Simple Summary
Cancer initiation and progression has been studied in purely genetic terms for decades. Implicit in this view is that all cells in a tissue are phenotypically identical until a single cell acquires a set of mutations that will eventually lead to malignancy. This model does not fully account for numerous clinical and epidemiological findings about cancer incidence and progression. Here, we summarize recent mathematical and biological insights that demonstrate how cells in a tissue can switch between dramatically different phenotypes independent of mutations. We explore how these insights, combined with our detailed understanding of oncogenic mutations, may answer key unexplained aspects of pancreatic ductal adenocarcinoma initiation. Importantly, such a combined model allows for a more nuanced understanding of pre-malignancy, and points the way towards early detection and intervention approaches in high-risk patients.
Abstract
While much of the research in oncogenesis and cancer therapy has focused on mutations in key cancer driver genes, more recent work suggests a complementary non-genetic paradigm. This paradigm focuses on how transcriptional and phenotypic heterogeneity, even in clonally derived cells, can create sub-populations associated with oncogenesis, metastasis, and therapy resistance. We discuss this complementary paradigm in the context of pancreatic ductal adenocarcinoma. A better understanding of cellular transcriptional heterogeneity and its association with oncogenesis can lead to more effective therapies that prevent tumor initiation and slow progression.
1. Introduction
1.1. Genetic Origins of Cancer
The accumulation of mutations in the cells of origin of a tumor is a key step in the initiation and maintenance of oncogenesis [1,2]. This is certainly true of pancreatic ductal adenocarcinoma (PDAC), where a great majority of PDAC tumors contain mutations in the KRAS gene. Mouse models where KRASG12D is induced in the pancreas grow tumors that mimic key clinical aspects of human PDAC tumor progression [3]. Intraductal papillary mucinous neoplasms (IPMNs) and pancreatic intraepithelial neoplasms (PanINs), which are common precursor lesions to PDAC in humans, harbor KRASG12D mutations in nearly 40–60% (IPMN) and 90% (PanIN) of cases [4,5]. While the cell-of-origin in PDAC is debated, PDACs can be derived from both pancreatic acinar cells (which make up nearly 90% of the pancreas) and ductal cells in mouse models [6].
1.2. Genetics Does Not Completely Explain Cancer Initiation
This purely genetic view of PDAC initiation implicitly assumes that every pancreatic epithelial cell is equally likely to initiate PDAC until one of them acquires the requisite oncogenic mutations. The role of factors such as diet, smoking and family history in increasing PDAC risk [7] are thought to ultimately change mutation rates in epithelial cells. This is thought to occur either directly through germline mutations or indirectly via tissue inflammation that causes increased epithelial cell division. This line of thinking cannot easily explain certain clinical observations, such as the fact that pancreatic main-duct IPMNs are more likely to lead to PDAC than branch-duct IPMNs [5], that PDAC arises more often in the head of the pancreas than the tail [8], or that a single bout of pancreatitis elevates the risk of PDAC for up to ten years after recovery [9]. These observations may be understood via notions of phenotypic heterogeneity across clonally derived cells. Decades of theoretical and experimental work have demonstrated that clonally derived cells exhibit phenotypic heterogeneity and where the heredity of a cellular state can be encoded by gene regulatory network dynamics [10]. In accordance, it has been shown that precursor cells are more susceptible to specific driver mutations than differentiated cells, and such oncogenic competence is correlated with the chromatin state of the developmental program [11]. Observations of pancreatic epithelial cells during injury and homeostasis, for instance, suggest that different sub-populations of epithelial cells divide during our lifetime [8]. These observations and theories suggest that thinking about cancer initiation through the lens of cellular states (quantified via transcriptomics or other methods) may provide a fruitful unification of the purely genetic and the purely systems-biology views of cancer, and lead to newer therapeutic avenues.
1.3. Stochastic Gene Expression and Regulatory Networks Result in Transcriptional Heterogeneity within a Cell Type
A regulatory network refers to the set of interactions, transcriptional or otherwise, between all the genes in a cell. Regulatory networks influence the transcriptional state of a cell, which can be defined as the global gene expression profile of the cell. Early mathematical models [12,13] of gene regulation suggested that each cell type in a multicellular organism can be thought of as a stable transcriptional state. Stability implies that a cell is impervious to many perturbations, i.e., minor changes in its microenvironment, or stochastic changes within a cell, do not dramatically alter its transcriptomic identity or phenotype. The observed statistical clustering of cells by type in single-cell RNA-seq atlases from multiple species support this notion, though statistically distinguishing between different states of a given cell type on the one hand, and different cell types on the other, can be a challenge in such datasets [14]. A cell of a given type is “attracted” towards one of its stable states when it is perturbed by either minor changes in its microenvironment [15] or the fluctuating expression of genes [16] within the cell. Accordingly, at a given point in time, all cells of a given type exhibit transcriptional heterogeneity. Some cell types are more “plastic” than others, i.e., they are able to switch between dramatically different transcriptional states, often under the influence of external signals. The amount of transcriptomic (and ultimately, phenotypic) variation across cells of a given type is a trait under the control of natural selection, with low variation (and robustness to changes in external stimuli) being desirable in some contexts, such as embryonic development [17], and high variation in others, such as immune responses [18]. This pervasive transcriptional heterogeneity has implications not only in development and homeostasis, but also in cancer.
1.4. Transcriptional Heterogeneity in Cancer
Cancer is characterized by major transcriptional/epigenetic changes not only in cells of origin but also in other cell types that jointly constitute the tumor microenvironment (TME). The cancer phenotype is ultimately a result of dynamically changing interactions between various cell types in dynamically varying states. Much of the research in oncogenesis, metastasis, drug resistance and relapse has focused on protein-coding mutations. However, a growing body of literature clearly points to an alternate mechanism centered around transcriptional heterogeneity and cellular plasticity that sets the stage for oncogenesis and plays critical roles in tumor progression, metastasis, and therapeutic response. As an example, an early study of normal human mammary epithelial cell culture showed that these cells interconverted between two transcriptionally and phenotypically different states marked by the expression of CD44. CD44hi cells were more stem-like, and additionally, oncogenically transformed CD44hi cells formed tumors in mice more rapidly than transformed CD44low cells [19]. The presence of such populations is dependent on cell culture conditions [20]. Sorted CD44low cell populations diversified over time and switched to a CD44hi state. Furthermore, mammary epithelial cells that detach from the matrix in 3D cultures exhibit gene expression profiles similar to those in pre-malignant breast lesions [21]. As yet another example, using a mouse model of tumor progression from pre-neoplastic hyperplasia to lung adenocarcinoma, Marjanovic et al. identified a high plasticity cell state (HPCS) in pre-malignant TIGIT-positive lung lesions exhibiting high growth and differentiation potential towards a malignant state [22]. HPCS in human lung adenocarcinoma tumors is associated with poor survival and greater resistance to chemotherapy in preclinical studies. Non-genetic variation in expression also plays a key role in therapy resistance, with vemurafenib resistance in melanoma being a prominent example. A fraction of cells in clonally derived vemurafenib-sensitive cell lines can stochastically express AXL and resist vemurafenib treatment [23] without acquiring a mutation. Thus, non-genetic heterogeneity can prime epithelial cells for oncogenic transformation and some cancer cells to escape therapy. It is likely that this phenotype can be fixed in a cell population through epigenetic or genetic alterations.
2. Transcriptional Heterogeneity and Pancreatic Ductal Adenocarcinoma Initiation
2.1. Acinar Heterogeneity in Pancreas Homeostasis
An early single-cell RNA-seq study of 108 pancreatic acinar cells from mice [24] detected a sub-population marked by a high expression of STMN1 (a protein that regulates microtubule assembly [25]) that constituted 1% of acinar cells but expanded to 30% during response to pancreatic injury. Another single-cell RNA-seq study of the human pancreas [26] found an acinar sub-population expressing the REG3A protein, a secreted bacterial C-type lectin, which is up-regulated in pancreatitis patients [27] and is involved in the transdifferentiation of acinar cells to ductal cells. A more detailed single-nucleus RNA-seq study of the human pancreas [28] confirmed the presence of REG3A-expressing acinar cells in the homeostatic pancreas.
The presence of cellular heterogeneity among acinar cells, and specifically, the presence of REG3A-expressing cells in the homeostatic pancreas, raises the question of whether there exists an acinar sub-population that has higher oncogenic potential. Our group developed a set of statistical tests to detect such “edge cells” [29] in an extensive re-analysis of all available pancreas and PDAC single-cell RNA-seq data [30]. We found that acinar cells, but not ductal cells, from histologically normal pancreas tissues possessed an edge sub-population. Edge cells were statistically farther (in a transcriptomic space) from the average acinar cell, while transcriptionally “drifting” towards a PDAC state. We found that these edge cells up-regulated STMN1 in addition to the transcription factors (TFs) SOX9 and PTF1A, which mark multipotent progenitors in embryonic pancreatic tissue [31]. Remarkably, we also found edge cells in varying proportions across different healthy human pancreases from single-cell RNA-seq datasets, with a strong positive correlation between the fraction of edge-like cells and the donor’s age. Crucially, through somatic variant calling, we ruled out the possibility that edge-like cells were clonally derived. A later study [32] found that inducing KRASG12D expression in mouse pancreatic acinar cells exhibiting a high level of telomerase (Terthi acinar cells) generated larger numbers of acinar progeny than Tertlow acinar cells, implying that Terthi cells better fit in an oncogenic context. However, as single-cell RNA-seq was not carried out in this study, it is unclear if Terthi cells are identical to the edge sub-population we detected.
2.2. Alternative Non-Genetic Paradigm to Oncogenesis
Our finding that the fraction of edge-like acinar cells in the pancreas increases with age while not being descended from a single ancestor cell may present an alternative to the prevalent paradigm of oncogenesis. Transcriptional changes induced by oncogenic mutations are believed to be a key step in cancer initiation. In tissues such as the skin, colon, and esophagus epithelium [33,34,35] stem-cell clones (i.e., stem cells and their progeny) accumulate mutations over time, with some mutations being oncogenic in nature. However, the existence of progenitor or stem cells within the adult pancreas is controversial. Current lineage-tracing studies [24,32] show acinar cells to be non-cycling during homeostasis until injury induction. Recovery from injury involves acinar cell plasticity, where there is a large but transient increase in the fraction of cycling acinar cells, eventually leading to a homeostatic state devoid of cycling acinar cells. This raises the question of the sufficiency of oncogenic mutations for PDAC initiation. An early mouse model showed that inducing KRASG12V expression in adult acinar cells did not lead to pre-malignant lesions in the pancreas [36], while KRASG12D expression in acinar cells resulted in PanIN formation in adult mice [37]. Remarkably, a more recent study found that when KrasG12D was expressed in a small fraction of acinar cells in mice, they were eliminated by their wild-type counterparts [38]. This is believed to be due to cell competition [39], a phenomenon seen in development where “fitter” epithelial cells can induce apoptosis in their less-fit neighbors. Thus, the presence of a KRASG12D mutation in a cell does not necessarily lead to oncogenic transformation. This is because the survival of a KRASG12D cell requires it to be fitter than its wild-type counterparts, which is in turn likely to depend on the tissue microenvironment. Tissue inflammation during pancreatitis resolution cooperates with KRASG12D-induced transcriptional changes, resulting in PDAC [40]. The inflammation likely provides the microenvironment in which KRASG12D cells outcompete KRASWT cells and proceed towards malignancy. Our edge-cell signature was up-regulated across all bulk RNA-seq samples of whole pancreatic extracts in mice recovering from pancreatitis induced by caerulein administration, as well as in single-nucleus RNA-seq data of acinar cells in chronic pancreatitis patients [30]. The magnitude of up-regulation was more pronounced in mice bearing KRASG12D mutations than in KRASWT mice. This link between inflammation and cancer initiation is in line with chronic pancreatitis being a risk factor for PDAC and may be interpreted within the paradigm of a tumor as a wound that does not heal [41].
2.3. Aging Microenvironment, Edge Cells, Increased Oncogenesis
In human pancreatic islet cells, aging is accompanied by phenotype drift and overall increase in inter-cell transcriptomic variation [42] as the accumulated mutations match signatures of aging. Similar observations have been made across organs in the Tabula Muris Senis cohort [43] of single-cell RNA-seq data across organs over the mouse lifespan. Cells from older mice are easier to oncogenically transform [44], as the deteriorating microenvironment’s aging can promote oncogenesis either via mechanisms related to senescence or stiffening of the extracellular matrix [45,46]. Our analysis finds an accumulation of edge-like cells with ageing but does not provide any obvious explanation. Future work will have to tease apart the contribution of microenvironmental changes with age and other cell-autonomous factors, such as changes in mitochondrial function, DNA methylation and other epigenetic marks underlying this trend.
2.4. Tissue-Specific Oncogenic Effects and Links between KRASG12D Mutation and Edge State
The question of why the KRASG12D mutation is a common driver of PDAC is unclear. KRASG12D mutations are common only in lung, pancreatic and colorectal adenocarcinomas and are thus not as much of a pan-cancer driver when compared to TP53 loss. As a rule, most driver mutations tend to be tissue-specific [47] and the reasons for this are not established. Our analysis reveals an insight into why KRASG12D in particular may drive PDAC. We discovered [30] that transcriptomic differences between edge and non-edge acinar cells in mice were highly correlated to transcriptomic differences between acinar cells from KRASG12D and KRASWT mice (Figure 1C). This suggests that the edge state is transcriptionally very similar to the KRASG12D-driven state, which may mean that the edge state is susceptible to oncogenic transformation in a similar manner to KRASG12D induction. It is also possible that the KRASG12D mutation may either increase the rate of transition from the non-edge to the edge state or increase the stability of the edge state. Further experiments and analyses are required to determine whether KRASG12D is the only mutation that has this effect, or whether others that commonly occur in pre-malignant pancreatic lesions, such as GNAS [4], have similar effects.
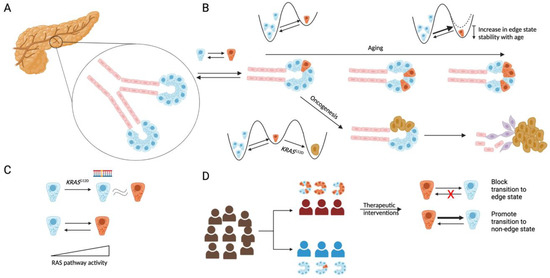
Figure 1.
(A) Schematic of the human pancreas, with the exocrine compartment consisting of acinar cells (blue) and ductal cells (red). (B) Acinar cells can switch to an edge state (brown) characterized by low expression of acinar identity genes. The fraction of edge cells increases with age, which may be due to an increased transition rate to the edge state or an increase in the stability of the edge state, or a combination thereof. Cells in the edge state are predisposed to malignant transformation with the acquisition of a KRASG12D mutation, leading to malignancy, with malignant cells existing in different phenotypic states (purple or brown) (C) Edge cells have high Ras activity, and the edge-like state phenocopies (at a transcriptomic level) the KRASG12D mutation in mice. (D) Acinar cells likely exist in an edge state in patients that are at a high risk for PDAC. Therapeutic interventions to block transitions to an edge state, or promote transitions away from the edge state, may lower PDAC risk.
As mentioned above, traits that lead to high gene expression heterogeneity can undergo natural selection if it confers survival benefits to an organism. Given the relationships between the edge state, pancreatitis resolution, and PDAC, it is worth speculating on whether the edge state represents a mal-adaptation or an adaptation. On the one hand, aging is associated with an increase in transcriptional heterogeneity [42,43], suggesting increasing dysregulation over this process. This is consistent with the notion that natural selection is less effective in purging mutations, whose effect is apparent late in life [48]. On the other hand, if cells in the edge state are primed to respond to pancreatic injury, then selection might maintain the ability of acinar cells to switch to an edge state to allow for quicker injury resolution. When the pancreas is challenged with injury after recovering from an earlier one, the second injury causes far less damage [49]. Interestingly, this study found that this priming for injury response is coupled with a higher susceptibility to KRAS-driven oncogenesis than cells from an uninjured pancreas. A similar observation has been made in mouse skin, where successive injuries are responded to much more quickly than the first injury [50], although the oncogenic potential of these epithelial cells was not evaluated.
3. Perspective and Future Directions
The transcriptional state of a cell is a consequence of regulatory interactions between genes as well as cell-intrinsic and cell-extrinsic stochastic fluctuations in gene expression [51,52], both of which can be influenced by genetics as well as epigenetics. Given that the acinar edge cells, to the best of our knowledge, are not induced by specific mutations, the heritability of the edge state needs to be better investigated, i.e., if an edge acinar cell were to divide, would its daughter cells also stay in an edge state? Over how many cell divisions does the edge state persist? If the edge state is a non-dividing state, how long does an edge state persist before transitioning to a non-edge state? Epigenetic memory can be maintained over tens of cell divisions [53,54,55]. Is that sufficient to affect a fate decision? Carefully designed lineage tracing experiments combined with single-cell multi-omic profiling are needed to definitively answer these questions.
Early detection is a challenge in PDAC treatment, and indeed in most cancers. Early detection is particularly challenging in the case of PDAC given the relatively low incidence rate of the disease [9]. Given that 90% of PDAC cases are sporadic and do not involve familial history or inherited genetic disease [56], defining a high-risk cohort for screening is a challenge (Figure 1D). However, given that the edge state potentially sits at the cross-roads of pancreatitis and PDAC, the genes up-regulated within the edge state may serve as potential biomarkers of PDAC. The ability of computational methods to impute gene expression within the pancreas and other tissues based on RNA-seq and epigenetic data from blood withdrawn [57] might provide non-invasively accessible biomarkers related to the edge state.
The development of an early detection test is likely to lead to a decrease in PDAC-related mortality but does not immediately suggest a therapy. Aside from the development of an early detection test, ongoing works point to possible early interventions amongst patients with a high risk of PDAC. In the case of colorectal cancer, conditions such as ulcerative colitis and Crohn’s disease are known to increase cancer risk [58,59]. Patients who managed these conditions through the administration of anti-TNF antibodies had a lower risk of colorectal cancer [60,61]. This perhaps suggests a general principle that reversing the state of inflammation within a tissue may reduce cancer risk, which may also partly explain the lower risk of lung, colorectal and bladder cancer in smokers who have reduced cigarette usage [62,63,64]. The prioritization of drugs that can effectively revert a pathological transcriptomic state has led to repurposed drugs for cancer and other diseases [65]. Drugs that reverse the edge signature may thus represent candidates that lower PDAC risk in high-risk cohorts. Future research on effective ways to reverse cellular states presents a promising complementary avenue to combat cancer-related mortality.
Author Contributions
V.G. and S.H. jointly conceived and wrote this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded, in part, by U.S. National Cancer Institute grant 1-ZIA-BC011979-02 and was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, NIH.
Acknowledgments
We would like to thank Chi-Ping Day and Efsun Arda for their feedback.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alkhayyat, M.; Abureesh, M.; Gill, A.; Khoudari, G.; Saleh, M.A.; Mansoor, E.; Regueiro, M. Lower Rates of Colorectal Cancer in Patients with Inflammatory Bowel Disease Using Anti-TNF Therapy. Inflamm. Bowel Dis. 2020, 27, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Almanzar, N.; Antony, J.; Baghel, A.S.; Bakerman, I.; Bansal, I.; Barres, B.A.; Beachy, P.A.; Berdnik, D.; Bilen, B.; Brown-field, D.; et al. A single-cell transcriptomic atlas characterizes ageing tissues in the mouse. Nature 2020, 583, 590–595. [Google Scholar] [CrossRef]
- Alonso-Curbelo, D.; Ho, Y.-J.; Burdziak, C.; Maag, J.L.V.; Iv, J.P.M.; Chandwani, R.; Chen, H.-A.; Tsanov, K.M.; Barriga, F.M.; Luan, W.; et al. A gene–environment-induced epigenetic program initiates tumorigenesis. Nature 2021, 590, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Arda, H.E.; Benitez, C.M.; Kim, S.K. Gene Regulatory Networks Governing Pancreas Development. Dev. Cell 2013, 25, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.; Doll, R. The Age Distribution of Cancer and a Multi-stage Theory of Carcinogenesis. Br. J. Cancer 1954, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Baggiolini, A.; Callahan, S.J.; Montal, E.; Weiss, J.M.; Trieu, T.; Tagore, M.M.; Tischfield, S.E.; Walsh, R.M.; Suresh, S.; Fan, Y.; et al. Developmental chromatin programs determine oncogenic competence in melanoma. Science 2021, 373, eabc1048. [Google Scholar] [CrossRef]
- Bahcecioglu, G.; Yue, X.; Howe, E.; Guldner, I.; Stack, M.S.; Nakshatri, H.; Zhang, S.; Zorlutuna, P. Aged Breast Extracellular Matrix Drives Mammary Epithelial Cells to an Invasive and Cancer-Like Phenotype. Adv. Sci. 2021, 8, 2100128. [Google Scholar] [CrossRef]
- Baker, N.E. Emerging mechanisms of cell competition. Nat. Rev. Genet. 2020, 21, 683–697. [Google Scholar] [CrossRef]
- Basu, M.; Wang, K.; Ruppin, E.; Hannenhalli, S. Predicting tissue-specific gene expression from whole blood transcriptome. Sci. Adv. 2021, 7, eabd6991. Available online: http://Dx.Doi.Org/10.1517/14728222.2011.620951 (accessed on 22 March 2023). [CrossRef]
- Belletti, B.; Baldassarre, G. Stathmin: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin. Ther. Targets 2011, 15, 1249–1266. [Google Scholar] [CrossRef] [PubMed]
- Bheda, P.; Gómez, D.A.; Becker, N.B.; Becker, J.; Stavrou, E.; Kukhtevich, I.; Höfer, T.; Maerkl, S.; Charvin, G.; Marr, C.; et al. Single-Cell Tracing Dissects Regulation of Maintenance and Inheritance of Transcriptional Reinduction Memory. Mol. Cell 2020, 78, 915–925.e7. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Brueckmann, I.; Scheel, C.; Kaestli, A.J.; Wiggins, P.A.; Rodrigues, L.O.; Brooks, M.; Reinhardt, F.; Su, Y.; Polyak, K.; et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proc. Natl. Acad. Sci. USA 2011, 108, 7950–7955. [Google Scholar] [CrossRef] [PubMed]
- Charkaoui, M.; Hajage, D.; Tubach, F.; Beaugerie, L.; Kirchgesner, J. Impact of Anti-tumour Necrosis Factor Agents on the Risk of Colorectal Cancer in Patients with Ulcerative Colitis: Nationwide French Cohort Study. J. Crohn’s Colitis 2021, 16, 893–899. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Ma, L.; Paik, H.; Sirota, M.; Wei, W.; Chua, M.-S.; So, S.; Butte, A.J. Reversal of cancer gene expression correlates with drug efficacy and reveals therapeutic targets. Nat. Commun. 2017, 8, 16022. [Google Scholar] [CrossRef] [PubMed]
- DeGregori, J.; Weinberg, R.A.; DeGregori, M. Adaptive Oncogenesis, A New Understanding of How cancer Evolve Inside Us; Harvard University Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Del Poggetto, E.; Ho, I.-L.; Balestrieri, C.; Yen, E.-Y.; Zhang, S.; Citron, F.; Shah, R.; Corti, D.; Diaferia, G.R.; Li, C.-Y.; et al. Epithelial memory of inflammation limits tissue damage while promoting pancreatic tumorigenesis. Science 2021, 373, eabj0486. [Google Scholar] [CrossRef]
- Dragotakes, Q.; Stouffer, K.M.; Fu, M.S.; Sella, Y.; Youn, C.; Yoon, O.I.; De Leon-Rodriguez, C.M.; Freij, J.B.; Bergman, A.; Casadevall, A. Macrophages use a bet-hedging strategy for antimicrobial activity in phagolysosomal acidification. J. Clin. Investig. 2020, 130, 3805–3819. [Google Scholar] [CrossRef]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal—Redux. Cancer Immunol. Res. 2015, 3, 1–11. [Google Scholar] [CrossRef]
- Enge, M.; Arda, H.E.; Mignardi, M.; Beausang, J.; Bottino, R.; Kim, S.K.; Quake, S.R. Single-Cell Analysis of Human Pancreas Reveals Transcriptional Signatures of Aging and Somatic Mutation Patterns. Cell 2017, 171, 321–330.e14. [Google Scholar] [CrossRef]
- Fane, M.; Weeraratna, A.T. How the ageing microenvironment influences tumour progression. Nat. Rev. Cancer 2019, 20, 89–106. [Google Scholar] [CrossRef]
- Flowers, B.M.; Xu, H.; Mulligan, A.S.; Hanson, K.J.; Seoane, J.A.; Vogel, H.; Curtis, C.; Wood, L.D.; Attardi, L.D. Cell of Origin Influences Pancreatic Cancer Subtype. Cancer Discov. 2021, 11, 660–677. [Google Scholar] [CrossRef]
- Gopalan, V.; Singh, A.; Mehrabadi, F.R.; Wang, L.; Ruppin, E.; Arda, H.E.; Hannenhalli, S. A Transcriptionally Distinct Subpopulation of Healthy Acinar Cells Exhibit Features of Pancreatic Progenitors and PDAC. Cancer Res. 2021, 81, 3958–3970. [Google Scholar] [CrossRef]
- Guerra, C.; Schuhmacher, A.J.; Cañamero, M.; Grippo, P.J.; Verdaguer, L.; Pérez-Gallego, L.; Dubus, P.; Sandgren, E.P.; Barbacid, M. Chronic Pancreatitis Is Essential for Induction of Pancreatic Ductal Adenocarcinoma by K-Ras Oncogenes in Adult Mice. Cancer Cell 2007, 11, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.B.; Pastushenko, I.; Skibinski, A.; Blanpain, C.; Kuperwasser, C. Phenotypic Plasticity: Driver of Cancer Initiation, Progression, and Therapy Resistance. Cell Stem Cell 2018, 24, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Habbe, N.; Shi, G.; Meguid, R.A.; Fendrich, V.; Esni, F.; Chen, H.; Feldmann, G.; Stoffers, D.A.; Konieczny, S.F.; Leach, S.D.; et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell tar-geting of oncogenic Kras in adult mice. Proc. Natl. Acad. Sci. USA 2008, 105, 18913–18918. [Google Scholar] [CrossRef] [PubMed]
- Haigis, K.M.; Cichowski, K.; Elledge, S.J. Tissue-specificity in cancer: The rule, not the exception. Science 2019, 363, 1150–1151. [Google Scholar] [CrossRef] [PubMed]
- Halpern, K.B.; Shenhav, R.; Massalha, H.; Toth, B.; Egozi, A.; E Massasa, E.; Medgalia, C.; David, E.; Giladi, A.; E Moor, A.; et al. Paired-cell sequencing enables spatial gene expression mapping of liver endothelial cells. Nat. Biotechnol. 2018, 36, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Hill, W.; Zaragkoulias, A.; Salvador-Barbero, B.; Parfitt, G.J.; Alatsatianos, M.; Padilha, A.; Porazinski, S.; Woolley, T.E.; Morton, J.P.; Sansom, O.J.; et al. EPHA2-dependent outcompetition of KRASG12D mutant cells by wild-type neighbors in the adult pancreas. Curr. Biol. 2021, 31, 2550–2560.e5. [Google Scholar] [CrossRef] [PubMed]
- Huang, S. Non-genetic heterogeneity of cells in development: More than just noise. Development 2009, 136, 3853–3862. [Google Scholar] [CrossRef]
- Huang, S. On the intrinsic inevitability of cancer: From foetal to fatal attraction. Semin. Cancer Biol. 2011, 21, 183–199. [Google Scholar] [CrossRef]
- Jiang, Z.; White, R.A.; Wang, T.C. Adult Pancreatic Acinar Progenitor-like Populations in Regeneration and Cancer. Trends Mol. Med. 2020, 26, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Matthaei, H.; Wu, J.; Hong, S.M.; Yu, J.; Borges, M.; Hruban, R.H.; Maitra, A.; Kinzler, K.; Vogelstein, B.; et al. Presence of somatic mutations in most early-stage pancreatic intraepithelial neoplasia. Gastroenterology 2012, 142, 730–733.e9. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S. Homeostasis and Differentiation in Random Genetic Control Networks. Nature 1969, 224, 177–178. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Li, Q.; Wennborg, A.; Aurell, E.; Dekel, E.; Zou, J.-Z.; Xu, Y.; Huang, S.; Ernberg, I. Dynamics inside the cancer cell attractor reveal cell heterogeneity, limits of stability, and escape. Proc. Natl. Acad. Sci. USA 2016, 113, 2672–2677. [Google Scholar] [CrossRef]
- Little, S.C.; Tikhonov, M.; Gregor, T. Precise Developmental Gene Expression Arises from Globally Stochastic Transcriptional Activity. Cell 2013, 154, 789–800. [Google Scholar] [CrossRef]
- Longo, D.L.; Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic Adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Marjanovic, N.D.; Hofree, M.; Chan, J.E.; Canner, D.; Wu, K.; Trakala, M.; Hartmann, G.G.; Smith, O.C.; Kim, J.Y.; Evans, K.V.; et al. Emergence of a High-Plasticity Cell State during Lung Cancer Evolution. Cancer Cell 2020, 38, 229–246.e13. [Google Scholar] [CrossRef]
- Martincorena, I.; Fowler, J.C.; Wabik, A.; Lawson, A.R.J.; Abascal, F.; Hall, M.W.J.; Cagan, A.; Murai, K.; Mahbubani, K.; Stratton, M.R.; et al. Somatic mutant clones colonize the human esophagus with age. Science 2018, 362, 911–917. [Google Scholar] [CrossRef]
- Martincorena, I.; Roshan, A.; Gerstung, M.; Ellis, P.; Van Loo, P.; McLaren, S.; Wedge, D.C.; Fullam, A.; Alexandrov, L.B.; Tubio, J.M.; et al. Tumor evolution. High burden and pervasive positive selection of somatic mutations in normal human skin. Science 2015, 348, 880–886. [Google Scholar] [CrossRef]
- Muraro, M.J.; Dharmadhikari, G.; Grün, D.; Groen, N.; Dielen, T.; Jansen, E.; van Gurp, L.; Engelse, M.A.; Carlotti, F.; de Koning, E.J.; et al. A Single-Cell Transcriptome Atlas of the Human Pancreas. Cell Syst. 2016, 3, 385–394.e3. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Larsen, S.B.; Gomez, N.C.; Alaverdyan, K.; Sendoel, A.; Yuan, S.; Polak, L.; Kulukian, A.; Chai, S.; Fuchs, E. Inflammatory memory sensitizes skin epithelial stem cells to tissue damage. Nature 2017, 550, 475–480. [Google Scholar] [CrossRef]
- Neuhöfer, P.; Roake, C.M.; Kim, S.J.; Lu, R.J.; West, R.B.; Charville, G.W.; Artandi, S.E. Acinar cell clonal expansion in pancreas homeostasis and carcinogenesis. Nature 2021, 597, 715–719. [Google Scholar] [CrossRef]
- Nicholson, A.M.; Olpe, C.; Hoyle, A.; Thorsen, A.-S.; Rus, T.; Colombé, M.; Brunton-Sim, R.; Kemp, R.; Marks, K.; Quirke, P.; et al. Fixation and Spread of Somatic Mutations in Adult Human Colonic Epithelium. Cell Stem Cell 2018, 22, 909–918.e8. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, R.; Morikawa, T.; Kuchiba, A.; Lochhead, P.; Yamauchi, M.; Liao, X.; Imamura, Y.; Nosho, K.; Shima, K.; Kawachi, I.; et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am. J. Epidemiol. 2013, 178, 84–100. [Google Scholar] [CrossRef]
- Nowell, P.C. The clonal evolution of tumor cell populations. Science 1976, 194, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in ulcerative colitis: A Scandinavian population-based cohort study. Lancet 2020, 395, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Olén, O.; Erichsen, R.; Sachs, M.C.; Pedersen, L.; Halfvarson, J.; Askling, J.; Ekbom, A.; Sørensen, H.T.; Ludvigsson, J.F. Colorectal cancer in Crohn’s disease: A Scandinavian population-based cohort study. Lancet Gastroenterol. Hepatol. 2020, 5, 475–484. [Google Scholar] [CrossRef]
- Ozbudak, E.M.; Thattai, M.; Kurtser, I.; Grossman, A.D.; van Oudenaarden, A. Regulation of noise in the expression of a single gene. Nat. Genet. 2002, 31, 69–73. [Google Scholar] [CrossRef]
- Pérez–Mancera, P.A.; Guerra, C.; Barbacid, M.; Tuveson, D.A. What We Have Learned About Pancreatic Cancer from Mouse Models. Gastroenterology 2012, 142, 1079–1092. [Google Scholar] [CrossRef]
- Pisco, A.O.; Fouquier d’Herouel, A.; Huang, S. Conceptual Confusion: The case of Epigenetics. BioRxiv 2016, 053009. [Google Scholar] [CrossRef]
- Rando, O.J.; Verstrepen, K.J. Timescales of Genetic and Epigenetic Inheritance. Cell 2007, 128, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef]
- Reitsma, M.; Kendrick, P.; Anderson, J.; Arian, N.; Feldman, R.; Gakidou, E.; Gupta, V. Reexamining Rates of Decline in Lung Cancer Risk after Smoking Cessation. A Meta-analysis. Ann. Am. Thorac. Soc. 2020, 17, 1126–1132. [Google Scholar] [CrossRef]
- Rink, M.; Furberg, H.; Zabor, E.C.; Xylinas, E.; Babjuk, M.; Pycha, A.; Lotan, Y.; Karakiewicz, P.I.; Novara, G.; Robinson, B.D.; et al. Impact of Smoking and Smoking Cessation on Oncologic Outcomes in Primary Non–muscle-invasive Bladder Cancer. Eur. Urol. 2013, 63, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.M.; Dunagin, M.C.; Torborg, S.R.; Torre, E.A.; Emert, B.; Krepler, C.; Beqiri, M.; Sproesser, K.; Brafford, P.A.; Xiao, M.; et al. Rare cell variability and drug-induced reprogramming as a mode of cancer drug resistance. Nature 2017, 546, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, S.M.; Emert, B.L.; Hueros, R.A.R.; Cote, C.; Harmange, G.; Schaff, D.L.; Sizemore, A.E.; Gupte, R.; Torre, E.; Singh, A.; et al. Memory Sequencing Reveals Heritable Single-Cell Gene Expression Programs Associated with Distinct Cellular Behaviors. Cell 2020, 182, 947–959.e17. [Google Scholar] [CrossRef] [PubMed]
- Svensson, V.; Beltrame, E.D.V.; Pachter, L. A curated database reveals trends in single-cell transcriptomics. Database 2020, 2020, baaa073. [Google Scholar] [CrossRef]
- Tao, Y.; Kang, B.; Petkovich, D.A.; Bhandari, Y.R.; In, J.; Stein-O’Brien, G.; Kong, X.; Xie, W.; Zachos, N.; Maegawa, S.; et al. Aging-like Spontaneous Epigenetic Silencing Facilitates Wnt Activation, Stemness, and BrafV600E-Induced Tumorigenesis. Cancer Cell 2019, 35, 315–328.e6. [Google Scholar] [CrossRef]
- Tosti, L.; Hang, Y.; Debnath, O.; Tiesmeyer, S.; Trefzer, T.; Steiger, K.; Ten, F.W.; Lukassen, S.; Ballke, S.; Kühl, A.A.; et al. Single-Nucleus and In Situ RNA–Sequencing Reveal Cell Topographies in the Human Pancreas. Gastroenterology 2021, 160, 1330–1344.e11. [Google Scholar] [CrossRef]
- Waddington, C.H. Canalization of Development and the Inheritance of Acquired Characters. Nature 1942, 150, 563–565. [Google Scholar] [CrossRef]
- Wang, C.-C.; Bajikar, S.S.; Jamal, L.; Atkins, K.A.; Janes, K.A. A time- and matrix-dependent TGFBR3–JUND–KRT5 regulatory circuit in single breast epithelial cells and basal-like premalignancies. Nat. Cell Biol. 2014, 16, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Wollny, D.; Zhao, S.; Everlien, I.; Lun, X.; Brunken, J.; Brüne, D.; Ziebell, F.; Tabansky, I.; Weichert, W.; Marciniak-Czochra, A.; et al. Single-Cell Analysis Uncovers Clonal Acinar Cell Heterogeneity in the Adult Pancreas. Dev. Cell 2016, 39, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Corredor, A.L.G.; Messina-Pacheco, J.; Li, Q.; Zogopoulos, G.; Kaddour, N.; Wang, Y.; Shi, B.-Y.; Gregorieff, A.; Liu, J.-L.; et al. REG3A/REG3B promotes acinar to ductal metaplasia through binding to EXTL3 and activating the RAS-RAF-MEK-ERK signaling pathway. Commun. Biol. 2021, 4, 688. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).