Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
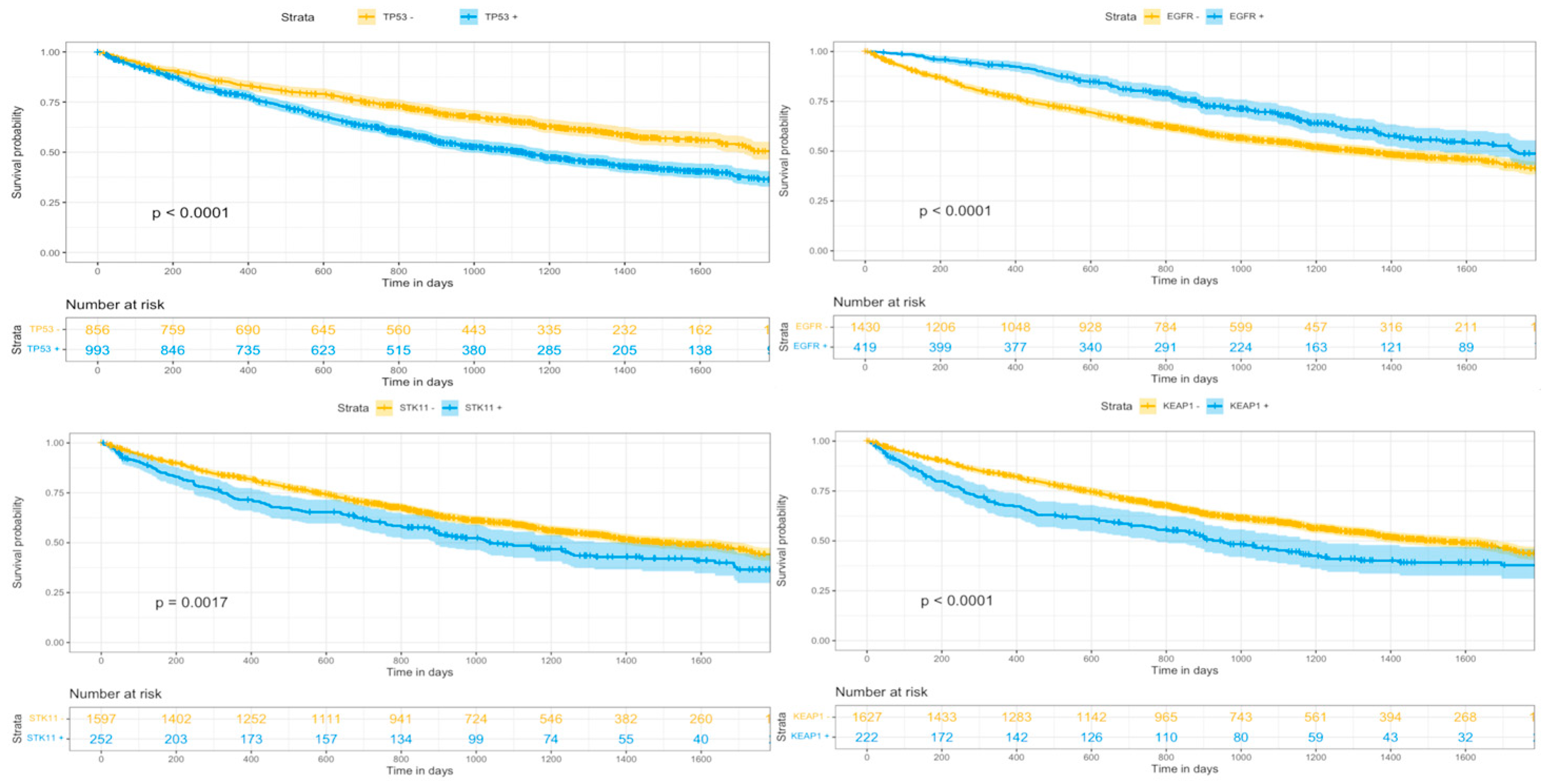
3. Results
3.1. Patient Characteristics
3.2. Imaging Studies and Race
3.3. Imaging-Derived Response and Genomic & Racial Diversity
4. Discussion
4.1. Disparity in Imaging Studies and Race
4.2. Genomics, Racial Diversity, and Image Derived Response
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AACR | American Association for Cancer Research |
| CT | Computed Tomography |
| MR | Magnetic Resonance |
| NSCLC | Non-Small Cell Lung Cancer |
| OR | Odds Ratio |
| OS | Overall Survival |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PSF | Progression Free Survival |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee in collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics 2021; Canadian Cancer Society: Toronto, ON, Canada, 2021. [Google Scholar]
- Henley, S.J.; Ward, E.M.; Scott, S.; Ma, J.; Anderson, R.N.; Firth, A.U.; Thomas, C.C.; Islami, F.; Weir, H.K.; Lewis, D.R.; et al. Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer 2020, 126, 2225–2249. [Google Scholar] [CrossRef] [PubMed]
- Kutikova, L.; Bowman, L.; Chang, S.; Long, S.R.; Obasaju, C.; Crown, W.H. The economic burden of lung cancer and the associated costs of treatment failure in the United States. Lung Cancer 2005, 50, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.K.; Will, B.P.; Berthelot, J.M.; Wolfson, M.C. The economics of lung cancer management in Canada. Lung Cancer 1996, 14, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.D.; Lathan, C.; Sholl, L.; Ducar, M.; Vega, M.; Sunkavalli, A.; Lin, L.; Hanna, M.; Schubert, L.; Thorner, A.; et al. Comparison of Prevalence and Types of Mutations in Lung Cancers Among Black and White Populations. JAMA Oncol. 2017, 3, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Zavala, V.A.; Bracci, P.M.; Carethers, J.M.; Carvajal-Carmona, L.; Coggins, N.B.; Cruz-Correa, M.R.; Davis, M.; de Smith, A.J.; Dutil, J.; Figueiredo, J.C.; et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 2021, 124, 315–332. [Google Scholar] [CrossRef]
- Mitchell, A.P.; Bach, P.B. Use of Positron Emission Tomography Imaging: Another Nonbiological Source of Racial Disparities in US Cancer Care. J. Natl. Cancer Inst. 2020, 112, 1177–1178. [Google Scholar] [CrossRef]
- Ryan, B.M. Lung cancer health disparities. Carcinogenesis 2018, 39, 741–751. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gonzalez, A.V.; Jantz, M.A.; Margolis, M.L.; Gould, M.K.; Tanoue, L.T.; Harris, L.J.; Detterbeck, F.C. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143 (Suppl. 5), e211S–e250S. [Google Scholar] [CrossRef]
- Postmus, P.E.; Kerr, K.M.; Oudkerk, M.; Senan, S.; Waller, D.A.; Vansteenkiste, J.; Escriu, C.; Peters, S. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28 (Suppl. 4), iv1–iv21. [Google Scholar] [CrossRef]
- Ung, Y.C.; Souter, L.H.; Darling, G.; Dobranowski, J.; Donohue, L.; Leighl, N.; Ellis, P.M. Follow-Up and Surveillance of Curatively Treated Lung Cancer Patients; Program in Evidence-Based Care Evidence-Based Series No.: 26-3 IN REVIEW; Cancer Care Ontario: Toronto, ON, Canada, 2014. [Google Scholar]
- Schneider, B.J.; Ismaila, N.; Aerts, J.; Chiles, C.; Daly, M.E.; Detterbeck, F.C.; Hearn, J.W.; Katz, S.I.; Leighl, N.B.; Levy, B.; et al. Lung Cancer Surveillance After Definitive Curative-Intent Therapy: ASCO Guideline. J. Clin. Oncol. 2020, 38, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Gould, M.K.; Ellen, M.S.; Wagner, T.H.; Xu, X.; Ghaus, S.J.; Wallace, R.B.; Provenzale, D.; Au, D.H. Disparities in lung cancer staging with positron emission tomography in the Cancer Care Outcomes Research and Surveillance (CanCORS) study. J. Thorac. Oncol. 2011, 6, 875–883. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Karam, S.D.; Bradley, C.J. Ethnic Disparities in Imaging Utilization at Diagnosis of Non-Small Cell Lung Cancer. J. Natl. Cancer Inst. 2020, 112, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Maziak, D.E.; Darling, G.E.; Inculet, R.I.; Gulenchyn, K.Y.; Driedger, A.A.; Ung, Y.C.; Miller, J.D.; Gu, C.S.; Cline, K.J.; Evans, W.K.; et al. Positron emission tomography in staging early lung cancer: A randomized trial. Ann. Intern. Med. 2009, 151, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Christiani, D.C. East meets West: Ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin. J. Cancer 2011, 30, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, J.; Zhang, S.; Wang, M.; Yang, S.; Li, N.; Wu, G.; Liu, W.; Liao, G.; Cai, K.; et al. Molecular Epidemiology of EGFR Mutations in Asian Patients with Advanced Non-Small-Cell Lung Cancer of Adenocarcinoma Histology-Mainland China Subset Analysis of the PIONEER study. PLoS ONE 2015, 10, e0143515. [Google Scholar] [CrossRef]
- Lusk, C.M.; Watza, D.; Dyson, G.; Craig, D.; Ratliff, V.; Wenzlaff, A.S.; Lonardo, F.; Bollig-Fischer, A.; Bepler, G.; Purrington, K.; et al. Profiling the Mutational Landscape in Known Driver Genes and Novel Genes in African American Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2019, 25, 4300–4308. [Google Scholar] [CrossRef]
- Bollig-Fischer, A.; Chen, W.; Gadgeel, S.M.; Wenzlaff, A.S.; Cote, M.L.; Schwartz, A.G.; Bepler, G. Racial diversity of actionable mutations in non-small cell lung cancer. J. Thorac. Oncol. 2015, 10, 250–255. [Google Scholar] [CrossRef]
- Consortium APG. AACR Project GENIE: Powering precision medicine through an international consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Suga, J.M.; Nguyen, D.V.; Mohammed, S.M.; Brown, M.; Calhoun, R.; Yoneda, K.; Gandara, D.R.; Lara, P.N., Jr. Racial disparities on the use of invasive and noninvasive staging in patients with non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 1772–1778. [Google Scholar] [CrossRef]
- Japuntich, S.J.; Krieger, N.H.; Salvas, A.L.; Carey, M.P. Racial Disparities in Lung Cancer Screening: An Exploratory Investigation. J. Natl. Med. Assoc. 2018, 110, 424–427. [Google Scholar] [CrossRef]
- Tanner, N.T.; Gebregziabher, M.; Hughes Halbert, C.; Payne, E.; Egede, L.E.; Silvestri, G.A. Racial Differences in Outcomes within the National Lung Screening Trial. Implications for Widespread Implementation. Am. J. Respir. Crit. Care Med. 2015, 192, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, M.; Navani, N. Fulfilling the Dream. Toward Reducing Inequalities in Lung Cancer Screening. Am. J. Respir. Crit. Care Med. 2015, 192, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Quadrelli, S.; Lyons, G.; Colt, H.; Chimondeguy, D.; Buero, A. Clinical characteristics and prognosis of incidentally detected lung cancers. Int. J. Surg. Oncol. 2015, 2015, 287604. [Google Scholar] [CrossRef]
- Sharma, D.; Newman, T.G.; Aronow, W.S. Lung cancer screening: History, current perspectives, and future directions. Arch. Med. Sci. 2015, 11, 1033–1043. [Google Scholar] [CrossRef]
- Kunitomo, Y.; Bade, B.; Gunderson, C.G.; Akgün, K.M.; Brackett, A.; Cain, H.; Tanoue, L.; Bastian, L.A. Racial Differences in Adherence to Lung Cancer Screening Follow-up: A Systematic Review and Meta-analysis. Chest 2022, 161, 266–275. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Miller, K.D.; Goding Sauer, A.; Jemal, A.; Siegel, R.L. Cancer statistics for African Americans, 2019. CA Cancer J. Clin. 2019, 69, 211–233. [Google Scholar] [CrossRef] [PubMed]
- Tannenbaum, S.L.; Koru-Sengul, T.; Zhao, W.; Miao, F.; Byrne, M.M. Survival disparities in non-small cell lung cancer by race, ethnicity, and socioeconomic status. Cancer J. 2014, 20, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, B.; Li, Q.; Xu, X.; Li, X.; You, X.; Yu, Z. Genetic profile of non-small cell lung cancer (NSCLC): A hospital-based survey in Jinhua. Mol. Genet. Genom. Med. 2020, 8, e1398. [Google Scholar] [CrossRef]
- Adderley, H.; Blackhall, F.H.; Lindsay, C.R. KRAS-mutant non-small cell lung cancer: Converging small molecules and immune checkpoint inhibition. EBioMedicine 2019, 41, 711–716. [Google Scholar] [CrossRef]
- Dearden, S.; Stevens, J.; Wu, Y.L.; Blowers, D. Mutation incidence and coincidence in non small-cell lung cancer: Meta-analyses by ethnicity and histology (mutMap). Ann. Oncol. 2013, 24, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Zhu, Q.G.; Ding, X.X.; Lin, S.; Zhao, J.; Guan, L.; Li, T.; He, B.; Zhang, H.Q. Prognostic value of EGFR and KRAS in resected non-small cell lung cancer: A systematic review and meta-analysis. Cancer Manag. Res. 2018, 10, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Kehl, K.L.; Riely, G.J.; Lepisto, E.M.; Lavery, J.A.; Warner, J.L.; LeNoue-Newton, M.L.; Sweeney, S.M.; Rudolph, J.E.; Brown, S.; Yu, C.; et al. Correlation Between Surrogate End Points and Overall Survival in a Multi-institutional Clinicogenomic Cohort of Patients With Non-Small Cell Lung or Colorectal Cancer. JAMA Netw. Open 2021, 4, e2117547. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Zhan, J.; Zhao, X.; Chen, X.; Xiao, L.; Wu, K.; Ma, Y.; Li, M.; Yang, Y.; et al. Utility of comprehensive genomic profiling in directing treatment and improving patient outcomes in advanced non-small cell lung cancer. BMC Med. 2021, 19, 223. [Google Scholar] [CrossRef]
- Goldman, M.L.; Kim, C.; Chen, Z.; Calzada, O.; Churnetski, M.C.; Flowers, C.; Cohen, J.B. Surveillance imaging during first remission in follicular lymphoma does not impact overall survival. Cancer 2021, 127, 3390–3402. [Google Scholar] [CrossRef]
- Hess, L.M.; Brnabic, A.; Mason, O.; Lee, P.; Barker, S. Relationship between Progression-free Survival and Overall Survival in Randomized Clinical Trials of Targeted and Biologic Agents in Oncology. J. Cancer 2019, 10, 3717–3727. [Google Scholar] [CrossRef]

| n = 1849 | |
|---|---|
| Sex (n) | |
| Male | 42% (784) |
| Female | 58% (1065) |
| Race (n) | |
| White | 83.6% (1545) |
| Black | 5.1% (94) |
| Chinese | 4.7% (87) |
| Other Asian | 1.8% (33) |
| American Indian, Aleutian or Eskimo | 0.2% (4) |
| Hawaiian | 0.1% (2) |
| Other | 1.4% (25) |
| Unknown | 3.2% (59) |
| Age at Dx (Sd) | 64.4 (10.5) |
| NSCLC Type (n) | |
| Adenocarcinoma | 68% (1262) |
| Squamous Cell | 9% (170) |
| Other | 23% (417) |
| Stage at Dx (n) * | |
| I-III | 57% (1052) |
| IV | 43% (793) |
| Smoking History (n) | |
| Current | 14% (259) |
| Former <1 y | 12% (218) |
| Former >1 y | 51% (950) |
| Never | 23% (419) |
| Other Cancers (n) | 29% (540) |
| Cancer-Related Death (n) | 49% (912) |
| Progression (n) | |
| 2–4 mo | 10% (179) |
| 5–7 mo | 10% (187) |
| 10–14 mo | 17% (311) |
| Within the 1st year | 31% (572) |
| Chemotherapy (n) | 65% (1197) |
| Platinum Based Therapy (n) | 69% (828) |
| Complete Baseline Imaging (n) | 43% (516) |
| Progression (n) | |
| 2–4 mo | 12% (143) |
| 5–7 mo | 13% (152) |
| 10–14 mo | 22% (268) |
| Race (n = 1155 *) | Baseline Imaging | Baseline CT † | Baseline MR | Baseline PET | ||||
|---|---|---|---|---|---|---|---|---|
| + | − | + | − | + | − | + | − | |
| White | 421 (44%) | 540 (56%) | 421 (44%) | 540 (56%) | 667 (69%) | 294 (31%) | 676 (70%) | 285 (30%) |
| Non-White | 78 (40%) | 116 (60%) | 78 (40%) | 116 (60%) | 123 (63%) | 71 (36%) | 124 (64%) | 70 (36%) |
| Genetic Markers (N) | All (N = 1849) | White (N = 1545) * | Non-White (N = 245) * | p |
|---|---|---|---|---|
| TP53 | 53.7% (993) | 54.1% (836) | 50.6% (124) | 0.11 |
| KRAS | 30.8% (569) | 33.3% (514) | 17.9% (44) | 0.02 |
| EGFR | 22.7% (419) | 19.2% (296) | 44.1% (108) | <0.01 |
| STK11 | 13.6% (252) | 14.8% (229) | 7.3% (18) | 0.01 |
| KEAP1 | 12.0% (222) | 13.3% (206) | 5.3% (13) | <0.01 |
| KMT2D | 9.3% (172) | 9.9% (153) | 6.1% (15) | 0.06 |
| RBM10 | 6.9% (127) | 7.2% (112) | 5.3% (13) | 0.23 |
| PRKDC | 5.0% (92) | 5.4% (83) | 3.3% (8) | 0.53 |
| LRP1B | 1.0% (18) | 0.9% (15) | 0.8% (2) | 0.89 |
| GRM3 | 0.3% (6) | 0.3% (4) | 0.8% (2) | 0.85 |
| KRAS + STK11+ | 7.5% (140) | 8.5% (132) | 3.2% (8) | <0.01 |
| KRAS + KEAP1+ | 4.5% (85) | 5.4% (84) | 0.4% (1) | <0.01 |
| Predictors | All Patients | Chemotherapy Group | ||||
|---|---|---|---|---|---|---|
| Death | Death | |||||
| Hazard Ratio | 95% CI | p | Hazard Ratio | 95% CI | p | |
| Progression at 3 mo | 1.70 | 1.40–2.05 | <0.01 | 1.92 | 1.55–2.38 | <0.01 |
| Progression at 6 mo | 1.43 | 1.19–1.72 | <0.01 | 1.69 | 1.37–2.07 | <0.01 |
| Progression at 1 y | 0.87 | 0.74–1.01 | 0.07 | 0.97 | 0.82–1.15 | 0.72 |
| Race White | 1.09 | 0.90–1.32 | 0.37 | 1.07 | 0.85–1.33 | 0.57 |
| TP53 | 1.37 | 1.19–1.58 | <0.01 | 1.42 | 1.20–1.67 | 0.02 |
| KRAS | 1.25 | 1.08–1.47 | <0.01 | 1.26 | 1.05– 1.52 | 0.01 |
| EGFR | 0.69 | 0.58–0.83 | 0.03 | 0.70 | 0.58–0.86 | <0.01 |
| STK11 | 0.96 | 0.79–1.18 | 0.76 | 1.03 | 0.81–1.31 | 0.82 |
| KEAP1 | 1.10 | 0.89–1.36 | 0.36 | 1.17 | 0.91–1.49 | 0.22 |
| LRP1B | 0.39 | 0.22–0.70 | <0.01 | 0.35 | 0.19–0.67 | <0.01 |
| PRKDC | 1.15 | 0.86–1.54 | 0.36 | 1.06 | 0.73–1.52 | 0.77 |
| RBM10 | 1.12 | 0.83–1.50 | 0.47 | 1.15 | 0.83–1.59 | 0.41 |
| KMT2D | 1.10 | 0.87–1.38 | 0.44 | 0.95 | 0.72–1.26 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohan, A.; Kulanthaivelu, R.; Hinzpeter, R.; Liu, Z.A.; Ortega, C.; Leighl, N.; Metser, U.; Veit-Haibach, P., on behalf of the AACR Project GENIE BPC Core Team. Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database. Cancers 2023, 15, 2096. https://doi.org/10.3390/cancers15072096
Kohan A, Kulanthaivelu R, Hinzpeter R, Liu ZA, Ortega C, Leighl N, Metser U, Veit-Haibach P on behalf of the AACR Project GENIE BPC Core Team. Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database. Cancers. 2023; 15(7):2096. https://doi.org/10.3390/cancers15072096
Chicago/Turabian StyleKohan, Andres, Roshini Kulanthaivelu, Ricarda Hinzpeter, Zhihui Amy Liu, Claudia Ortega, Natasha Leighl, Ur Metser, and Patrick Veit-Haibach on behalf of the AACR Project GENIE BPC Core Team. 2023. "Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database" Cancers 15, no. 7: 2096. https://doi.org/10.3390/cancers15072096
APA StyleKohan, A., Kulanthaivelu, R., Hinzpeter, R., Liu, Z. A., Ortega, C., Leighl, N., Metser, U., & Veit-Haibach, P., on behalf of the AACR Project GENIE BPC Core Team. (2023). Disparity and Diversity in NSCLC Imaging and Genomics: Evaluation of a Mature, Multicenter Database. Cancers, 15(7), 2096. https://doi.org/10.3390/cancers15072096






