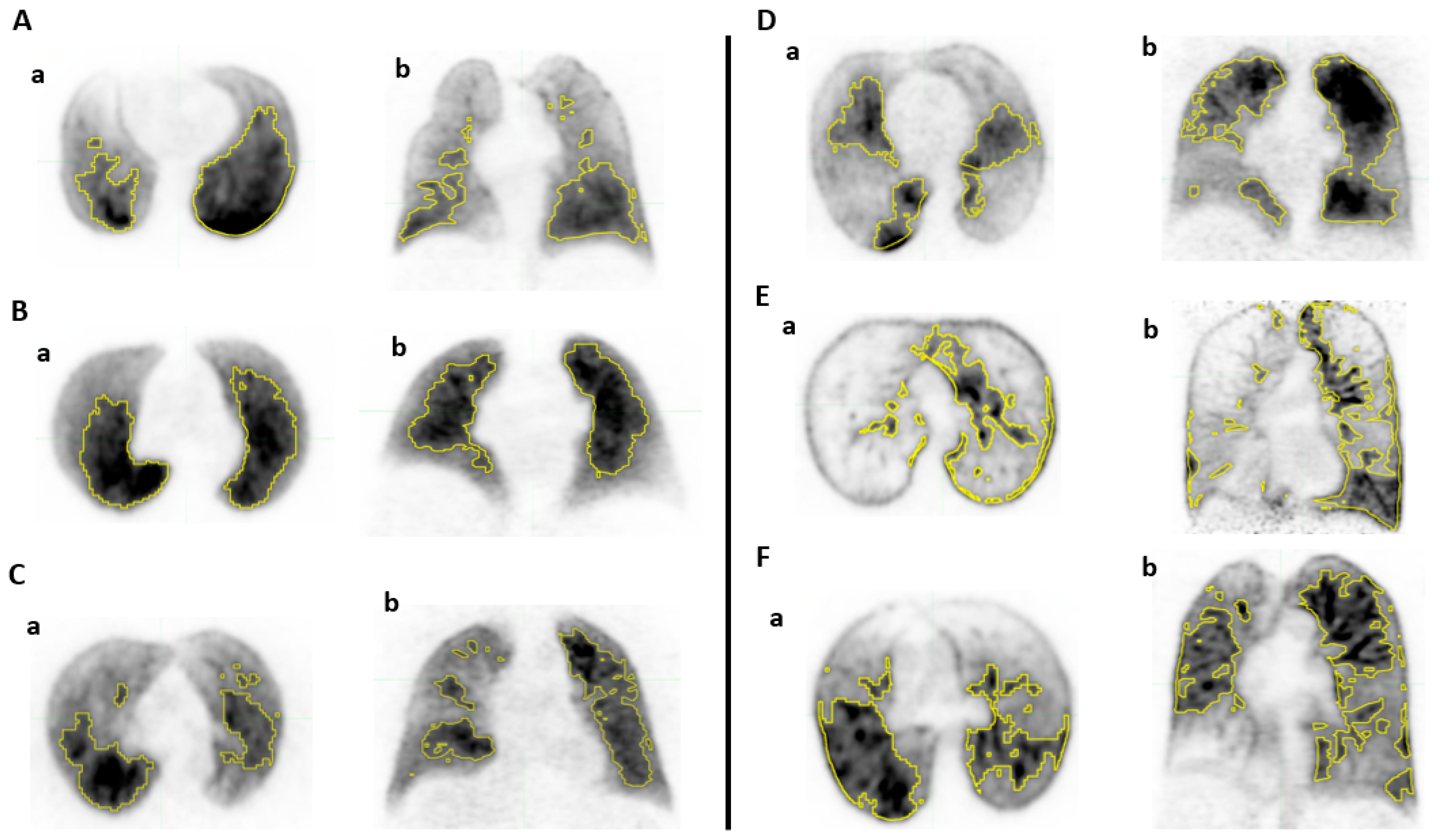
2.3. Lung Functional Volumes Delineation
2.4. Pulmonary Function Tests (PFTs)
2.5. Statistics
3. Results
3.1. Study Population
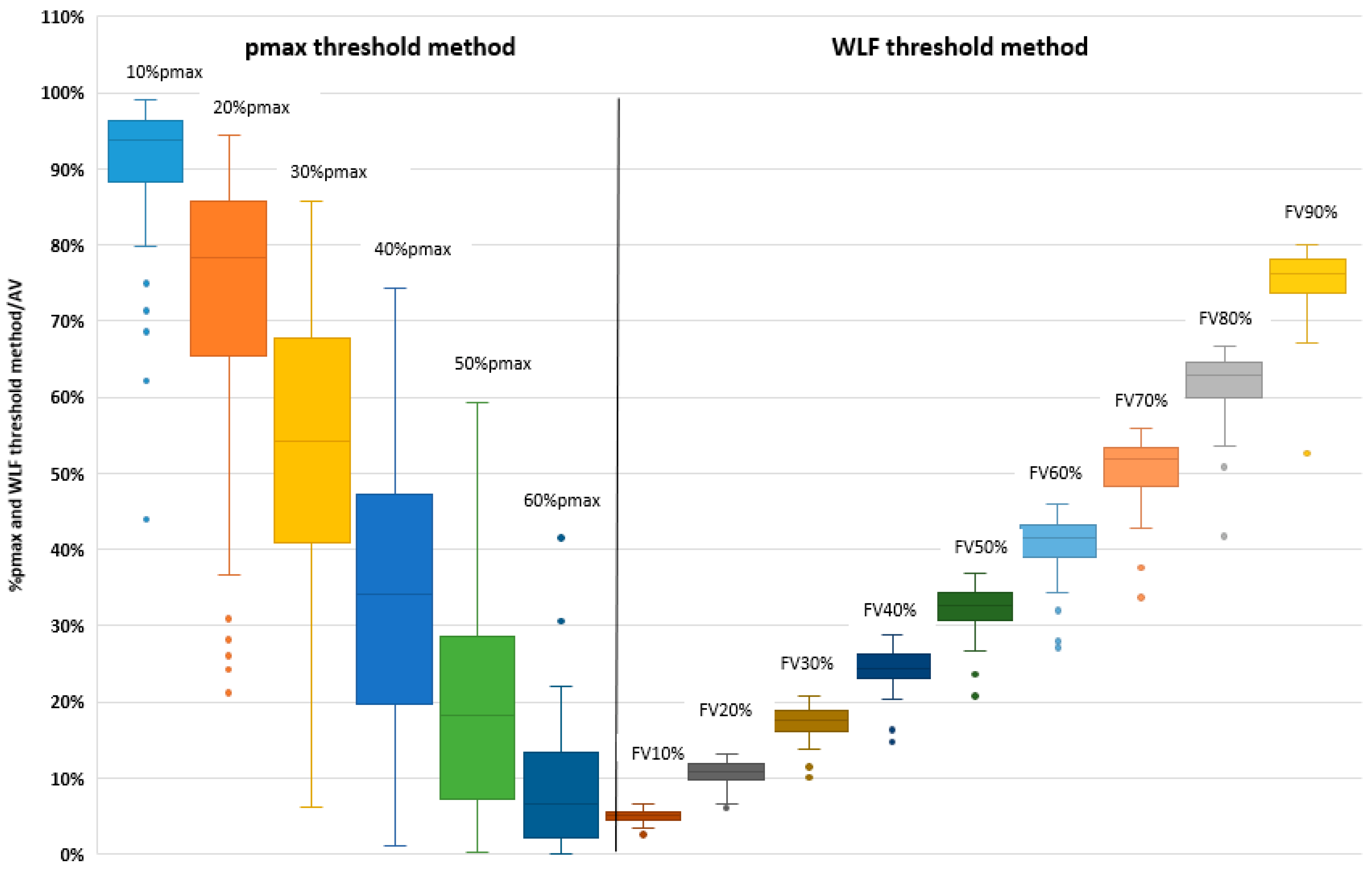
3.2. Pmax Threshold Method
3.3. Relative to Whole Lung Function Threshold Method
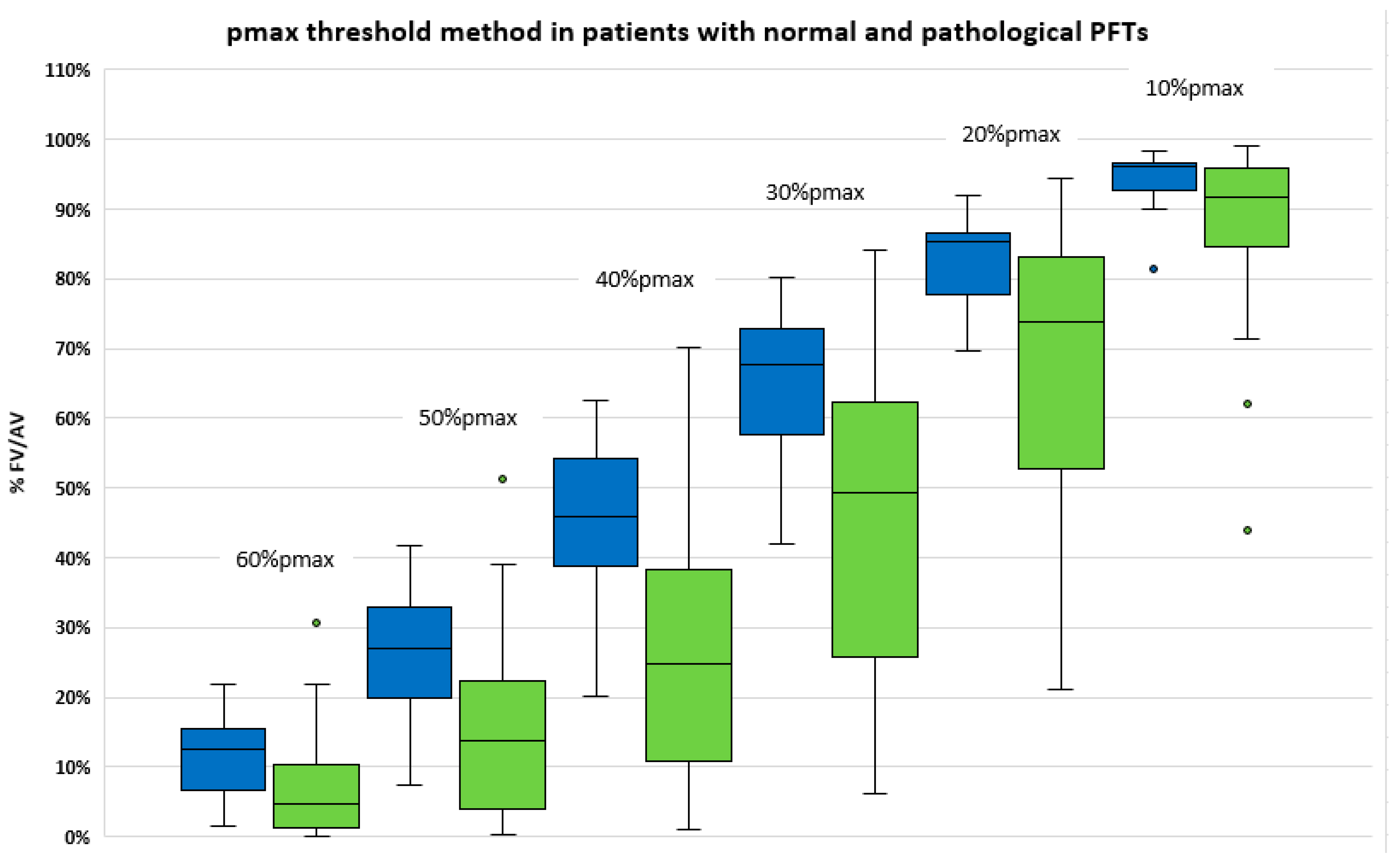
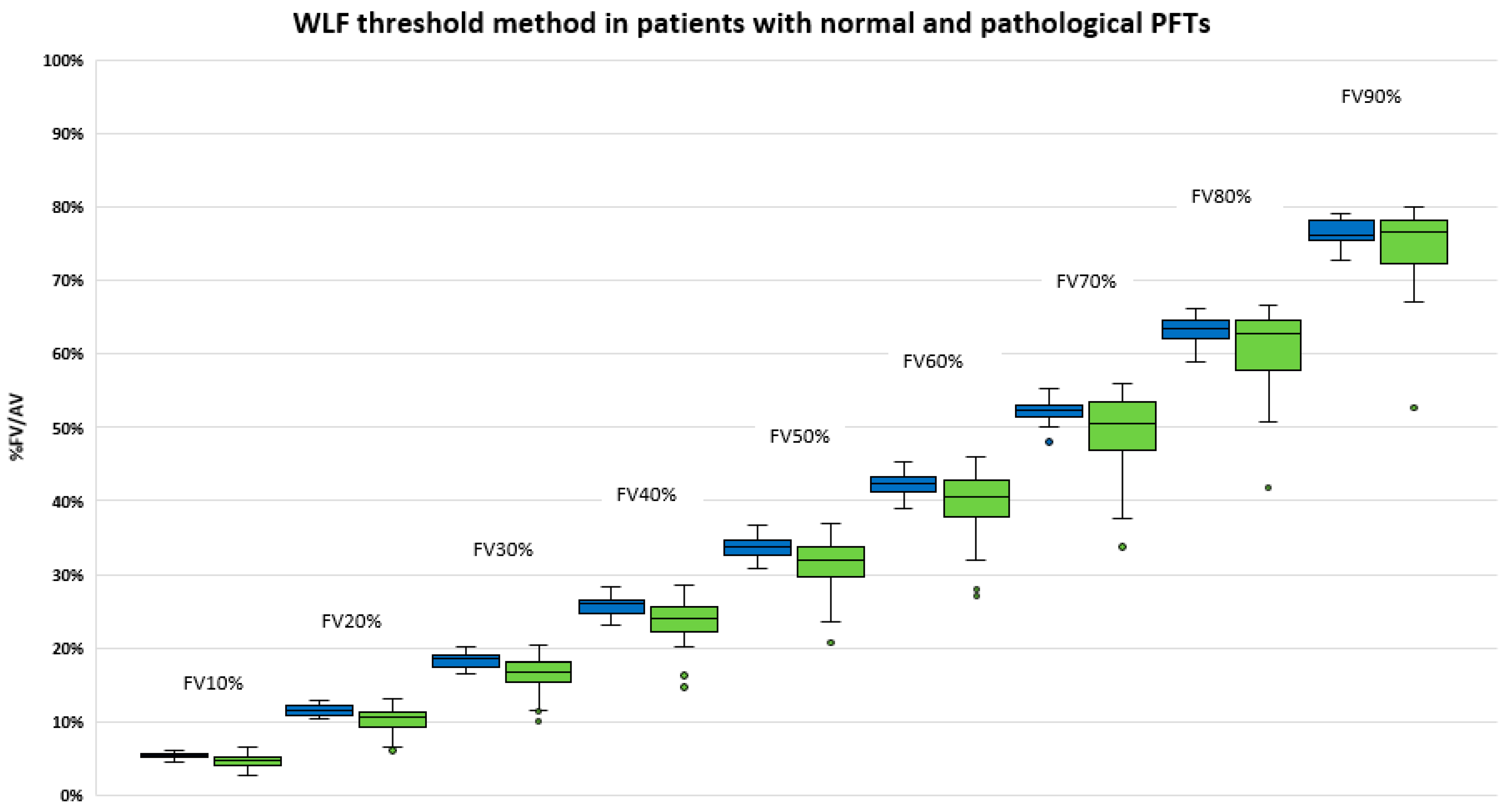
3.4. Comparison of Functional Volumes in Patients with or without Lung Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Le Roux, P.Y.; Hicks, R.J.; Siva, S.; Hofman, M.S. PET/CT Lung Ventilation and Perfusion Scanning using Galligas and Gallium-68-MAA. Semin. Nucl. Med. 2019, 49, 71–81. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Hennebicq, S.; Robin, P.; Tripier, R.; Salaün, P.-Y.; Le Roux, P.-Y. Radiopharmaceutical Labelling for Lung Ventilation/Perfusion PET/CT Imaging: A Review of Production and Optimization Processes for Clinical Use. Pharmaceuticals 2022, 15, 518. [Google Scholar] [CrossRef]
- Hicks, R.J.; Hofman, M.S. Is there still a role for SPECT-CT in oncology in the PET-CT era? Nat. Rev. Clin. Oncol. 2012, 9, 712–720. [Google Scholar] [CrossRef]
- Le Roux, P.Y.; Robin, P.; Salaun, P.Y. New developments and future challenges of nuclear medicine and molecular imaging for pulmonary embolism. Thromb. Res. 2018, 163, 236–241. [Google Scholar] [CrossRef]
- Hofman, M.S.; Beauregard, J.-M.; Barber, T.W.; Neels, O.C.; Eu, P.; Hicks, R.J. 68Ga PET/CT Ventilation–Perfusion Imaging for Pulmonary Embolism: A Pilot Study with Comparison to Conventional Scintigraphy. J. Nucl. Med. 2011, 52, 1513–1519. [Google Scholar] [CrossRef]
- Le Roux, P.-Y.; Iravani, A.; Callahan, J.; Burbury, K.; Eu, P.; Steinfort, D.P.; Lau, E.; Woon, B.; Salaun, P.Y.; Hicks, R.J.; et al. Independent and incremental value of ventilation/perfusion PET/CT and CT pulmonary angiography for pulmonary embolism diagnosis: Results of the PECAN pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1596–1604. [Google Scholar] [CrossRef]
- Le Roux, P.Y.; Leong, T.L.; Barnett, S.A.; Hicks, R.J.; Callahan, J.; Eu, P.; Manser, R.; Hofman, M.S. Gallium-68 perfusion positron emission tomography/computed tomography to assess pulmonary function in lung cancer patients undergoing surgery. Cancer Imaging 2016, 16, 24. [Google Scholar] [CrossRef]
- Siva, S.; Hardcastle, N.; Kron, T.; Bressel, M.; Callahan, J.; MacManus, M.; Shaw, M.; Plumridge, N.; Hicks, R.; Steinfort, D.; et al. Ventilation/Perfusion Positron Emission Tomography—Based Assessment of Radiation Injury to Lung. Int. J. Radiat. Oncol. 2015, 93, 408–417. [Google Scholar] [CrossRef]
- Siva, S.; Thomas, R.; Callahan, J.; Hardcastle, N.; Pham, D.; Kron, T.; Hicks, R.; MacManus, M.; Ball, D.L.; Hofman, M.S. High-resolution pulmonary ventilation and perfusion PET/CT allows for functionally adapted intensity modulated radiotherapy in lung cancer. Radiother. Oncol. 2015, 115, 157–162. [Google Scholar] [CrossRef]
- Bucknell, N.W.; Hardcastle, N.; Bressel, M.; Hofman, M.S.; Kron, T.; Ball, D.; Siva, S. Functional lung imaging in radiation therapy for lung cancer: A systematic review and meta-analysis. Radiother. Oncol. 2018, 129, 196–208. [Google Scholar] [CrossRef]
- Lucia, F.; Rehn, M.; Blanc-Béguin, F.; Le Roux, P.-Y. Radiation Therapy Planning of Thoracic Tumors: A Review of Challenges Associated with Lung Toxicities and Potential Perspectives of Gallium-68 Lung PET/CT Imaging. Front. Med. 2021, 8, 723748. [Google Scholar] [CrossRef]
- Le Roux, P.Y.; Siva, S.; Steinfort, D.P.; Callahan, J.; Eu, P.; Irving, L.B.; Hicks, R.J.; Hofman, M.S. Correlation of 68Ga Ventilation-Perfusion PET/CT with Pulmonary Function Test Indices for Assessing Lung Function. J. Nucl. Med. 2015, 56, 1718–1723. [Google Scholar] [CrossRef]
- Le Roux, P.Y.; Siva, S.; Callahan, J.; Claudic, Y.; Bourhis, D.; Steinfort, D.P.; Hicks, R.J.; Hofman, M.S. Automatic delineation of functional lung volumes with (68)Ga-ventilation/perfusion PET/CT. EJNMMI Res. 2017, 7, 82. [Google Scholar] [CrossRef]
- Lucia, F.; Hamya, M.; Pinot, F.; Goasduff, G.; Blanc-Béguin, F.; Bourhis, D.; Pradier, O.; Lucia, A.-S.; Hennebicq, S.; Mauguen, M.; et al. A Feasibility Study of Functional Lung Volume Preservation during Stereotactic Body Radiotherapy Guided by Gallium-68 Perfusion PET/CT. Cancers 2023, 15, 1726. [Google Scholar] [CrossRef]
- Blanc-Béguin, F.; Masset, J.; Robin, P.; Tripier, R.; Hennebicq, S.; Guilloux, V.; Vriamont, C.; Warnier, C.; Cogulet, V.; Eu, P.; et al. Fully Automated 68Ga-Labeling and Purification of Macroaggregated Albumin Particles for Lung Perfusion PET Imaging. Front. Nucl. Med. 2021, 1, 778191. [Google Scholar] [CrossRef]
- Decramer, M.; Janssens, W. Chronic obstructive pulmonary disease and comorbidities. Lancet Respir. Med. 2013, 1, 73–83. [Google Scholar] [CrossRef]
| Patients Characteristics | ||
|---|---|---|
| General Characteristics | No. of Patients (%) | |
| No. of patients | 60 | |
| Age (y) | 69 (IQ 63.0–72.3) | |
| Men | 31 (52%) | |
| Women | 29 (48%) | |
| Performans status | ||
| PS 0 | 27 (45%) | |
| PS 1 | 23 (39%) | |
| PS 2 | 8 (13%) | |
| Unknown | 2 (3%) | |
| Tobacco smoking | ||
| Unknown | 2 (3%) | |
| Never-smoker | 15 (25%) | |
| Former or current smoker | 43 (72%) | |
| Previous thoracic treatment | 33 (55%) | |
| Radiotherapy | 19 (32%) | |
| Lung surgery | 14 (23%) | |
| PFTs | ||
| Not performed or not interpretable | 12 (20%) | |
| Normal | 15 (25%) | |
| Abnormal (FEV1/FVC < 0.7 or DLCO < 0.6) | 33 (55%) | |
| Pmax Threshold Method | ||
| Volume (% of the AV) Median (IQ) | Activity (% of Total Activity in the AV) Median (IQ) | |
| 10%pmax | 93.8 (88.2–96.4) | 98.9 (97.4–99.4) |
| 20%pmax | 78.2 (65.3–85.8) | 92.0 (86.5–95.2) |
| 30%pmax | 54.2 (40.8–67.7) | 75.5 (62.1–84.0) |
| 40%pmax | 34.1 (19.6–47.3) | 55.3 (34.0–66.9) |
| 50%pmax | 18.3 (7.1–28.5) | 32.3 (14.0–45.3) |
| 60%pmax | 6.6 (2.2–13.3) | 14.5 (5.6–24.3) |
| WLF Threshold Method | ||
| Volume (% of the AV) Median (IQ) | Corresponding %pmax threshold Median (IQ) | |
| FV10% | 5.0 (4.4–5.5) | 63.0 (52.8–69.3) |
| FV20% | 10.9 (9.7–11.8) | 55.5 (46.0–62.3) |
| FV30% | 17.6 (16.1–18.8) | 51.0 (41.0–57.0) |
| FV40% | 24.4 (23.0–26.3) | 46.5 (37.0–52.0) |
| FV50% | 32.6 (30.7–34.2) | 42.0 (33.8–48.0) |
| FV60% | 41.4 (39.0–43.1) | 38.0 (30.8–43.3) |
| FV70% | 51.9 (48.3–53.3) | 33.0 (26.5–38.3) |
| FV80% | 62.8 (60.0–64.5) | 27.0 (22.8–33.0) |
| FV90% | 76.1 (73.7–78.2) | 21.0 (17.0–26.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinot, F.; Bourhis, D.; Bourbonne, V.; Floch, R.; Mauguen, M.; Blanc-Béguin, F.; Schick, U.; Hamya, M.; Abgral, R.; Le Gal, G.; et al. New Automated Method for Lung Functional Volumes Delineation with Lung Perfusion PET/CT Imaging. Cancers 2023, 15, 2166. https://doi.org/10.3390/cancers15072166
Pinot F, Bourhis D, Bourbonne V, Floch R, Mauguen M, Blanc-Béguin F, Schick U, Hamya M, Abgral R, Le Gal G, et al. New Automated Method for Lung Functional Volumes Delineation with Lung Perfusion PET/CT Imaging. Cancers. 2023; 15(7):2166. https://doi.org/10.3390/cancers15072166
Chicago/Turabian StylePinot, Fanny, David Bourhis, Vincent Bourbonne, Romain Floch, Maelle Mauguen, Frédérique Blanc-Béguin, Ulrike Schick, Mohamed Hamya, Ronan Abgral, Grégoire Le Gal, and et al. 2023. "New Automated Method for Lung Functional Volumes Delineation with Lung Perfusion PET/CT Imaging" Cancers 15, no. 7: 2166. https://doi.org/10.3390/cancers15072166
APA StylePinot, F., Bourhis, D., Bourbonne, V., Floch, R., Mauguen, M., Blanc-Béguin, F., Schick, U., Hamya, M., Abgral, R., Le Gal, G., Salaün, P.-Y., Lucia, F., & Le Roux, P.-Y. (2023). New Automated Method for Lung Functional Volumes Delineation with Lung Perfusion PET/CT Imaging. Cancers, 15(7), 2166. https://doi.org/10.3390/cancers15072166





